Vitamin D Supplementation is Associated with Increased Glutathione Peroxidase-1 Levels in Arab Adults with Prediabetes
Abstract
:1. Introduction
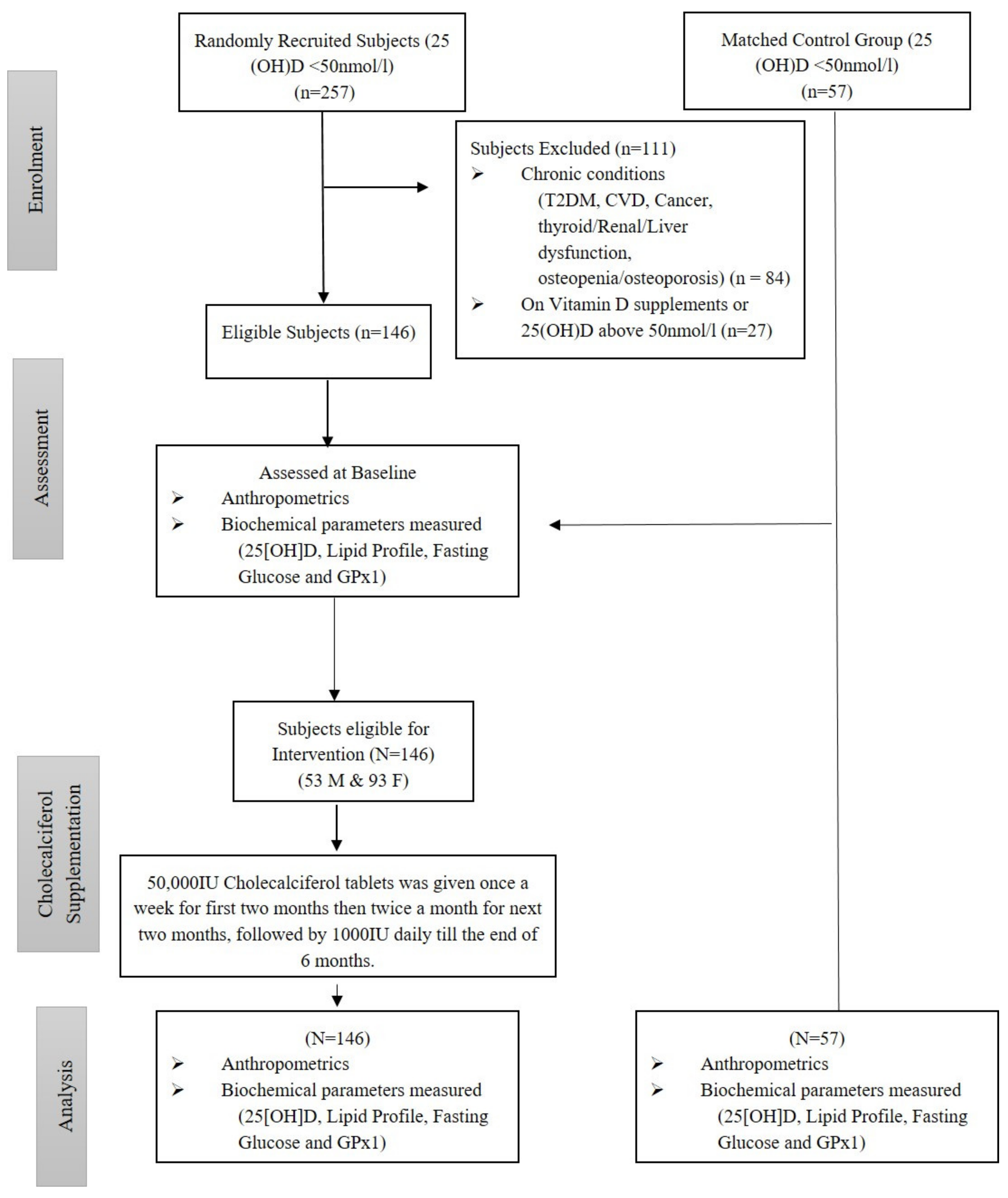
2. Materials and Methods
2.1. Participants
Exclusion Criteria
2.2. Anthropometry and Biochemical Assessments
2.3. Intervention
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cho, N.; Shaw, J.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pr. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Ansari, M.G.A.; Sabico, S.; Al-Saleh, Y.; Aljohani, N.J.; Alfawaz, H.; Alharbi, M.; Al-Othman, A.M.; Alokail, M.S.; Wimalawansa, S.J. Efficacy of different modes of vitamin D supplementation strategies in Saudi adolescents. J. Steroid Biochem. Mol. Biol. 2018, 180, 23–28. [Google Scholar] [CrossRef]
- Alwin Robert, A.; Abdulaziz Al Dawish, M.; Braham, R.; Ali Musallam, M.; Abdullah Al Hayek, A.; Hazza Al Kahtany, N. Type 2 diabetes mellitus in Saudi Arabia: Major challenges and possible solutions. Curr. Diabetes Rev. 2017, 13, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M. Vitamin D in Saudi Arabia: Prevalence, distribution and disease associations. J. Steroid Biochem. Mol. Biol. 2018, 175, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Lecture, K.W. Challenges in diabetes epidemiology form West to the rest. Diabetes Care 1992, 15, 232–252. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782. [Google Scholar] [CrossRef]
- Perez-Bravo, F.; Carrasco, E.; Gutierrez-Lopez, M.; Martinez, M.; Lopez, G.; de los Rios, M.G. Genetic predisposition and environmental factors leading to the development of insulin-dependent diabetes mellitus in Chilean children. J. Mol. Med. 1996, 74, 105–109. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.; Alokail, M.S.; Alkharfy, K.M.; Draz, H.M.; Agliardi, C.; Mohammed, A.K.; Guerini, F.R.; Clerici, M. Vitamin D receptor gene polymorphisms and HLA DRB1* 04 cosegregation in Saudi type 2 diabetes patients. J. Immunol. 2012, 188, 1325–1332. [Google Scholar] [CrossRef] [Green Version]
- Raciti, G.A.; Longo, M.; Parrillo, L.; Ciccarelli, M.; Mirra, P.; Ungaro, P.; Formisano, P.; Miele, C.; Béguinot, F. Understanding type 2 diabetes: From genetics to epigenetics. Acta Diabetol. 2015, 52, 821–827. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alkharfy, K.M.; Khan, N.; Mohammed, A.K.; Vinodson, B.; Ansari, M.G.A.; Alenad, A.; Alokail, M.S. Association of VDR-gene variants with factors related to the metabolic syndrome, type 2 diabetes and vitamin D deficiency. Gene 2014, 542, 129–133. [Google Scholar] [CrossRef]
- Lee, W.C.; Mokhtar, S.S.; Munisamy, S.; Yahaya, S.; Rasool, A.H.G. Vitamin D status and oxidative stress in diabetes mellitus. Cell Mol. Biol (Noisy-Le-Grand) 2018, 64, 60–69. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuki, A.; Sumida, Y.; Urakawa, H.; Gabazza, E.C.; Murashima, S.; Nakatani, K.; Yano, Y.; Adachi, Y. Increased oxidative stress is associated with serum levels of triglyceride, insulin resistance, and hyperinsulinemia in Japanese metabolically obese, normal-weight men. Diabetes Care 2004, 27, 631–632. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A. Oxidative stress and glycemic regulation. Metab. Clin. Exp. 2000, 49, 27–29. [Google Scholar] [CrossRef]
- Durackova, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459. [Google Scholar]
- Brunelli, E.; La Russa, D.; Pellegrino, D. Impaired Oxidative Status Is Strongly Associated with Cardiovascular Risk Factors. Oxid. Med. Cell Longev. 2017. [Google Scholar] [CrossRef] [Green Version]
- Ndrepepa, G. Myeloperoxidase - A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef]
- Lau, A.T.; Wang, Y.; Chiu, J.F. Reactive oxygen species: Current knowledge and applications in cancer research and therapeutic. J. Cell. Biochem. 2008, 104, 657–667. [Google Scholar] [CrossRef]
- Brown, N.S.; Bicknell, R. Hypoxia and oxidative stress in breast cancer Oxidative stress-its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 2001, 3, 323. [Google Scholar] [CrossRef] [Green Version]
- Bolaños, J.P.; Moro, M.A.; Lizasoain, I.; Almeida, A. Mitochondria and reactive oxygen and nitrogen species in neurological disorders and stroke: Therapeutic implications. Adv. Drug Deliv. Rev. 2009, 61, 1299–1315. [Google Scholar] [CrossRef] [PubMed]
- Gelderman, K.A.; Hultqvist, M.; Olsson, L.M.; Bauer, K.; Pizzolla, A.; Olofsson, P.; Holmdahl, R. Rheumatoid arthritis: The role of reactive oxygen species in disease development and therapeutic strategies. Antioxid. Redox Signal. 2007, 9, 1541–1568. [Google Scholar] [CrossRef] [PubMed]
- Tohari, A.M.; Alhasani, R.H.; Biswas, L.; Patnaik, S.R.; Reilly, J.; Zeng, Z.; Shu, X. Vitamin D Attenuates Oxidative Damage and Inflammation in Retinal Pigment Epithelial Cells. Antioxidant 2019, 8, 341. [Google Scholar] [CrossRef] [Green Version]
- Al-Daghri, N.M.; Mohammed, A.K.; Bukhari, I.; Rikli, M.; Abdi, S.; Ansari, M.G.A.; Sabico, S.; Hussain, S.D.; Alenad, A.; Al-Saleh, Y.; et al. Efficacy of vitamin D supplementation according to vitamin D-binding protein polymorphisms. Nutrition 2019, 63–64, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Brett, N.R.; Lavery, P.; Agellon, S.; Vanstone, C.A.; Goruk, S.; Field, C.J.; Weiler, H.A. Vitamin D Status and Immune Health Outcomes in a Cross-Sectional Study and a Randomized Trial of Healthy Young Children. Nutrition 2018, 10, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhumaidi, M.; Adnan, A.; Dewish, M. Vitamin d deficiency in patients with type-2 diabetes mellitus in southern region of saudi arabia. Maedica 2013, 8, 231. [Google Scholar]
- Svoren, B.M.; Volkening, L.K.; Wood, J.R.; Laffel, L.M. Significant vitamin D deficiency in youth with type 1 diabetes mellitus. J. Pediatrics 2009, 154, 132–134. [Google Scholar] [CrossRef] [Green Version]
- Di Cesar, D.J.; Ploutz-Snyder, R.; Weinstock, R.S.; Moses, A.M. Vitamin D deficiency is more common in type 2 than in type 1 diabetes. Diabetes Care 2006, 29, 174. [Google Scholar] [CrossRef]
- Tang, T.; Prior, S.; Li, K.; Ireland, H.; Bain, S.; Hurel, S.; Cooper, J.; Humphries, S.; Stephens, J. Association between the rs1050450 glutathione peroxidase-1 (C > T) gene variant and peripheral neuropathy in two independent samples of subjects with diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 417–425. [Google Scholar] [CrossRef]
- Matés, J.M.; Pérez-Gómez, C.; De Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Domínguez, C.; Ruiz, E.; Gussinye, M.; Carrascosa, A. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care 1998, 21, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Shukla, R.; Madhu, S.V.; Gambhir, J.K.; Prabhu, K.M. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin. Biochem. 2003, 36, 557–562. [Google Scholar] [CrossRef]
- Ndahimana, J.; Dorchy, H.; Vertongen, F. Erythrocyte and plasma antioxidant activity in diabetes mellitus type I. Presse Med. 1996, 25, 188–192. [Google Scholar] [PubMed]
- Mukhopadhyay, S.; Singh, M.; Chatterjee, M. Vitamin D3 as a modulator of cellular antioxidant defence in murine lymphoma. Nutr. Res. 2000, 20, 91–102. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Alkharfy, K.M.; Al-Othman, A.; El-Kholie, E.; Moharram, O.; Alokail, M.S.; Al-Saleh, Y.; Sabico, S.; Kumar, S.; Chrousos, G.P. Vitamin D supplementation as an adjuvant therapy for patients with T2DM: An 18-month prospective interventional study. Cardiovasc. Diabetol. 2012, 11, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Daghri, N.M.; Wani, K.; Sabico, S.; Garbis, S.D.; Chrousos, G.P.; Amer, O.E.; Ansari, M.G.A.; Al-Saleh, Y.; Aljohani, N.J.; Al-Attas, O.S. Sex-specific expression of apolipoprotein levels following replenishment of vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 180, 129–136. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Yousef, M.; Sabico, S.L.; Chrousos, G.P. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): A decade of an epidemic. BMC Med. 2011, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Al-Daghri, N.M.; Yakout, S.M.; Wani, K.; Khattak, M.N.K.; Garbis, S.D.; Chrousos, G.P.; Al-Attas, O.S.; Alokail, M.S. IGF and IGFBP as an index for discrimination between vitamin D supplementation responders and nonresponders in overweight Saudi subjects. Medicine 2018, 97. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Saleh, Y.; Aljohani, N.; Sulimani, R.; Al-Othman, A.M.; Alfawaz, H.; Fouda, M.; Al-Amri, F.; Shahrani, A.; Alharbi, M.; et al. Vitamin D status correction in Saudi Arabia: An experts’ consensus under the auspices of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases (ESCEO). Arch. Osteoporos 2017, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Kostoglou-Athanassiou, I.; Athanassiou, P.; Gkountouvas, A.; Kaldrymides, P. Vitamin D and glycemic control in diabetes mellitus type 2. Adv. Endocrinol. Metab. 2013, 4, 122–128. [Google Scholar] [CrossRef]
- Mitri, J.; Dawson-Hughes, B.; Hu, F.B.; Pittas, A.G. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: The Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am. J. Clin. Nutr 2011, 94, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Lucas, T.S.; Duncan, A.M.; Rabasa-Lhoret, R.; Veith, R.; Gibbs, A.L.; Badawi, A.; Wolever, T.M.S. Effect of vitamin D supplementation on oral glucose tolerance in individuals with low vitamin D status and increased risk for developing type 2 diabetes (EVIDENCE): A double-blind, randomized, placebo-controlled clinical trial. Diabetes Obes. Metab. 2017, 19, 133–141. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ciglia, L.; Chatterjee, R.; Desouza, C.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef] [Green Version]
- Saif-Elnasr, M.; Ibrahim, I.M.; Alkady, M.M. Role of Vitamin D on glycemic control and oxidative stress in type 2 diabetes mellitus. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2017, 22, 22. [Google Scholar] [CrossRef]
- Colak, E.; Majkić-Singh, N.; Stanković, S.; Srecković-Dimitrijević, V.; Djordjević, P.; Lalić, K.; Lalić, N. Parameters of antioxidative defense in type 2 diabetic patients with cardiovascular complications. Ann. Med. 2005, 37, 613–620. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Torzewski, M.; Hafner, G.; Tiret, L.; Smieja, M.; Cambien, F.; Meyer, J.; Lackner, K.J. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. New Engl. J. Med. 2003, 349, 1605–1613. [Google Scholar] [CrossRef] [Green Version]
- Espinola-Klein, C.; Rupprecht, H.J.; Bickel, C.; Schnabel, R.; Genth-Zotz, S.; Torzewski, M.; Lackner, K.; Munzel, T.; Blankenberg, S. Glutathione peroxidase-1 activity, atherosclerotic burden, and cardiovascular prognosis. Am. J. Cardiol. 2007, 99, 808–812. [Google Scholar] [CrossRef]
- Harijith, A.; Ebenezer, D.L.; Natarajan, V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front. Physiol. 2014, 5, 352. [Google Scholar] [CrossRef]
- Dzik, K.; Skrobot, W.; Flis, D.J.; Karnia, M.; Libionka, W.; Kloc, W.; Kaczor, J.J. Vitamin D supplementation attenuates oxidative stress in paraspinal skeletal muscles in patients with low back pain. Eur. J. Appl. Physiol. 2018, 118, 143–151. [Google Scholar] [CrossRef]
- Saedisomeolia, A.; Taheri, E.; Djalali, M.; Djazayeri, A.; Qorbani, M.; Rajab, A.; Larijani, B. Vitamin D status and its association with antioxidant profiles in diabetic patients: A cross-sectional study in Iran. Indian J. Med. Sci. 2013, 67, 29–37. [Google Scholar]
- Baez-Duarte, B.G.; Zamora-Ginez, I.; Mendoza-Carrera, F.; Ruiz-Vivanco, G.; Torres-Rasgado, E.; Gonzalez-Mejia, M.E.; Garcia-Zapien, A.; Flores-Martinez, S.E.; Perez-Fuentes, R. Serum levels of glutathione peroxidase 3 in overweight and obese subjects from central Mexico. Arch. Med. Res. 2012, 43, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Al-Saleh, Y.; Aljohani, N.; Alokail, M.; Al-Attas, O.; Alnaami, A.M.; Sabico, S.; Alsulaimani, M.; Al-Harbi, M.; Alfawaz, H.; et al. Vitamin D deficiency and cardiometabolic risks: A juxtaposition of Arab adolescents and adults. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Alokail, M.S.; Manousopoulou, A.; Heinson, A.; Al-Attas, O.; Al-Saleh, Y.; Sabico, S.; Yakout, S.; Woelk, C.H.; Chrousos, G.P.; et al. Sex-specific vitamin D effects on blood coagulation among overweight adults. Eur. J. Clin. Investig. 2016, 46, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Intervention | Control | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Change | p-Value | Before | After | Change | p-Value | ||
| N (M/F) | 146 (53/93) | 57 (25/32) | |||||||
| Total Cholestrol (mmol/L) | 5.1 ± 1.2 | 5.12 ± 1.2 | −0.02 (−0.2–0.16) | 0.52 | 5.07 ± 1.0 | 5.51 ± 1.2 | 0.44 (0.13–0.75) | 0.03 | 0.19 |
| HDL-Cholestrol (mmol/L) | 1.0 ± 0.4 | 1.15 ± 0.40 | 0.11 (0.02–0.19) | 0.14 | 1.05 ± 0.5 | 1.30 ± 0.4 | 0.22 (0.08–0.37) | <0.001 | 0.71 |
| LDL-Cholestrol (mmol/L) | 3.2 ± 0.9 | 3.14 ± 0.9 | −0.11 (−0.3–0.06) | 0.12 | 3.30 ± 0.8 | 3.44 ± 0.9 | 0.14 (−0.14–0.41) | 0.65 | 0.38 |
| Triglycerides (mmol/L) # | 1.5 (1.0–2.1) | 1.49 (1.1–1.9) | 0.01 (−0.4–0.4) | 0.56 | 1.18 (0.8–1.8) | 1.51 (0.9–2.3) | 0.24 (−0.26–0.9) | 0.12 | 0.29 |
| Glucose (mmol/L) | 5.5 ± 0.9 | 5.6 ± 0.9 | 0.07 (−0.1–0.23) | 0.04 | 5.35 ± 0.10 | 5.48 ± 0.8 | 0.13 (−0.17–0.44) | 0.98 | 0.55 |
| CRP (µg/mL)# | 20.6 (4.5–49.5) | 39.9 (14.9–75.9) | 0.27 (−10.5–4.5) | 0.39 | 17.7 (5.2–40) | 34.7 (8.0–80.4) | 0.13 (−20.1–17.7) | 0.22 | 0.24 |
| 25(OH)D (nmol/L) | 32.5 ± 11.6 | 66.2 ± 18.01 | 33.7 (30.6–36.8) | <0.01 | 31.9 ± 15.3 | 29.10 ± 12.4 | −2.8 (−6.2–0.8) | 0.01 | <0.001 |
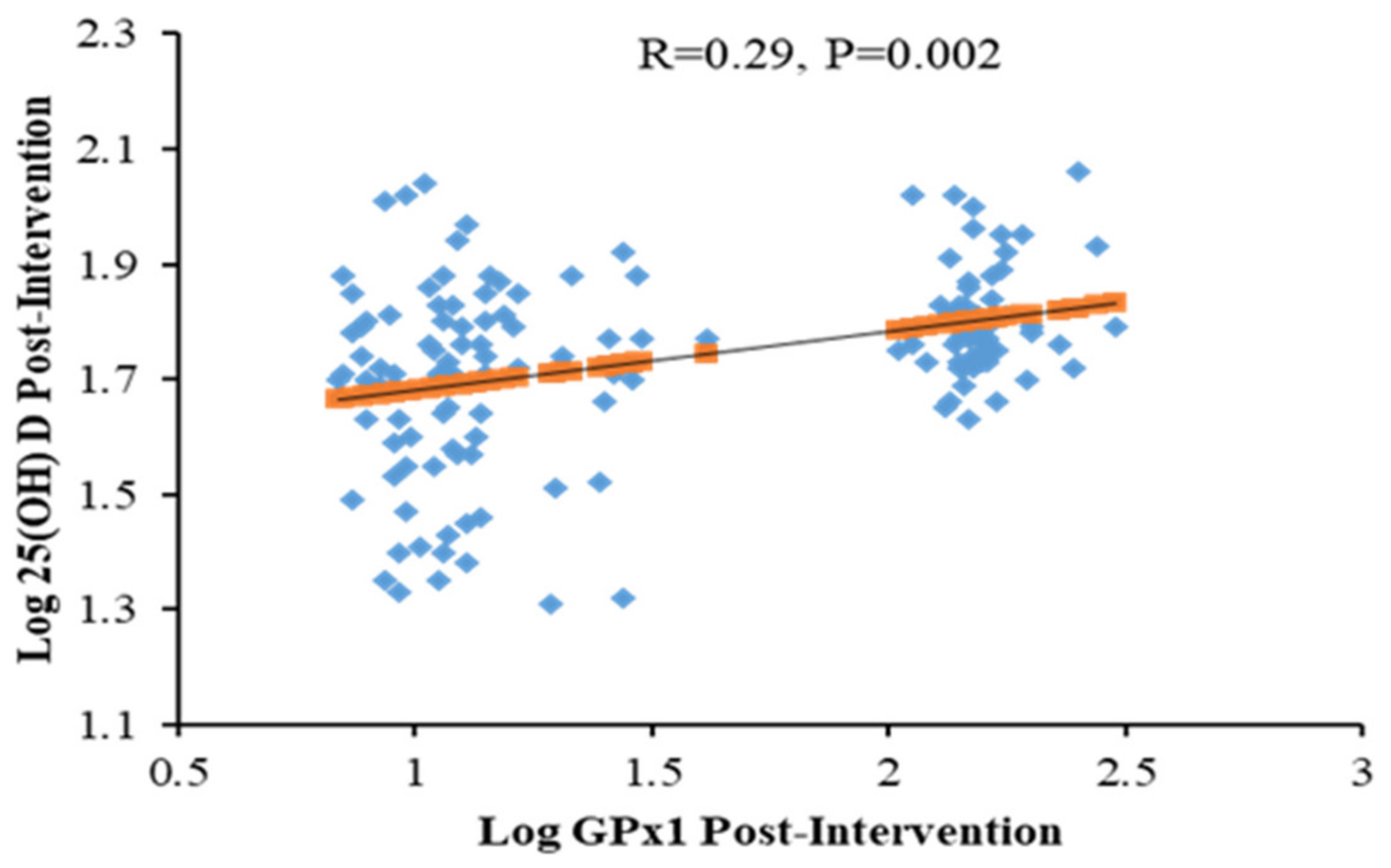
| GPx1 (ng/mL) | 17.3 (11.5–59.0) | 26.7 (11.4–59.9) | 7.5 (−1.7–8.2) | <0.01 | 14.6 (7.6–56) | 16.3 (8.5–66.3) | 1.9 (−4.5–6) | 0.15 | 0.01 |
| Parameter | Males | Females | Group Effect | Group Effect (Adjusted for age & BMI) | ||
|---|---|---|---|---|---|---|
| Intervention (N = 53) | Control (N = 25) | Intervention (N = 93) | Control (N = 32) | |||
| 25 (OH) D (nmol/Ll) | ||||||
| Baseline | 34.9 ± 10.8 | 38.6 ± 13.7 | 30.9 ± 14.5 | 29.2 ± 16.2 | <0.001 | <0.001 |
| 6 months | 60.1 ± 20.2 | 32.8 ± 9.5 | 54.3 ± 24.5 | 27.4 ± 12.9 | ||
| Change (1st–3rd) percentile | 26.0 (19.8–32.2) | −2.5 (-22.4–7.3) | 21.1 (15.1–26.1) | −1.2 (−10.9–7.9) | ||
| Time effect | <0.001 | <0.001 | ||||
| Time effect (Adjusted) | <0.001 | <0.001 | ||||
| GPx1 (ng/mL) | < 0.001 | <0.023 | ||||
| Baseline | 24.6 (14–140) | 16.6 (13.4–23.5) | 14.9 (11.1–41.6) | 11.1 (9.8–13.) | ||
| 6 months | 30.1 (12–140) | 18.6 (10.2–27.2) | 16.1 (10.1–44.5) | 11.5 (11.5–14.1) | ||
| Change (1st–3rd) percentile | 8.9 (2.9–14.9) | 2.04 (−5.7–6.4) | 6.9 (2.7–11.1) | 0.16 (−1.6–2.8) | ||
| Time effect | 0.004 | 0.06 | ||||
| Time effect (Adjusted) | 0.002 | 0.57 | ||||
| Parameters | All | Intervention | Control |
|---|---|---|---|
| N | 203 | 146 | 57 |
| Age (year) | 0.18 * | 0.19 | 0.05 |
| Body Mass Index (BMI) (kg/m2) | −0.20 * | −0.17 | 0.21 |
| Waist Hip Ratio (WHR) | 0.20 | 0.25 ** | 0.25 |
| Systolic BP (mmHg) | 0.19 * | 0.21 * | 0.20 |
| Diastolic BP (mmHg) | 0.16 | 0.26 ** | 0.14 |
| Total Cholesterol (mmol/L) | −0.11 | −0.19 * | −0.04 |
| HDL-Cholesterol (mmol/L) | 0.12 | 0.15 | 0.18 |
| LDL-Cholesterol (mmol/L) | −0.26 ** | −0.22 * | −0.20 |
| Triglycerides (mmol/L) # | 0.10 | 0.01 | 0.33 * |
| Glucose (mmol/L) | 0.16 | 0.04 | 0.16 |
| 25(OH) D (nmol/L) | 0.06 | 0.03 | 0.20 |
| CRP (µg/mL) # | −0.10 | −0.08 | 0.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, M.G.A.; Sabico, S.; Clerici, M.; Khattak, M.N.K.; Wani, K.; Al-Musharaf, S.; Amer, O.E.; Alokail, M.S.; Al-Daghri, N.M. Vitamin D Supplementation is Associated with Increased Glutathione Peroxidase-1 Levels in Arab Adults with Prediabetes. Antioxidants 2020, 9, 118. https://doi.org/10.3390/antiox9020118
Ansari MGA, Sabico S, Clerici M, Khattak MNK, Wani K, Al-Musharaf S, Amer OE, Alokail MS, Al-Daghri NM. Vitamin D Supplementation is Associated with Increased Glutathione Peroxidase-1 Levels in Arab Adults with Prediabetes. Antioxidants. 2020; 9(2):118. https://doi.org/10.3390/antiox9020118
Chicago/Turabian StyleAnsari, Mohammed Ghouse Ahmed, Shaun Sabico, Mario Clerici, Malak Nawaz Khan Khattak, Kaiser Wani, Sara Al-Musharaf, Osama Emam Amer, Majed S. Alokail, and Nasser M. Al-Daghri. 2020. "Vitamin D Supplementation is Associated with Increased Glutathione Peroxidase-1 Levels in Arab Adults with Prediabetes" Antioxidants 9, no. 2: 118. https://doi.org/10.3390/antiox9020118






