Beneficial Effects of Fermented Papaya Preparation (FPP®) Supplementation on Redox Balance and Aging in a Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Immun’Âge®-FPP® (Fermented Papaya Preparation)
2.2. In Vivo Studies
2.3. Total Antioxidant Power Assay (PAO Test Kit)
2.4. Ascorbic Acid Assay
2.5. Collection and Processing of Murine Plasma from Blood Samples
2.5.1. Total Antioxidant Power Assay (PAO Test kit)
2.5.2. Superoxide Dismutase (SOD) Activity Assay
2.5.3. Reduced Glutathione (GSH) Detection and Quantification Assay
2.5.4. Total Reactive Oxygen Species (ROS) Assay
2.5.5. Detection of telomerase by ELISA Assay
2.6. Bone Marrow Cells Recovery from Mice
2.7. Ovarian Germ Cells Recovery from Mice
2.8. Detection of Telomeres by PNA Kit/FITC for Flow Cytometry
2.9. Statistical Analysis
3. Results
3.1. Evaluation of FPP®-Supplemented Water Effectiveness
3.2. Early Treatment with FPP®: from 6 to 51 Weeks of Age
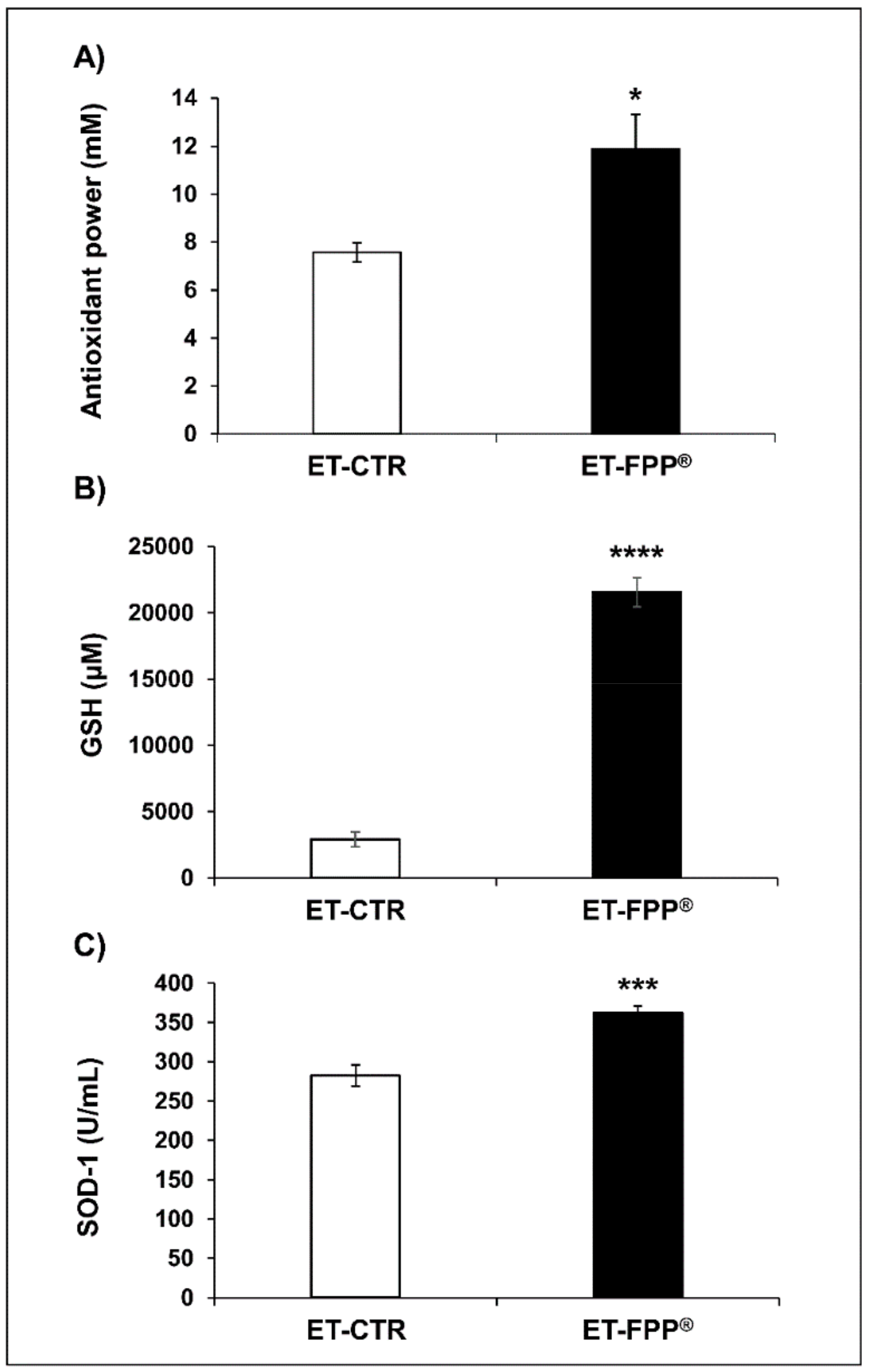
3.2.1. Oral Administration of FFP® Increases Plasma Levels of Antioxidants
3.2.2. Oral Administration of FFP® Reduces Plasma Levels of ROS
3.2.3. Oral Administration of FFP® Increases Plasmatic Telomerase Activity
3.2.4. Oral Administration of FFP® Increases Telomeres Length in Bone Marrow Cells and Ovarian Germ Cells
3.3. Late Treatment with FPP®: from 51 to 96 Weeks of Age
3.3.1. Oral Administration of FFP® Increases Plasma Levels of Antioxidants
3.3.2. Oral Administration of FFP® Reduces Plasma Levels of ROS
3.3.3. Oral Administration of FFP® Increases Plasmatic Telomerase Activity
3.3.4. Oral Administration of FFP® Increases Telomeres Length in Bone Marrow Cells and Ovarian Germ Cells
3.4. Comparison of FPP® Effectiveness between Early Treatment and Late Treatment Supplementation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nuraida, L. A review: Health Promoting Lactic Acid Bacteria in Traditional Indonesian Fermented Foods. Food Sci. Hum. Wellness 2015, 4, 47–55. [Google Scholar] [CrossRef]
- Frias, J.; Martinez-Villaluenga, C.; Peñas, E. Fermented Foods in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Terefe, N.S. Food Fermentation. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; p. 978008100596503420. [Google Scholar]
- Altay, F.; Karbancıoğlu-Güler, F.; Daskaya-Dikmen, C.; Heperkan, D. A Review on Traditional Turkish Fermented Non-Alcoholic Beverages: Microbiota, Fermentation Process and Quality Characteristics. Int. J. Food Microbiol. 2013, 167, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Kim, J.-C.; Moon, H.; Yang, J.-Y.; Kim, M. Determination of Sodium Contents in Traditional Fermented Foods in Korea. J. Food Compos. Anal. 2017, 56, 110–114. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health Benefits of Fermented Foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Tamang, J.P. Fermented Foods and Beverages of the World; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4200-9495-4. [Google Scholar]
- Rubio, C.A.; Schmidt, P.T. Severe Defects in the Macrophage Barrier to Gut Microflora in Inflammatory Bowel Disease and Colon Cancer. Anticancer Res. 2018, 38, 3811–3815. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, Gut Microbiota, and Their Influence on Host Health and Disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Howarth, G.S.; Wang, H. Role of Endogenous Microbiota, Probiotics and Their Biological Products in Human Health. Nutrients 2013, 5, 58–81. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sarrias, A.; Romo-Vaquero, M.; García-Villalba, R.; Cortés-Martín, A.; Selma, M.V.; Espín, J.C. The Endotoxemia Marker Lipopolysaccharide-Binding Protein is Reduced in Overweight-Obese Subjects Consuming Pomegranate Extract by Modulating the Gut Microbiota: A Randomized Clinical Trial. Mol. Nutr. Food Res. 2018, 62, 1800160. [Google Scholar] [CrossRef] [PubMed]
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One Health, Fermented Foods and Gut Microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Mackowiak, P.A. Recycling Metchnikoff: Probiotics, the Intestinal Microbiome and the Quest for Long Life. Front. Public Health 2013, 1, 52. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Lynch, D.B.; Jeffery, I.B.; Cusack, S.; O’Connor, E.M.; O’Toole, P.W. Diet-Microbiota-Health Interactions in Older Subjects: Implications for Healthy Aging. Interdiscip. Top Gerontol. 2015, 40, 141–154. [Google Scholar]
- Aruoma, O.I.; Hayashi, Y.; Marotta, F.; Mantello, P.; Rachmilewitz, E.; Montagnier, L. Applications and Bioefficacy of the Functional Food Supplement Fermented Papaya Preparation. Toxicology 2010, 278, 6–16. [Google Scholar] [CrossRef]
- Irigaray, P.; Catherine, G.; Carine, H.; Pierre, M.; Dominique, B. Beneficial Effects of a Fermented Papaya Preparation for the Treatment of Electrohypersensitivity Self-Reporting Patients: Results of a Phase I-II Clinical Trial with Special Reference to Cerebral Pulsation Measurement and Oxidative Stress Analysis. Funct. Foods Health Dis. 2018, 8, 122–144. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Somanah, J.; Bourdon, E.; Rondeau, P.; Bahorun, T. Diabetes as a Risk Factor to Cancer: Functional Role of Fermented Papaya Preparation as Phytonutraceutical Adjunct in the Treatment of Diabetes and Cancer. Mutat. Res. Mol. Mech. Mutagen. 2014, 768, 60–68. [Google Scholar] [CrossRef]
- Dickerson, R.; Banerjee, J.; Rauckhorst, A.; Pfeiffer, D.R.; Gordillo, G.M.; Khanna, S.; Osei, K.; Roy, S. Does Oral Supplementation of a Fermented Papaya Preparation Correct Respiratory Burst Function of Innate Immune Cells in Type 2 Diabetes Mellitus Patients? Antioxid. Redox Signal. 2015, 22, 339–345. [Google Scholar] [CrossRef]
- Marotta, F.; Koike, K.; Lorenzetti, A.; Jain, S.; Signorelli, P.; Metugriachuk, Y.; Mantello, P.; Locorotondo, N. Regulating Redox Balance Gene Expression in Healthy Individuals by Nutraceuticals: A Pilot Study. Rejuvenation Res. 2010, 13, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, N.; Dang, N.H.; Kumagai, E.; Kondo, A.; Iwata, S.; Morimoto, C. Aqueous Extract of Carica Papaya Leaves Exhibits Anti-Tumor Activity and Immunomodulatory Effects. J. Ethnopharmacol. 2010, 127, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Fibach, E.; Rachmilewitz, E.A. The Effect of Fermented Papaya Preparation on Radioactive Exposure. Radiat. Res. 2015, 184, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Somanah, J.; Bourdon, E.; Rondeau, P.; Bahorun, T.; Aruoma, O.I. Relationship between Fermented Papaya Preparation Supplementation, Erythrocyte Integrity and Antioxidant Status in Pre-Diabetics. Food Chem. Toxicol. 2014, 65, 12–17. [Google Scholar] [CrossRef]
- Marotta, F.; Chui, D.H.; Jain, S.; Polimeni, A.; Koike, K.; Zhou, L.; Lorenzetti, A.; Shimizu, H.; Yang, H. Effect of a Fermented Nutraceutical on Thioredoxin Level and TNF-alpha Signalling in Cirrhotic Patients. J. Biol. Regul. Homeost. Agents 2011, 25, 37–45. [Google Scholar]
- Tomella, C.; Catanzaro, R.; Illuzzi, N.; Cabeca, A.; Zerbinati, N.; Celep, G.; Milazzo, M.; Sapienza, C.; Italia, A.; Lorenzetti, A.; et al. The Hidden Phenomenon of Oxidative Stress during Treatment of Subclinical-Mild Hypothyroidism: A Protective Nutraceutical Intervention. Rejuvenation Res. 2014, 17, 180–183. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Macchia, D.; Spada, M.; Fais, S. Oral Administration of Fermented Papaya (FPP®) Controls the Growth of a Murine Melanoma through the in Vivo Induction of a Natural Antioxidant Response. Cancers 2019, 11, 118. [Google Scholar] [CrossRef]
- Marotta, F.; Koike, K.; Lorenzetti, A.; Naito, Y.; Fayet, F.; Shimizu, H.; Marandola, P. Nutraceutical Strategy in Aging: Targeting Heat Shock Protein and Inflammatory Profile through Understanding Interleukin-6 Polymorphism. Ann. N. Y. Acad. Sci. 2007, 1119, 196–202. [Google Scholar] [CrossRef]
- Collard, E.; Roy, S. Improved Function of Diabetic Wound-Site Macrophages and Accelerated Wound Closure in Response to Oral Supplementation of a Fermented Papaya Preparation. Antioxid. Redox Signal. 2010, 13, 599–606. [Google Scholar] [CrossRef]
- Das, A.; Dickerson, R.; Das Ghatak, P.; Gordillo, G.M.; Chaffee, S.; Saha, A.; Khanna, S.; Roy, S. May Dietary Supplementation Augment Respiratory Burst in Wound-Site Inflammatory Cells? Antioxid. Redox Signal. 2018, 28, 401–405. [Google Scholar] [CrossRef]
- Dickerson, R.; Deshpande, B.; Gnyawali, U.; Lynch, D.; Gordillo, G.M.; Schuster, D.; Osei, K.; Roy, S. Correction of Aberrant NADPH Oxidase Activity in Blood-Derived Mononuclear Cells from Type II Diabetes Mellitus Patients by a Naturally Fermented Papaya Preparation. Antioxid. Redox Signal. 2012, 17, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Marotta, F.; Catanzaro, R.; Yadav, H.; Jain, S.; Tomella, C.; Polimeni, A.; Mantello, P. Functional foods in genomic medicine: a review of fermented papaya preparation research progress. Acta Biomed. 2012, 83, 21–29. [Google Scholar] [PubMed]
- Rimbach, G.; Guo, Q.; Akiyama, T.; Matsugo, S.; Moini, H.; Virgili, F.; Packer, L. Ferric Nitrilotriacetate Induced DNA and Protein Damage: Inhibitory Effect of a Fermented Papaya Preparation. Anticancer Res. 2000, 20, 2907–2914. [Google Scholar] [PubMed]
- Prus, E.; Fibach, E. The Antioxidant Effect of Fermented Papaya Preparation Involves Iron Chelation. J. Boil. Regul. Homeost. Agents 2012, 26, 203–210. [Google Scholar]
- Rimbach, G.; Park, Y.C.; Guo, Q.; Moini, H.; Qureshi, N.; Saliou, C.; Takayama, K.; Virgili, F.; Packer, L. Nitric Oxide Synthesis and TNF-alpha Secretion in RAW 264.7 Macrophages: Mode of Action of a Fermented Papaya Preparation. Life Sci. 2000, 67, 679–694. [Google Scholar] [CrossRef]
- Marotta, F.; Celep, G.S.; Cabeca, A.; Polimeni, A. Novel Concepts on Functional Foods and Nutrigenomics in Healthy Aging and Chronic Diseases: A Review of Fermented Papaya Preparation Research Progress. Funct. Foods Health Dis. 2012, 2, 120. [Google Scholar] [CrossRef]
- Santiago, L.A.; Osato, J.A.; Hiramatsu, M.; Mori, A. Fermented Papaya Preparation Quenched Free Radicals and Inhibited Lipids Peroxidation in Iron- Induced Epileptic Focus in Rats. Oxyg. Rad. 1992, 4, 405–408. [Google Scholar]
- Santiago, L.A.; Osato, J.A.; Ogawa, N.; Mori, A. Antioxidant Protection of Bio-Normalizer in Cerebral Ischaemia-Reperfusion Injury in the Gerbil. NeuroReport 1993, 4, 1031–1034. [Google Scholar] [CrossRef]
- Zhang, J.; Mori, A.; Chen, Q.; Zhao, B. Fermented Papaya Preparation Attenuates Beta-Amyloid Precursor Protein: Beta-Amyloid-Mediated Copper Neurotoxicity in Beta-Amyloid Precursor Protein and Beta-Amyloid Precursor Protein Swedish Mutation Overexpressing SH-SY5Y Cells. Neuroscience 2006, 143, 63–72. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Colognato, R.; Fontana, I.; Gartlon, J.; Migliore, L.; Koike, K.; Coecke, S.; Lamy, E.; Mersch-Sundermann, V.; Laurenza, I.; et al. Molecular Effects of Fermented Papaya Preparation on Oxidative Damage, MAP Kinase Activation and Modulation of the Benzo[a]Pyrene Mediated Genotoxicity. BioFactors 2006, 26, 147–159. [Google Scholar] [CrossRef]
- Marotta, F.; Weksler, M.; Marandola, P.; Naito, Y.; Yoshida, C.; Yoshioka, M. Nutraceutical Supplementation: Effect of a Fermented Papaya Preparation on Redox Status and DNA Damage in Healthy Elderly Individuals and Relationship with GSTM1 Genotype: A Randomized, Placebo-Controlled, Cross-Over Study. Ann. N. Y. Acad. Sci. 2006, 1067, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Marotta, F.; Pavasuthipaisit, K.; Yoshida, C.; Albergati, F.; Marandola, P. Relationship Between Aging and Susceptibility of Erythrocytes to Oxidative Damage: In View of Nutraceutical Interventions. Rejuvenation Res. 2006, 9, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Bertuccelli, G.; Zerbinati, N.; Marcellino, M.; Kumar, N.S.N.; He, F.; Tsepakolenko, V.; Cervi, J.; Lorenzetti, A.; Marotta, F. Effect of a Quality-Controlled Fermented Nutraceutical on Skin Aging Markers: An Antioxidant-Control, Double-Blind Study. Exp. Ther. Med. 2016, 11, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Marotta, F.; Marcellino, M.; Solimene, U.; Cuffari, B.; Yadav, H.; Khokhlov, A.N.; Lorenzetti, A.; Mantello, A.; Cervi, J.; Catanzaro, R. A 2-year Double-Blind RCT Follow-up Study with Fermented Papaya Preparation (FPP) Modulating Key Markers in Middle-Age Subjects with Clustered Neurodegenerative Disease-Risk Factors. Clin. Pharmacol. Biopharm. 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Barbagallo, M.; Marotta, F.; Dominguez, L.J. Oxidative Stress in Patients with Alzheimer’s Disease: Effect of Extracts of Fermented Papaya Powder. Mediat. Inflamm. 2015, 2015. [Google Scholar] [CrossRef]
- Bolner, A.; Micciolo, R.; Bosello, O.; Nordera, G. Effect of Papaya Supplementation on Oxidative Stress Markers in Parkinsons Disease. Oxid. Antioxid. Med. Sci. 2016, 5, 49. [Google Scholar] [CrossRef]
- Sharma, A.; Fish, B.L.; Moulder, J.E.; Medhora, M.; Baker, J.E.; Mader, M.; Cohen, E.P. Safety and Blood 546 Sample Volume and Quality of a Refined Retro-Orbital Bleeding Technique in Rats Using a Lateral Approach. Lab Anim. 2014, 43, 63–66. [Google Scholar] [CrossRef]
- Rizza, P.; Santini, S.M.; A Logozzi, M.; Lapenta, C.; Sestili, P.; Gherardi, G.; Lande, R.; Spada, M.; Parlato, S.; Belardelli, F.; et al. T-cell Dysfunctions in hu-PBL-SCID Mice Infected with Human Immunodeficiency Virus (HIV) Shortly after Reconstitution: In Vivo Effects of HIV on Highly Activated Human Immune Cells. J. Virol. 1996, 70, 7958–7964. [Google Scholar] [CrossRef]
- Santini, S.M.; Spada, M.; Parlato, S.; Logozzi, M.; Lapenta, C.; Proietti, E.; Belardelli, F.; Fais, S. Treatment of Severe Combined Immunodeficiency Mice with Anti-Murine Granulocyte Monoclonal Antibody Improves Human Leukocyte Xenotransplantation. Transplanttion 1998, 65, 416–420. [Google Scholar] [CrossRef]
- Fais, S.; Lapenta, C.; Santini, S.M.; Spada, M.; Parlato, S.; Logozzi, M.; Rizza, P.; Belardelli, F. Human Immunodeficiency Virus Type 1 Strains R5 and X4 Induce Different Pathogenic Effects in hu-PBL-SCID Mice, Depending on the State of Activation/Differentiation of Human Target Cells at the Time of Primary Infection. J. Virol. 1999, 73, 6453–6459. [Google Scholar] [CrossRef]
- Amend, S.R.; Valkenburg, K.C.; Pienta, K.J. Murine Hind Limb Long Bone Dissection and Bone Marrow Isolation. J. Vis. Exp. 2016, e53936. [Google Scholar] [CrossRef] [PubMed]
- Duselis, A.R.; Vrana, P.B. Retrieval of Mouse Oocytes. J. Vis. Exp. 2007, 185. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Glutathione-Ascorbic Acid Antioxidant System in Animals. J. Biol. Chem. 1994, 269, 9397–9400. [Google Scholar]
- Lenton, K.J.; Therriault, H.; Cantin, A.M.; Fülöp, T.; Payette, H.; Wagner, J.R. Direct Correlation of Glutathione and Ascorbate and Their Dependence on Age and Season in Human Lymphocytes. Am. J. Clin. Nutr. 2000, 71, 1194–1200. [Google Scholar] [CrossRef]
- Gebicki, J.M.; Nauser, T.; Domazou, A.; Steinmann, D.; Bounds, P.L.; Koppenol, W.H. Reduction of Protein Radicals by GSH and Ascorbate: Potential Biological Significance. Amino Acids 2010, 39, 1131–1137. [Google Scholar] [CrossRef]
- Domazou, A.S.; Koppenol, W.H.; Gebicki, J.M. Efficient Repair of Protein Radicals by Ascorbate. Free Radic. Biol. Med. 2009, 46, 1049–1057. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic Acid Metabolism and Functions: A Comparison of Plants and Mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Marotta, F.; Yoshida, C.; Barreto, R.; Naito, Y.; Packer, L. Oxidative-Inflammatory Damage in Cirrhosis: Effect of Vitamin E and a Fermented Papaya Preparation: Oxidative Damage in Stable Liver Cirrhosis. J. Gastroenterol. Hepatol. 2007, 22, 697–703. [Google Scholar] [CrossRef]
- Yabuta, S.; Masaki, M.; Shidoji, Y. Associations of Buccal Cell Telomere Length with Daily Intake of β-Carotene or α-Tocopherol Are Dependent on Carotenoid Metabolism-related Gene Polymorphisms in Healthy Japanese Adults. J. Nutr. Health Aging 2016, 20, 267–274. [Google Scholar] [CrossRef]
- Prasad, K.N.; Wu, M.; Bondy, S.C. Telomere Shortening during Aging: Attenuation by Antioxidants and Anti-Inflammatory Agents. Mech. Ageing Dev. 2017, 164, 61–66. [Google Scholar] [CrossRef]
- Jeon, J.S.; Oh, J.-J.; Kwak, H.C.; Yun, H.; Kim, H.C.; Kim, Y.-M.; Oh, S.J.; Kim, S.K. Age-Related Changes in Sulfur Amino Acid Metabolism in Male C57BL/6 Mice. Biomol. Ther. (Seoul) 2018, 26, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Unnikrishnan, A.; Deepa, S.S.; Liu, Y.; Li, Y.; Ikeno, Y.; Sosnowska, D.; Van Remmen, H.; Richardson, A. A New Role for Oxidative Stress in Aging: The Accelerated Aging Phenotype in Sod1-/- Mice Is Correlated to Increased Cellular Senescence. Redox Biol. 2017, 11, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Xiong, C.; Lucero, J.; Behrens, M.M.; Dugan, L.L.; Quick, K.L. Gender Differences in Free Radical Homeostasis during Aging: Shorter-Lived Female C57BL6 Mice Have Increased Oxidative Stress. Aging Cell 2006, 5, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Aliahmat, N.S.; Noor, M.R.M.; Yusof, W.J.W.; Makpol, S.; Ngah, W.Z.W.; Yusof, Y.A.M. Antioxidant Enzyme Activity and Malondialdehyde Levels Can Be Modulated by Piper Betle, Tocotrienol Rich Fraction and Chlorella Vulgaris in Aging C57BL/6 Mice. Clinics 2012, 67, 1447–1454. [Google Scholar] [CrossRef]








| FPP® in 500 mL Water (the Amount in a Bottle for Each Cage) | FPP® Drank by Mice Daily | |
|---|---|---|
| Total Antioxidant Power | 6.7 ± 0.6 M | 13.48 ± 0.9 mM |
| Ascorbic Acid | 192.2 ± 3.5 ng | 0.4 ± 0.03 ng |
| ET-FPP® | LT-FPP® | |
|---|---|---|
| Total Antioxidant Power | +56% | +1% |
| GSH | +640% | +34% |
| SOD-1 | +30% | +15% |
| Total ROS | −30% | −5% |
| Telomerase | +58% | +34% |
| Telomeres length of bone marrow cells | +300% | +101% |
| Telomeres length of ovarian germ cells | +174% | +19% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Andreotti, M.; Spada, M.; Macchia, D.; Fais, S. Beneficial Effects of Fermented Papaya Preparation (FPP®) Supplementation on Redox Balance and Aging in a Mouse Model. Antioxidants 2020, 9, 144. https://doi.org/10.3390/antiox9020144
Logozzi M, Di Raimo R, Mizzoni D, Andreotti M, Spada M, Macchia D, Fais S. Beneficial Effects of Fermented Papaya Preparation (FPP®) Supplementation on Redox Balance and Aging in a Mouse Model. Antioxidants. 2020; 9(2):144. https://doi.org/10.3390/antiox9020144
Chicago/Turabian StyleLogozzi, Mariantonia, Rossella Di Raimo, Davide Mizzoni, Mauro Andreotti, Massimo Spada, Daniele Macchia, and Stefano Fais. 2020. "Beneficial Effects of Fermented Papaya Preparation (FPP®) Supplementation on Redox Balance and Aging in a Mouse Model" Antioxidants 9, no. 2: 144. https://doi.org/10.3390/antiox9020144
APA StyleLogozzi, M., Di Raimo, R., Mizzoni, D., Andreotti, M., Spada, M., Macchia, D., & Fais, S. (2020). Beneficial Effects of Fermented Papaya Preparation (FPP®) Supplementation on Redox Balance and Aging in a Mouse Model. Antioxidants, 9(2), 144. https://doi.org/10.3390/antiox9020144





