Antioxidant Effect of Cocoa By-Product and Cherry Polyphenol Extracts: A Comparative Study
Abstract
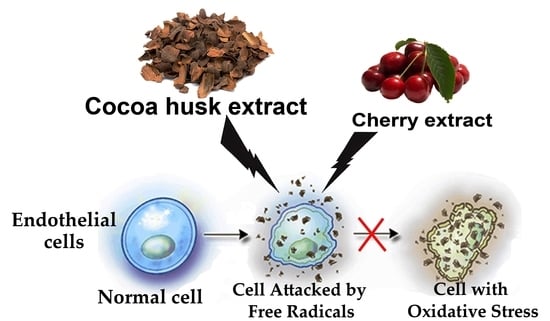
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Cocoa Bean and Husk Phenol Extraction
2.2.2. Cherry Extract (CE) Preparation and Characterization
2.3. HPLC-PDA/UVvis-ESI-MS/MS Analysis of Cocoa Extracts
2.4. Antioxidants Determination
2.5. Total Polyphenolic Content
2.6. HUVEC Isolation and Culture
2.7. Cell Treatment
2.8. Cell Viability
2.9. ROS Production
2.10. Permeation Study of CHE and CE
2.11. Cocoa Extract and CE Stability Studies
2.12. Statistical Analysis
3. Results
3.1. Phenolic Profile of Cocoa Extracts
3.2. Cherry and Cocoa By-Product Extracts Characterization
3.3. Dose- and Time-Dependent Effect of CHE and CE on HUVECs Viability
3.4. Protective Effect from Oxidative Stress
3.5. Antioxidant Activity
3.6. Permeation Study of CHE and CE
3.7. CHE and CE Stability Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chakraborti, T.; Ghosh, S.K.; Michael, J.R.; Batabyal, S.K.; Chakraborti, S. Targets of oxidative stress in cardiovascular system. Mol. Cell. Biochem. 1998, 187, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Pallardo, F.V.; Garcia de la Asuncion, J.; Vina, J. Mitochondria, oxidative stress and aging. Free Radic. Res. 2000, 32, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Berlett, B.S. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab. Rev. 1998, 30, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Harrison, D.G. Oxidative stress and vascular damage in hypertension. Coron. Artery Dis. 2001, 12, 455–461. [Google Scholar] [CrossRef]
- Zalba, G.; San Jose, G.; Moreno, M.U.; Fortuno, M.A.; Fortuno, A.; Beaumont, F.J.; Diez, J. Oxidative stress in arterial hypertension: Role of NAD(P)H oxidase. Hypertension 2001, 38, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.B.; Stuart, D.A.; Smith, N.L.; Lee, C.Y.; McHale, N.L.; Flanagan, J.A.; Ou, B.; Hurst, W.J. Antioxidant activity and polyphenol and procyanidin contents of selected commercially available cocoa-containing and chocolate products in the United States. J. Agric. Food Chem. 2006, 54, 4062–4068. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Stuart, D.A.; Ou, B.; Fan, E.; Ji, H.; Kou, Y. Determination of total procyanidins in selected chocolate and confectionery products using DMAC. J. AOAC Int. 2010, 93, 89–96. [Google Scholar]
- Martínez, R.; Torres, P.; Meneses, M.; Figueroa, J.; Pérez-Álvarez, J.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Oddoye, E.O.K.; Agyente-Badu, C.K.; Gyedu-Akoto, E. Cocoa and its by-products: Identification and utilization. In Chocolate in Health and Nutrition; Watson, R., Preedy, V., Zibadi, S., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 7, pp. 23–37. [Google Scholar]
- Pérez, E.; Méndez, A.; León, M.; Hernández, G.; Sívoli, L. Proximal composition and the nutritional and functional properties of cocoa by-products (pods and husks) for their use in the food industry. In Chocolate Cocoa Byproducts Technology, Rheology, Styling, and Nutrition; Pérez, E., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2015; pp. 219–234. [Google Scholar]
- Ortega, N.; Romero, M.-P.; Macia, A.; Reguant, J.; Anglès, N.; Morelló, J.; Motilva, M.-J. Comparative study of UPLC–MS/MS and HPLC–MS/MS to determine procyanidins and alkaloids in cocoa samples. J. Food Compos. Anal. 2010, 23, 298–305. [Google Scholar] [CrossRef]
- Counet, C.; Collin, S. Effect of the number of flavanol units on the antioxidant activity of procyanidin fractions isolated from chocolate. J. Agric. Food Chem. 2003, 51, 6816–6822. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ramiro, I.; Martin, M.A.; Ramos, S.; Bravo, L.; Goya, L. Comparative effects of dietary flavanols on antioxidant defences and their response to oxidant-induced stress on Caco2 cells. Eur. J. Nutr. 2011, 50, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matias, A.A.; Rosado-Ramos, R.; Nunes, S.L.; Figueira, I.; Serra, A.T.; Bronze, M.R.; Santos, C.N.; Duarte, C.M. Protective effect of a (Poly)phenol-rich extract derived from sweet cherries culls against oxidative cell damage. Molecules 2016, 21, 406. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.; Zambito, Y.; Di Colo, G.; D’Onofrio, C.; Fausto, C.; Balbarini, A.; Di Stefano, R. Red grape skin and seeds polyphenols: Evidence of their protective effects on endothelial progenitor cells and improvement of their intestinal absorption. Eur. J. Pharm. Biopharm. 2012, 80, 176–184. [Google Scholar] [CrossRef]
- Balestrieri, M.L.; Schiano, C.; Felice, F.; Casamassimi, A.; Balestrieri, A.; Milone, L.; Servillo, L.; Napoli, C. Effect of low doses of red wine and pure resveratrol on circulating endothelial progenitor cells. J. Biochem. 2008, 143, 179–186. [Google Scholar] [CrossRef]
- Lin, X.L.; Liu, Y.; Liu, M.; Hu, H.; Pan, Y.; Fan, X.J.; Hu, X.M.; Zou, W.W. Inhibition of hydrogen peroxide-induced human umbilical vein endothelial cells aging by allicin depends on Sirtuin1 activation. Med. Sci. Monit. 2017, 23, 563–570. [Google Scholar] [CrossRef] [Green Version]
- Felice, F.; Maragò, E.; Sebastiani, L.; Di Stefano, R. Apple juices from ancient Italian cultivars: A study on mature endothelial cells model. Fruits 2015, 70, 361–369. [Google Scholar] [CrossRef]
- Hafizah, A.H.; Zaiton, Z.; Zulkhairi, A.; Mohd Ilham, A.; Nor Anita, M.M.; Zaleha, A.M. Piper sarmentosum as an antioxidant on oxidative stress in human umbilical vein endothelial cells induced by hydrogen peroxide. J. Zhejiang Univ. Sci. B 2010, 11, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Berni, R.; Romi, M.; Cantini, C.; Hausman, J.-F.; Guerriero, G.; Cai, G. Functional molecules in locally-adapted crops: The case study of tomatoes, onions, and sweet cherry fruits from Tuscany in Italy. Front. Plant Sci. 2019, 9, 1983. [Google Scholar] [CrossRef] [Green Version]
- Hammerstone, J.F.; Lazarus, S.A.; Mitchell, A.E.; Rucker, R.; Schmitz, H.H. Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 1999, 47, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Bruna, C.; Eichholz, I.; Rohn, S.; Kroh, L.; Huyskens-Keil, S. Bioactive compounds and antioxidant activity of cocoa hulls (Theobroma cacao L.) from different origins. J. Appl. Botany Food Qual. 2009, 83, 9–13. [Google Scholar]
- Kim, H.; Keeney, P. (-)-Epicatechin Content in Fermented and Unfermented Cocoa Beans. J. Food Sci. 2006, 49, 1090–1092. [Google Scholar] [CrossRef]
- Urbanska, B.; Kowalska, J. Comparison of the total polyphenol content and antioxidant activity of chocolate obtained from roasted and unroasted cocoa beans from different regions of the World. Antioxidants 2019, 8, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racine, K.C.; Wiersema, B.D.; Griffin, L.E.; Essenmacher, L.A.; Lee, A.H.; Hopfer, H.; Lambert, J.D.; Stewart, A.C.; Neilson, A.P. Flavanol polymerization is a superior predictor of alpha-glucosidase inhibitory activity compared to flavanol or total polyphenol concentrations in cocoas prepared by variations in controlled fermentation and roasting of the same raw cocoa beans. Antioxidants 2019, 8, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Żyżelewicz, D.; Krysiak, W.; Oracz, J.; Sosnowska, D.; Budryn, G.; Nebesny, E. The influence of the roasting process conditions on the polyphenol content in cocoa beans, nibs and chocolates. Food Res. Int. 2016, 89, 918–929. [Google Scholar] [CrossRef]
- Beconcini, D.; Fabiano, A.; Zambito, Y.; Berni, R.; Santoni, T.; Piras, A.M.; Di Stefano, R. Chitosan-based nanoparticles containing cherry extract from Prunus avium L. to improve the resistance of endothelial cells to oxidative stress. Nutrients 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Braca, A.; Sinisgalli, C.; De Leo, M.; Muscatello, B.; Cioni, P.L.; Milella, L.; Ostuni, A.; Giani, S.; Sanogo, R. Phytochemical profile, antioxidant and antidiabetic activities of Adansonia digitata L. (Baobab) from Mali, as a source of health-promoting compounds. Molecules 2018, 23. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Beconcini, D.; Fabiano, A.; Di Stefano, R.; Macedo, M.H.; Felice, F.; Zambito, Y.; Sarmento, B. Cherry extract from Prunus avium L. to improve the resistance of endothelial cells to oxidative stress: Mucoadhesive chitosan vs. poly(lactic-co-glycolic acid) nanoparticles. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Mattii, L.; Braca, A.; Felice, F.; Di Stefano, R.; Zambito, Y. Nanoparticles based on quaternary ammonium-chitosan conjugate: A vehicle for oral administration of antioxidants contained in red grapes. J. Drug Deliv. Technol. 2016, 32, 291–297. [Google Scholar] [CrossRef]
- Legen, I.; Salobir, J.; Kerc, J. Comparison of different intestinal epithelia as models for absorption enhancement studies. Int. J. Pharm. 2005, 291, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.L.; Lozano-Sanchez, J.; Contreras-Gámez, M.; Legeai-Mallet, L.; Fernández-Arroyo, S.; Segura-Carretero, A. Isolation, comprehensive characterization and antioxidant activities of Theobroma cacao extract. J. Funct. Foods 2014, 10, 485–498. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Cienfuegos-Jovellanos, E.; Marin, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 55, 3926–3935. [Google Scholar] [CrossRef] [PubMed]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef]
- Ortega, N.; Romero, M.P.; Macia, A.; Reguant, J.; Angles, N.; Morello, J.R.; Motilva, M.J. Obtention and characterization of phenolic extracts from different cocoa sources. J. Agric. Food Chem. 2008, 56, 9621–9627. [Google Scholar] [CrossRef]
- Zambito, Y.; Zaino, C.; Uccello-Barretta, G.; Balzano, F.; Di Colo, G. Improved synthesis of quaternary ammonium-chitosan conjugates (N+ -Ch) for enhanced intestinal drug permeation. Eur. J. Pharm. Sci. 2008, 33, 343–350. [Google Scholar] [CrossRef]
- Kumar, V.; Khan, A.A.; Tripathi, A.; Dixit, P.K.; Bajaj, U.K. Role of oxidative stress in various diseases: Relevance of dietary antioxidants. J. Pharm. Exp. Ther. 2015, 4, 126–132. [Google Scholar]
- Usenik, V.; Fabčič, J.; Stampar, F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Relationship of the phytochemicals from coffee and cocoa by-products with their potential to modulate biomarkers of metabolic syndrome in vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.A.; Kwak, M.K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvano, F.; Fauci, L.; Lazzarino, G.; Fogliano, V.; Ritieni, A.; Ciappellano, S.; Battistini, N.C.; Tavazzi, B.; Galvano, G. Cyanidins: Metabolism and biological properties. J. Nutr. Biochem. 2004, 15, 2–11. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.-Y.; Li, H.-B. Fruits for Prevention and Treatment of Cardiovascular Diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef] [Green Version]






| Peak a | Compound | tR (min) | M | [M+HCOO]− | [M − H]− | ESI- MS/MS (Product Ions) (m/z) b | UV (λmax) |
|---|---|---|---|---|---|---|---|
| Phenols | |||||||
| 1 | N-caffeoyl aspartate | 17.8 | 295 | 294 | 276, 179, 132 | 252, 277, 305 | |
| 2 | procyanidin C (trimer I) | 19.4 | 866 | 865 | 847, 739, 713, 695, 577, 451, 407, 287 | 258, 277 | |
| 3 | procyanidin C (trimer II) | 20.7 | 866 | 865 | 847, 739, 713, 695, 577, 451, 407, 287 | 252, 279 | |
| 4 | procyanidin C (trimer III) | 21.7 | 866 | 865 | 847, 739, 713, 695, 577, 451, 407, 287 | 248, 280 | |
| 5 | procyanidin B (dimer I) | 23.0 | 578 | 577 | 451, 425, 407, 289 | 243, 279 | |
| 6 | procyanidin B (dimer II) | 24.1 | 578 | 577 | 451, 425, 407, 289 | 243, 279 | |
| 7 | procyanidin C (trimer IV) | 26.3 | 866 | 865 | 847, 739, 713, 695, 577, 451, 407, 287 | 244, 280 | |
| 8 | procyanidin C (trimer V) | 27.2 | 866 | 865 | 847, 739, 713, 695, 577, 451, 407, 287 | 242, 279 | |
| 9 | catechin/epicatechin | 28.6 | 290 | 335 | 289 | 271, 245, 205, 179 | 240, 279 |
| 10 | procyanidin B (dimer III) | 36.0 | 578 | 577 | 451, 425, 407, 289 | 277 | |
| 11 | procyanidin C (trimer VI) | 36.4 | 866 | 865 | 739, 713, 695, 577, 451, 407, 287 | 277 | |
| 12 | procyanidin C (trimer VII) | 37.0 | 866 | 865 | 739, 713, 695, 577, 451, 407, 287 | 278 | |
| 13 | quercetin 3-O-glucoside | 42.7 | 464 | 463 | 301, 179 | 268, 355 | |
| 14 | quercetin 3-O-arabinoside | 44.2 | 434 | 433 | 301, 179 | 267, 354 |
| Extract | Antioxidant Content a (mmol Fe2+/100 g FW) | TPC b (mg GAE/100g FW) |
|---|---|---|
| CE | 2.19 ± 0.09 | 402.5 ± 8.4 |
| CHE | 2.50 ± 0.01 | 7105 ± 96.9 * |
| Extract | Flux 102 (µg cm−2min−1) | Papp a 104 (cm min−1) | T4h b (%) |
|---|---|---|---|
| CE | 0.41 ± 0.03 | 2.64 ± 0.02 | 5.75 ± 0.07 |
| CHE | 14.50 ± 1.33 * | 5.18 ± 0.47 * | 8.58 ± 2.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felice, F.; Fabiano, A.; De Leo, M.; Piras, A.M.; Beconcini, D.; Cesare, M.M.; Braca, A.; Zambito, Y.; Di Stefano, R. Antioxidant Effect of Cocoa By-Product and Cherry Polyphenol Extracts: A Comparative Study. Antioxidants 2020, 9, 132. https://doi.org/10.3390/antiox9020132
Felice F, Fabiano A, De Leo M, Piras AM, Beconcini D, Cesare MM, Braca A, Zambito Y, Di Stefano R. Antioxidant Effect of Cocoa By-Product and Cherry Polyphenol Extracts: A Comparative Study. Antioxidants. 2020; 9(2):132. https://doi.org/10.3390/antiox9020132
Chicago/Turabian StyleFelice, Francesca, Angela Fabiano, Marinella De Leo, Anna Maria Piras, Denise Beconcini, Maria Michela Cesare, Alessandra Braca, Ylenia Zambito, and Rossella Di Stefano. 2020. "Antioxidant Effect of Cocoa By-Product and Cherry Polyphenol Extracts: A Comparative Study" Antioxidants 9, no. 2: 132. https://doi.org/10.3390/antiox9020132
APA StyleFelice, F., Fabiano, A., De Leo, M., Piras, A. M., Beconcini, D., Cesare, M. M., Braca, A., Zambito, Y., & Di Stefano, R. (2020). Antioxidant Effect of Cocoa By-Product and Cherry Polyphenol Extracts: A Comparative Study. Antioxidants, 9(2), 132. https://doi.org/10.3390/antiox9020132