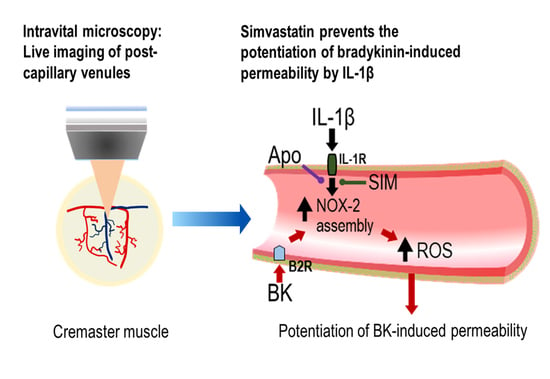
Redox Regulation of Microvascular Permeability: IL-1β Potentiation of Bradykinin-Induced Permeability Is Prevented by Simvastatin
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Isolation of the Cremaster Skeletal Muscle Preparation
2.2. Superfusion of Cremaster Muscle Preparation
2.3. Measurement of Post-Capillary Venule Permeability to FITC-Albumin
2.4. Role of Nitric Oxide and Reactive Oxygen Species in Microvascular Permeability
2.5. Bradykinin- and IL-1β-Induced Increases in Microvascular Permeability
2.6. Inhibition of NADPH Oxidase Assembly
2.7. Pretreatment of Animals with Simvastatin
2.8. Inhibition of Heme Oxygenase-1 with Tin Protophoryrin IX (SnPP)
2.9. Reagents
2.10. Statistical Analysis
3. Results
3.1. Role of NO and Reactive Oxygen Species in Modulating Basal Microvascular Permeability
3.2. Histamine- and Bradykinin-Induced Microvascular Permeability Is Mediated by Different Signaling Pathways
3.3. Bradykinin-Induced Microvascular Permeability Is Potentiated by IL-1β
3.4. A Role for NADPH Oxidase and Reactive Oxygen Species in the Potentiation of Bradykinin-Induced Microvascular Permeability by IL-1β
3.5. Pretreatment of Animals with Simvastatin
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nussberger, J.; Cugno, M.; Cicardi, M. Bradykinin-mediated angioedema. N. Engl. J. Med. 2002, 347, 621–622. [Google Scholar] [CrossRef]
- Bas, M.; Adams, V.; Suvorava, T.; Niehues, T.; Hoffmann, T.K.; Kojda, G. Nonallergic angioedema: Role of bradykinin. Allergy 2007, 62, 842–856. [Google Scholar] [CrossRef]
- Obtulowicz, K. Bradykinin-mediated angioedema. Pol. Arch. Med. Wewn. 2016, 126, 76–85. [Google Scholar] [CrossRef][Green Version]
- Zausinger, S.; Lumenta, D.B.; Pruneau, D.; Schmid-Elsaesser, R.; Plesnila, N.; Baethmann, A. Effects of LF 16-0687 Ms, a bradykinin B(2) receptor antagonist, on brain edema formation and tissue damage in a rat model of temporary focal cerebral ischemia. Brain Res. 2002, 950, 268–278. [Google Scholar] [CrossRef]
- Ruiz, S.; Vardon-Bounes, F.; Buleon, M.; Guilbeau-Frugier, C.; Seguelas, M.H.; Conil, J.M.; Girolami, J.P.; Tack, I.; Minville, V. Kinin B1 receptor: A potential therapeutic target in sepsis-induced vascular hyperpermeability. J. Transl. Med. 2020, 18, 174. [Google Scholar] [CrossRef]
- Othman, R.; Vaucher, E.; Couture, R. Bradykinin Type 1 Receptor—Inducible Nitric Oxide Synthase: A New Axis Implicated in Diabetic Retinopathy. Front. Pharmacol. 2019, 10, 300. [Google Scholar] [CrossRef]
- Jacobson, J.R.; Barnard, J.W.; Grigoryev, D.N.; Ma, S.F.; Tuder, R.M.; Garcia, J.G. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L1026–L1032. [Google Scholar] [CrossRef]
- Nagaraja, T.N.; Knight, R.A.; Croxen, R.L.; Konda, K.P.; Fenstermacher, J.D. Acute neurovascular unit protection by simvastatin in transient cerebral ischemia. Neurol. Res. 2006, 28, 826–830. [Google Scholar] [CrossRef]
- Beziaud, T.; Ru Chen, X.; El Shafey, N.; Frechou, M.; Teng, F.; Palmier, B.; Beray-Berthat, V.; Soustrat, M.; Margaill, I.; Plotkine, M.; et al. Simvastatin in traumatic brain injury: Effect on brain edema mechanisms. Crit. Care Med. 2011, 39, 2300–2307. [Google Scholar] [CrossRef]
- Yang, D.; Knight, R.A.; Han, Y.; Karki, K.; Zhang, J.; Ding, C.; Chopp, M.; Seyfried, D.M. Vascular recovery promoted by atorvastatin and simvastatin after experimental intracerebral hemorrhage: Magnetic resonance imaging and histological study. J. Neurosurg. 2011, 114, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Regoli, D. Neurohumoral regulation of precapillary vessels: The kallikrein-kinin system. J. Cardiovasc. Pharmacol. 1984, 6, S401–S412. [Google Scholar] [CrossRef]
- Teuwen, L.A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Roche, J.A.; Roche, R. A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 7265–7269. [Google Scholar] [CrossRef]
- Van de Veerdonk, F.L.; Netea, M.G.; van Deuren, M.; van der Meer, J.W.; de Mast, Q.; Bruggemann, R.J.; van der Hoeven, H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife 2020, 9, e57555. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Penninger, J.M. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp. Physiol. 2008, 93, 543–548. [Google Scholar] [CrossRef]
- Shimizu, S.; Ishii, M.; Yamamoto, T.; Kawanishi, T.; Momose, K.; Kuroiwa, Y. Bradykinin induces generation of reactive oxygen species in bovine aortic endothelial cells. Res. Commun. Chem. Pathol. Pharmacol. 1994, 84, 301–314. [Google Scholar]
- Woodfin, A.; Hu, D.E.; Sarker, M.; Kurokawa, T.; Fraser, P. Acute NADPH oxidase activation potentiates cerebrovascular permeability response to bradykinin in ischemia-reperfusion. Free Radic. Biol. Med. 2011, 50, 518–524. [Google Scholar] [CrossRef]
- Sarker, M.H.; Hu, D.E.; Fraser, P.A. Acute effects of bradykinin on cerebral microvascular permeability in the anaesthetized rat. J. Physiol. 2000, 528, 177–187. [Google Scholar] [CrossRef]
- Sobey, C.G. Bradykinin B2 receptor antagonism: A new direction for acute stroke therapy? Br. J. Pharmacol. 2003, 139, 1369–1371. [Google Scholar] [CrossRef]
- Touzani, O.; Boutin, H.; Chuquet, J.; Rothwell, N. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J. Neuroimmunol. 1999, 100, 203–215. [Google Scholar] [CrossRef]
- Cavalli, G.; De Luca, G.; Campochiaro, C.; Della-Torre, E.; Ripa, M.; Canetti, D.; Oltolini, C.; Castiglioni, B.; Tassan Din, C.; Boffini, N.; et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol. 2020, 2, e325–e331. [Google Scholar] [CrossRef]
- Ucciferri, C.; Auricchio, A.; Di Nicola, M.; Potere, N.; Abbate, A.; Cipollone, F.; Vecchiet, J.; Falasca, K. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol. 2020. [Google Scholar] [CrossRef]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.; Ross, R.; Frydas, I.; Kritas, S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 327–331. [Google Scholar]
- Stoll, L.L.; McCormick, M.L.; Denning, G.M.; Weintraub, N.L. Antioxidant effects of statins. Timely Top. Med. Cardiovasc. Dis. 2005, 9, E1. [Google Scholar] [CrossRef]
- Freitas, F.; Estato, V.; Reis, P.; Castro-Faria-Neto, H.C.; Carvalho, V.; Torres, R.; Lessa, M.A.; Tibirica, E. Acute simvastatin treatment restores cerebral functional capillary density and attenuates angiotensin II-induced microcirculatory changes in a model of primary hypertension. Microcirculation 2017, 24. [Google Scholar] [CrossRef]
- Margaritis, M.; Sanna, F.; Antoniades, C. Statins and oxidative stress in the cardiovascular system. Curr. Pharm. Des. 2017. [Google Scholar] [CrossRef]
- Ali, F.; Zakkar, M.; Karu, K.; Lidington, E.A.; Hamdulay, S.S.; Boyle, J.J.; Zloh, M.; Bauer, A.; Haskard, D.O.; Evans, P.C.; et al. Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow. J. Biol. Chem. 2009, 284, 18882–18892. [Google Scholar] [CrossRef]
- Chartoumpekis, D.; Ziros, P.G.; Psyrogiannis, A.; Kyriazopoulou, V.; Papavassiliou, A.G.; Habeos, I.G. Simvastatin lowers reactive oxygen species level by Nrf2 activation via PI3K/Akt pathway. Biochem. Biophys. Res. Commun. 2010, 396, 463–466. [Google Scholar] [CrossRef]
- Gueler, F.; Park, J.K.; Rong, S.; Kirsch, T.; Lindschau, C.; Zheng, W.; Elger, M.; Fiebeler, A.; Fliser, D.; Luft, F.C.; et al. Statins attenuate ischemia-reperfusion injury by inducing heme oxygenase-1 in infiltrating macrophages. Am. J. Pathol. 2007, 170, 1192–1199. [Google Scholar] [CrossRef]
- Hsu, H.H.; Ko, W.J.; Hsu, J.Y.; Chen, J.S.; Lee, Y.C.; Lai, I.R.; Chen, C.F. Simvastatin ameliorates established pulmonary hypertension through a heme oxygenase-1 dependent pathway in rats. Respir. Res. 2009, 10, 32. [Google Scholar] [CrossRef]
- Makabe, S.; Takahashi, Y.; Watanabe, H.; Murakami, M.; Ohba, T.; Ito, H. Fluvastatin protects vascular smooth muscle cells against oxidative stress through the Nrf2-dependent antioxidant pathway. Atherosclerosis 2010, 213, 377–384. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Kopacz, A.; Kloska, D.; Zagrapan, B.; Neumayer, C.; Grochot-Przeczek, A.; Huk, I.; Brostjan, C.; Dulak, J.; Jozkowicz, A. Simvastatin Treatment Upregulates HO-1 in Patients with Abdominal Aortic Aneurysm but Independently of Nrf2. Oxidative Med. Cell. Longev. 2018, 2018, 2028936. [Google Scholar] [CrossRef]
- Chambers, D.J.; Fallouh, H.B. Cardioplegia and cardiac surgery: Pharmacological arrest and cardioprotection during global ischemia and reperfusion. Pharmacol. Ther. 2010, 127, 41–52. [Google Scholar] [CrossRef]
- Fraser, P.A.; Dallas, A.D.; Davies, S. Measurement of filtration coefficient in single cerebral microvessels of the frog. J. Physiol. 1990, 423, 343–361. [Google Scholar] [CrossRef]
- Ximenes, V.F.; Kanegae, M.P.; Rissato, S.R.; Galhiane, M.S. The oxidation of apocynin catalyzed by myeloperoxidase: Proposal for NADPH oxidase inhibition. Arch. Biochem. Biophys. 2007, 457, 134–141. [Google Scholar] [CrossRef]
- Kaizu, T.; Tamaki, T.; Tanaka, M.; Uchida, Y.; Tsuchihashi, S.; Kawamura, A.; Kakita, A. Preconditioning with tin-protoporphyrin IX attenuates ischemia/reperfusion injury in the rat kidney. Kidney Int. 2003, 63, 1393–1403. [Google Scholar] [CrossRef]
- Cambridge, H.; Brain, S.D. Mechanism of bradykinin-induced plasma extravasation in the rat knee joint. Br. J. Pharmacol. 1995, 115, 641–647. [Google Scholar] [CrossRef]
- Feletou, M.; Bonnardel, E.; Canet, E. Bradykinin and changes in microvascular permeability in the hamster cheek pouch: Role of nitric oxide. Br. J. Pharmacol. 1996, 118, 1371–1376. [Google Scholar] [CrossRef][Green Version]
- Holland, J.A.; Pritchard, K.A.; Pappolla, M.A.; Wolin, M.S.; Rogers, N.J.; Stemerman, M.B. Bradykinin induces superoxide anion release from human endothelial cells. J. Cell. Physiol. 1990, 143, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Wambi-Kiesse, C.O.; Katusic, Z.S. Inhibition of copper/zinc superoxide dismutase impairs NO.-mediated endothelium-dependent relaxations. Am. J. Physiol. 1999, 276, H1043–H1048. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, S.; Ishida, S.; Gute, D.C.; Korthuis, R.J. Bradykinin-induced proinflammatory signaling mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2676–H2686. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Vanhoutte, P.M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989, 3, 2007–2018. [Google Scholar] [CrossRef]
- Nizamutdinova, I.T.; Maejima, D.; Nagai, T.; Bridenbaugh, E.; Thangaswamy, S.; Chatterjee, V.; Meininger, C.J.; Gashev, A.A. Involvement of histamine in endothelium-dependent relaxation of mesenteric lymphatic vessels. Microcirculation 2014, 21, 640–648. [Google Scholar] [CrossRef]
- Manitsopoulos, N.; Orfanos, S.E.; Kotanidou, A.; Nikitopoulou, I.; Siempos, I.; Magkou, C.; Dimopoulou, I.; Zakynthinos, S.G.; Armaganidis, A.; Maniatis, N.A. Inhibition of HMGCoA reductase by simvastatin protects mice from injurious mechanical ventilation. Respir. Res. 2015, 16, 24. [Google Scholar] [CrossRef]
- Yang, C.H.; Kao, M.C.; Shih, P.C.; Li, K.Y.; Tsai, P.S.; Huang, C.J. Simvastatin attenuates sepsis-induced blood-brain barrier integrity loss. J. Surg. Res. 2015, 194, 591–598. [Google Scholar] [CrossRef]
- Tuuminen, R.; Sahanne, S.; Loukovaara, S. Low intravitreal angiopoietin-2 and VEGF levels in vitrectomized diabetic patients with simvastatin treatment. Acta Ophthalmol. 2014, 92, 675–681. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, H. Simvastatin increases circulating endothelial progenitor cells and reduces the formation and progression of diabetic retinopathy in rats. Exp. Eye Res. 2012, 105, 1–8. [Google Scholar] [CrossRef]
- Caldwell, R.B.; Bartoli, M.; Behzadian, M.A.; El-Remessy, A.E.; Al-Shabrawey, M.; Platt, D.H.; Liou, G.I.; Caldwell, R.W. Vascular endothelial growth factor and diabetic retinopathy: Role of oxidative stress. Curr. Drug Targets 2005, 6, 511–524. [Google Scholar] [CrossRef]
- Dell’Omo, G.; Bandinelli, S.; Penno, G.; Pedrinelli, R.; Mariani, M. Simvastatin, capillary permeability, and acetylcholine-mediated vasomotion in atherosclerotic, hypercholesterolemic men. Clin. Pharmacol. Ther. 2000, 68, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuw Amerongen, G.P.; Vermeer, M.A.; Negre-Aminou, P.; Lankelma, J.; Emeis, J.J.; van Hinsbergh, V.W. Simvastatin improves disturbed endothelial barrier function. Circulation 2000, 102, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D.; Haas, M.J.; Batejko, O.; Hovsepyan, M.; Feman, S.S. Statins ameliorate endothelial barrier permeability changes in the cerebral tissue of streptozotocin-induced diabetic rats. Diabetes 2005, 54, 2977–2982. [Google Scholar] [CrossRef] [PubMed]
- Liesmaa, I.; Kokkonen, J.O.; Kovanen, P.T.; Lindstedt, K.A. Lovastatin induces the expression of bradykinin type 2 receptors in cultured human coronary artery endothelial cells. J. Mol. Cell. Cardiol. 2007, 43, 593–600. [Google Scholar] [CrossRef]
- Blum, C.B. Comparison of properties of four inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Am. J. Cardiol. 1994, 73, 3d–11d. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, J.K. Pleiotropic effects of statins—Basic research and clinical perspectives. Circ. J. Off. J. Jpn. Circ. Soc. 2010, 74, 818–826. [Google Scholar]
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam. Clin. Pharmacol. 2005, 19, 117–125. [Google Scholar] [CrossRef]
- Kunz, M.; Nussberger, J.; Holtmannspötter, M.; Bitterling, H.; Plesnila, N.; Zausinger, S. Bradykinin in blood and cerebrospinal fluid after acute cerebral lesions: Correlations with cerebral edema and intracranial pressure. J. Neurotrauma 2013, 30, 1638–1644. [Google Scholar] [CrossRef]
- Dobrivojevic, M.; Spiranec, K.; Sindic, A. Involvement of bradykinin in brain edema development after ischemic stroke. Pflug. Arch. Eur. J. Physiol. 2015, 467, 201–212. [Google Scholar] [CrossRef]
- Eder, C. Mechanisms of interleukin-1beta release. Immunobiology 2009, 214, 543–553. [Google Scholar] [CrossRef]
- Yang, D.; Elner, S.G.; Bian, Z.M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye Res. 2007, 85, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Warboys, C.M.; Toh, H.B.; Fraser, P.A. Role of NADPH oxidase in retinal microvascular permeability increase by RAGE activation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Ziff, M. Increased superoxide anion release from human endothelial cells in response to cytokines. J. Immunol. 1986, 137, 3295–3298. [Google Scholar] [PubMed]
- Wagner, A.H.; Kohler, T.; Ruckschloss, U.; Just, I.; Hecker, M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arter. Thromb. Vasc. Biol. 2000, 20, 61–69. [Google Scholar] [CrossRef]
- Endres, M.; Laufs, U. Effects of statins on endothelium and signaling mechanisms. Stroke 2004, 35, 2708–2711. [Google Scholar] [CrossRef]
- Chen, W.; Pendyala, S.; Natarajan, V.; Garcia, J.G.; Jacobson, J.R. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L575–L583. [Google Scholar] [CrossRef]
- Wassmann, S.; Laufs, U.; Baumer, A.T.; Muller, K.; Konkol, C.; Sauer, H.; Bohm, M.; Nickenig, G. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: Involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol. Pharmacol. 2001, 59, 646–654. [Google Scholar] [CrossRef]
- Delbosc, S.; Morena, M.; Djouad, F.; Ledoucen, C.; Descomps, B.; Cristol, J.P. Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are able to reduce superoxide anion production by NADPH oxidase in THP-1-derived monocytes. J. Cardiovasc. Pharmacol. 2002, 40, 611–617. [Google Scholar] [CrossRef]
- Hordijk, P.L. Regulation of NADPH oxidases: The role of Rac proteins. Circ. Res. 2006, 98, 453–462. [Google Scholar] [CrossRef]
- Maack, C.; Kartes, T.; Kilter, H.; Schafers, H.J.; Nickenig, G.; Bohm, M.; Laufs, U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation 2003, 108, 1567–1574. [Google Scholar] [CrossRef]
- Ma, T.; Xue, Y. RhoA-mediated potential regulation of blood-tumor barrier permeability by bradykinin. J. Mol. Neurosci. 2010, 42, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wojciak-Stothard, B.; Potempa, S.; Eichholtz, T.; Ridley, A.J. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J. Cell Sci. 2001, 114, 1343–1355. [Google Scholar] [PubMed]
- Boland, A.J.; Gangadharan, N.; Kavanagh, P.; Hemeryck, L.; Kieran, J.; Barry, M.; Walsh, P.T.; Lucitt, M. Simvastatin Suppresses Interleukin Iβ Release in Human Peripheral Blood Mononuclear Cells Stimulated With Cholesterol Crystals. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Miyazaki, T.; Hirano, M.; Akiyama, Y.; Mimura, T. Simvastatin inhibits production of interleukin 6 (IL-6) and IL-8 and cell proliferation induced by tumor necrosis factor-alpha in fibroblast-like synoviocytes from patients with rheumatoid arthritis. J. Rheumatol. 2006, 33, 463–471. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, F.; Tibiriçá, E.; Singh, M.; Fraser, P.A.; Mann, G.E. Redox Regulation of Microvascular Permeability: IL-1β Potentiation of Bradykinin-Induced Permeability Is Prevented by Simvastatin. Antioxidants 2020, 9, 1269. https://doi.org/10.3390/antiox9121269
Freitas F, Tibiriçá E, Singh M, Fraser PA, Mann GE. Redox Regulation of Microvascular Permeability: IL-1β Potentiation of Bradykinin-Induced Permeability Is Prevented by Simvastatin. Antioxidants. 2020; 9(12):1269. https://doi.org/10.3390/antiox9121269
Chicago/Turabian StyleFreitas, Felipe, Eduardo Tibiriçá, Mita Singh, Paul A. Fraser, and Giovanni E. Mann. 2020. "Redox Regulation of Microvascular Permeability: IL-1β Potentiation of Bradykinin-Induced Permeability Is Prevented by Simvastatin" Antioxidants 9, no. 12: 1269. https://doi.org/10.3390/antiox9121269
APA StyleFreitas, F., Tibiriçá, E., Singh, M., Fraser, P. A., & Mann, G. E. (2020). Redox Regulation of Microvascular Permeability: IL-1β Potentiation of Bradykinin-Induced Permeability Is Prevented by Simvastatin. Antioxidants, 9(12), 1269. https://doi.org/10.3390/antiox9121269