Anti–Inflammatory and Antioxidant Properties of the Ethanol Extract of Clerodendrum Cyrtophyllum Turcz in Copper Sulfate-Induced Inflammation in Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the Ethanol Extract of Clerodendrum cyrtophyllum Turcz (EE)
2.2.1. Plant Collection
2.2.2. Preparation of Total Extract
2.3. Fish and Experimental Conditions
2.4. Experimental Design
2.4.1. Preparation of Test Samples
2.4.2. Protective Effect of EE against CuSO4 Toxicity in Zebrafish Larvae
2.4.3. Anti-Oxidant Effect of EE
In Vitro Antioxidant Tests: Measurement of DPPH Radical Scavenging Capacity
Cu2+ Chelation Ability
In Vivo Antioxidant Test
Quantitative Real–Time PCR
Total RNA Extraction, DNase Treatment and Reverse Transcription
2.4.4. Anti-Inflammatory Effect of EE
2.5. Data Presentation and Statistical Analyses
3. Results
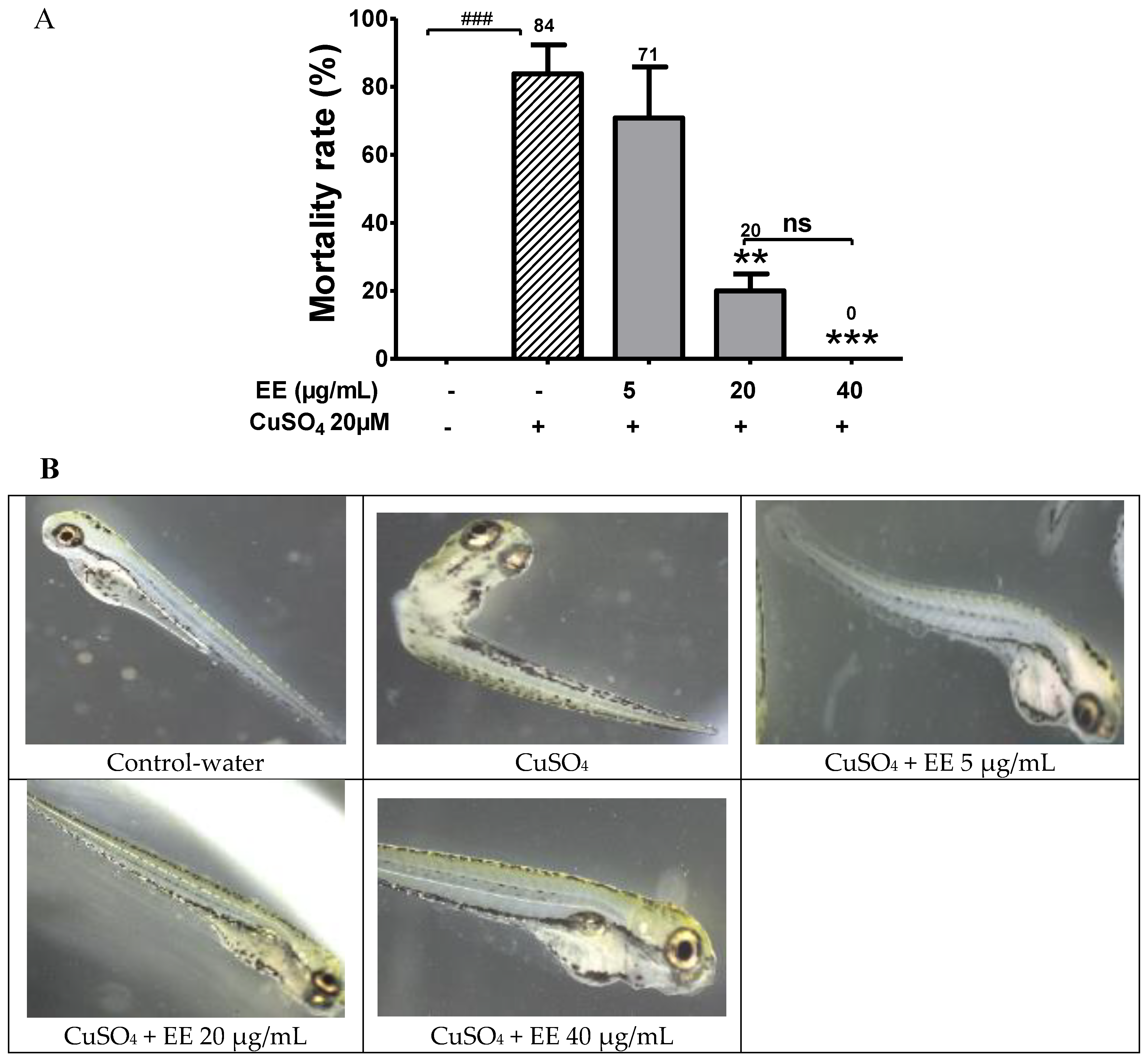
3.1. Protective Activity of EE against CuSO4 Toxicity in Zebrafish Larvae
3.2. Antioxidant Effect
3.2.1. In Vitro Antioxidant Activity of EE
Effect of the Ethanol Extract of C. cyrtophyllum Leaves (EE) on DPPH Radical Scavenging
Effect of the Ethanol Extract of C. cyrtophyllum Leaves (EE) on Cu2+ Chelation Ability
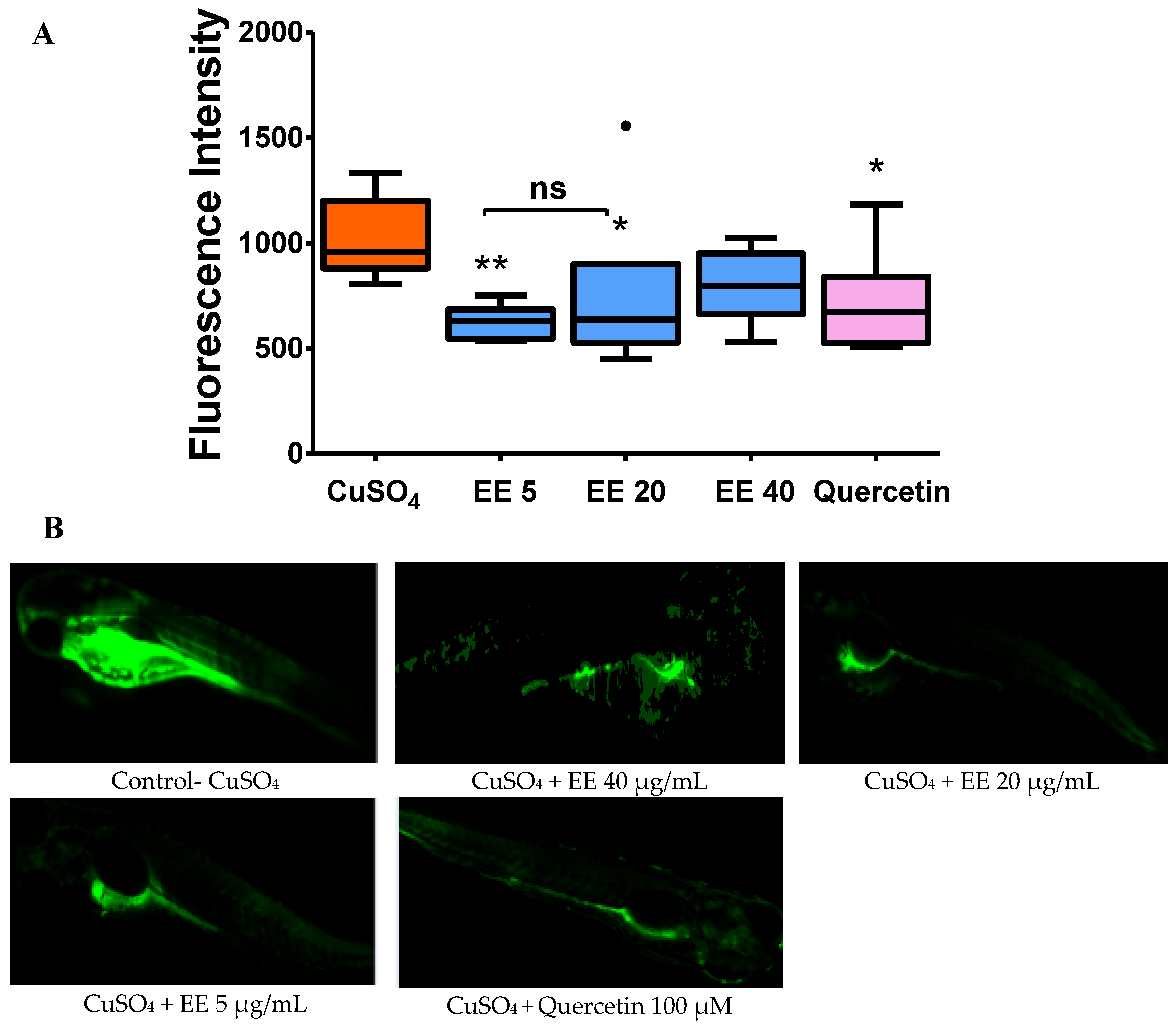
3.2.2. Effect of the Ethanol Extract of C. cyrtophyllum Leaves (EE) on the Generation of Reactive Oxygen Species
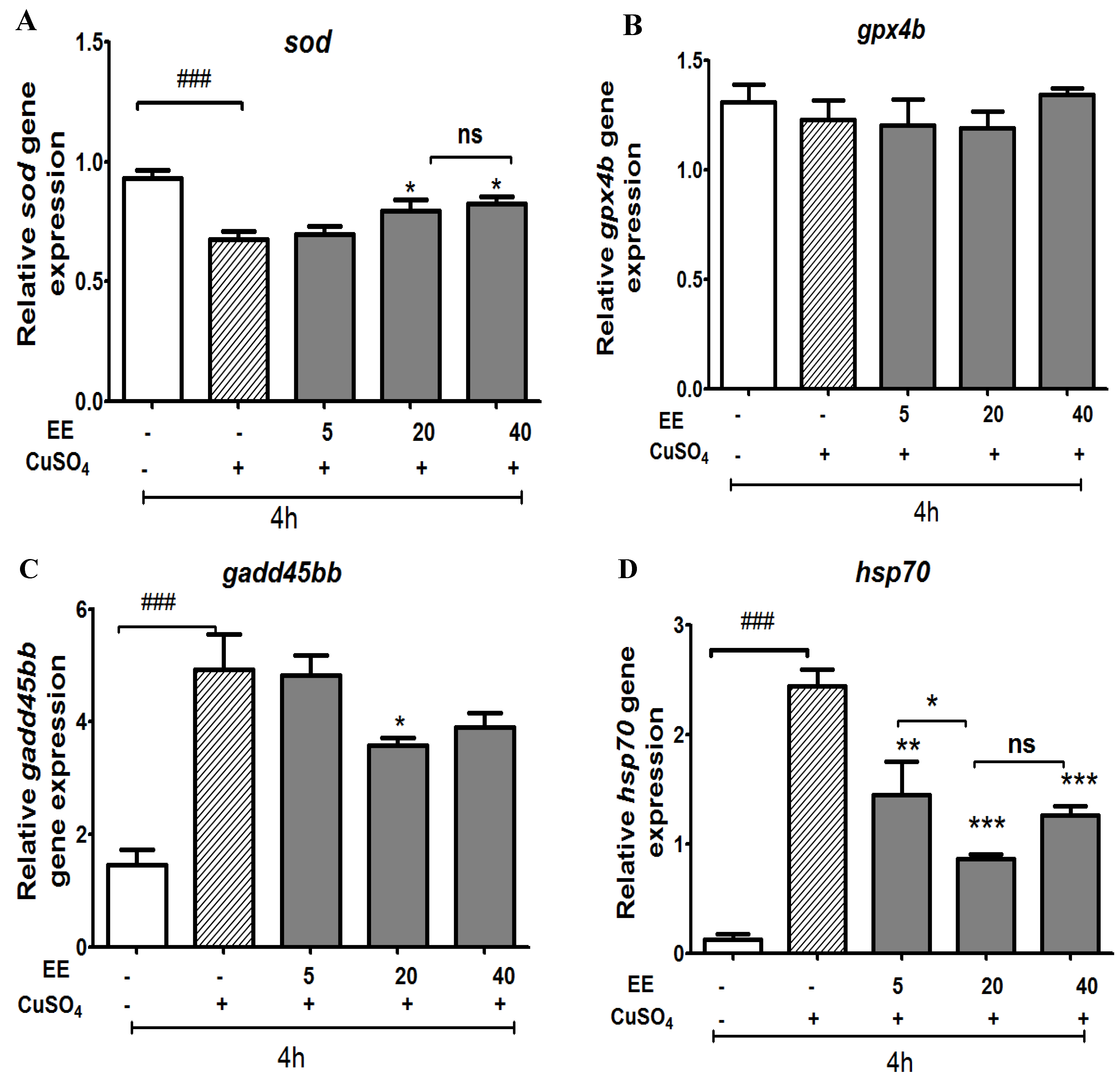
3.2.3. Effect of the Ethanol Extract of C. cyrtophyllum Leaves (EE) on Antioxidant Gene Expression
3.3. Anti-Inflammatory Effect of EE
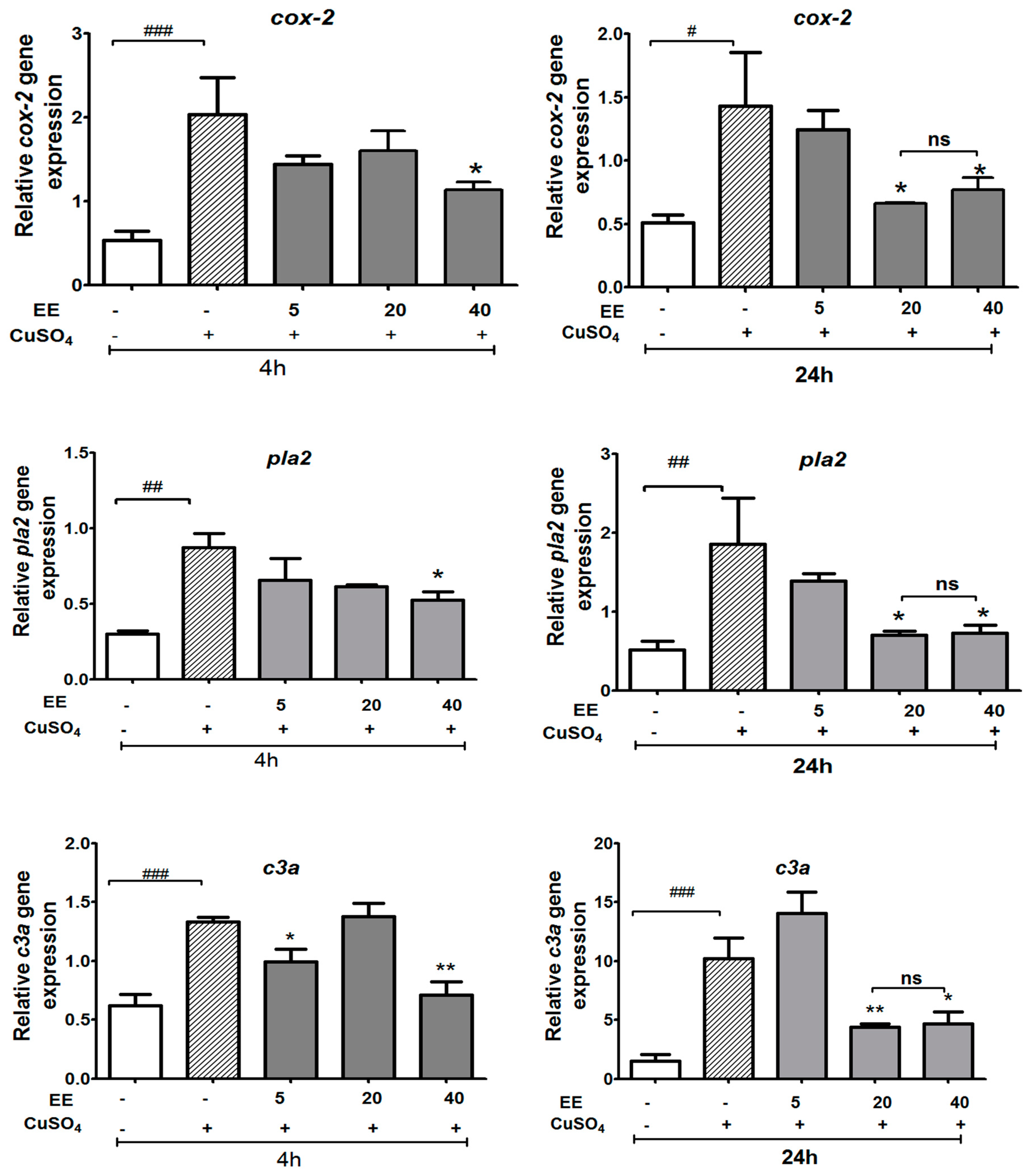
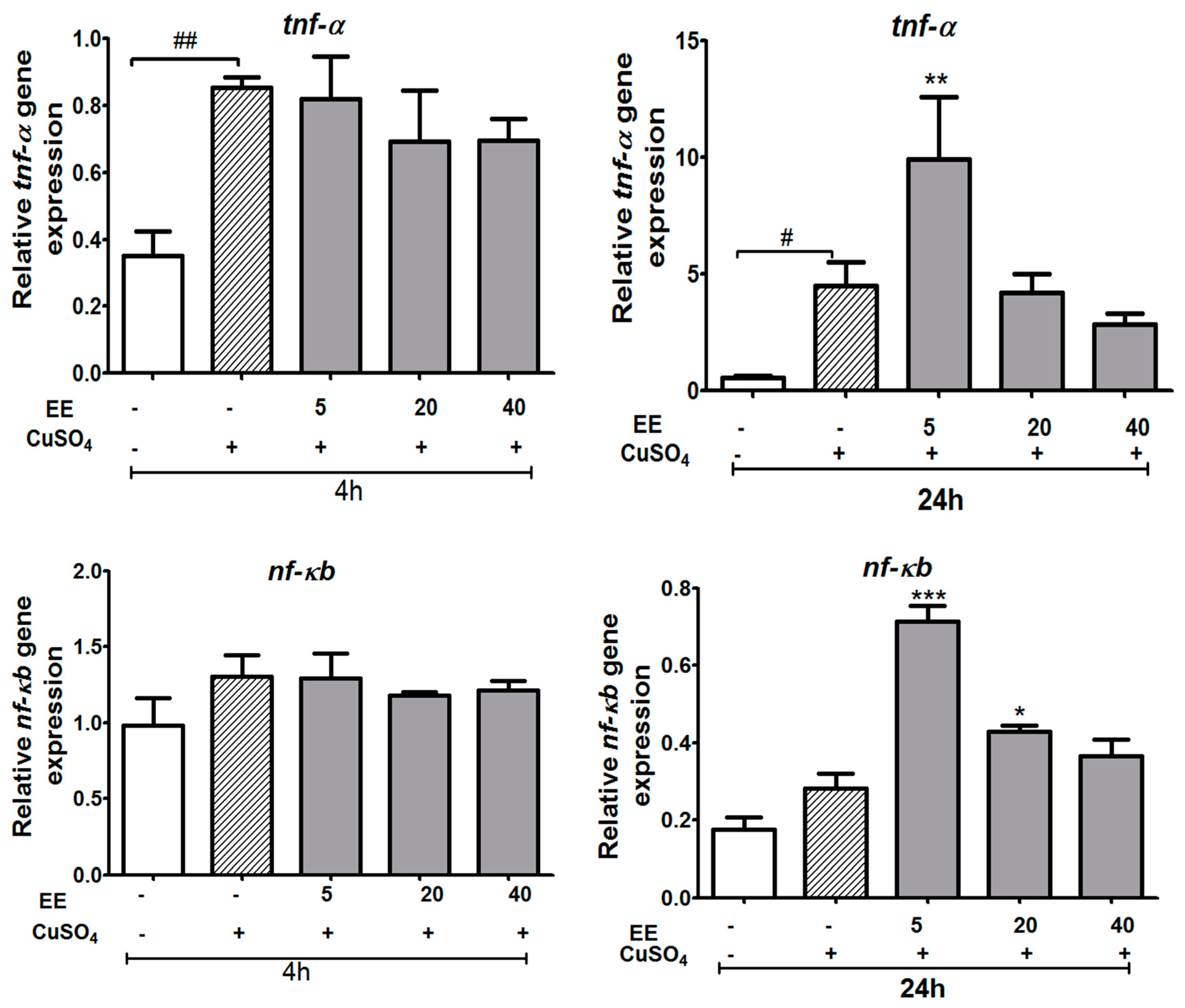
3.3.1. Effect of the Ethanol Extract of C. cyrtophyllum Turcz Leaves on the Expressions of c3a, cox-2, and pla2 Genes
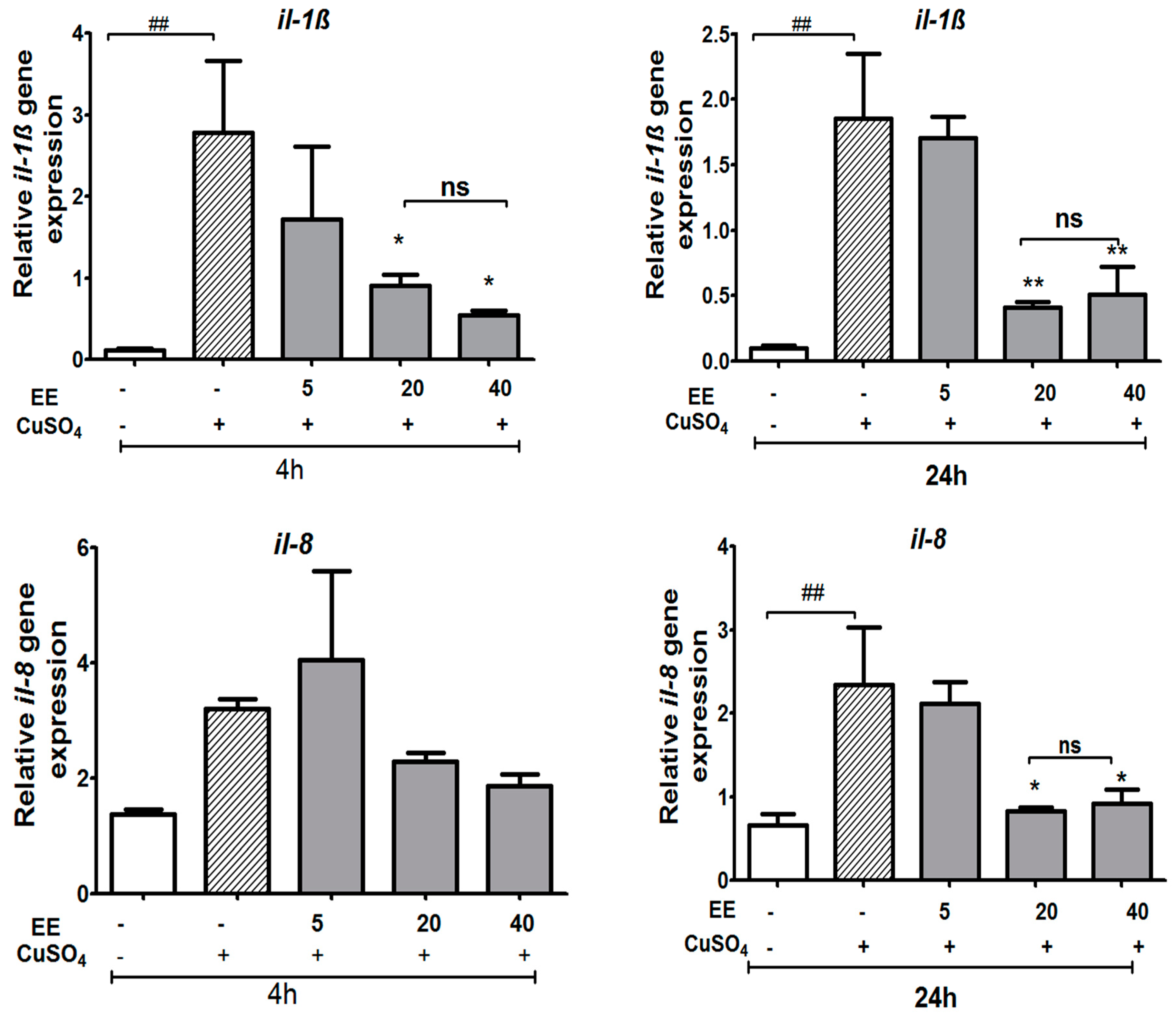
3.3.2. The EE Inhibited the Transcription of CuSO4-Induced Pro-Inflammatory Cytokines in Zebrafish Larvae
3.3.3. Effect of the Ethanol Extract (EE) of C. cyrtophyllum Turcz Leaves on Expression of the mpo Gene
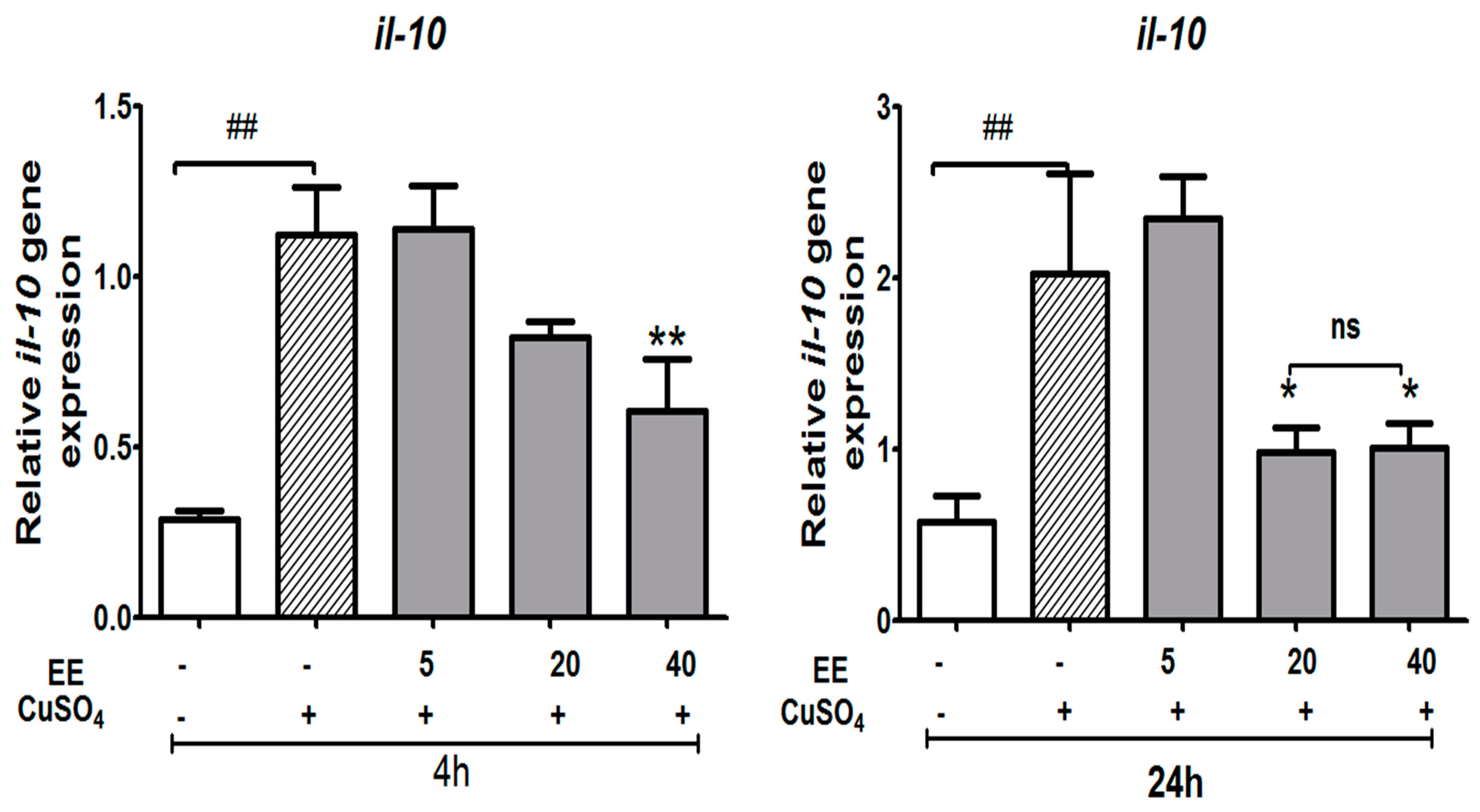
3.3.4. Effect of the Ethanol Extract (EE) of C. cyrtophyllum Turcz leaves on the Expression of Anti-Inflammatory Cytokine il-10
4. Discussion
4.1. Protective Activity of the Ethanol Extract of C. cyrtophyllum against CuSO4 Toxicity
4.2. Antioxidant and Anti-Inflammatory Activity of the Ethanol Extract of C. cyrtophyllum
4.3. The Relation between Dose and Response
4.4. The Potential Area of Application of EE
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fialkow, L.; Wang, Y.; Downey, G.P. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic. Biol. Med. 2007, 42, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Karin, M. Nuclear factor-kB—A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Hsu, C.; Wen, Z.; Lin, C.; Agoramoorthy, G. Zebrafish: A Complete Animal Model for In Vivo Drug Discovery and Development. Curr. Drug Metab. 2009, 10, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Pereira, T.C.B.; Campos, M.M.; Bogo, M.R. Copper toxicology, oxidative stress and inflammation using zebrafish as experimental model. J. Appl. Toxicol. 2016, 36, 876–885. [Google Scholar] [CrossRef]
- Nhuong, L.C.; van Long, N.; Hoa, N.D. Chemical constituents and antioxidant activity of flavonoids from Clerodendron cyrtophyllum Turcz. Vietnam. Pharm. J. 2006, 46, 30–33. [Google Scholar]
- Kar, P.; Goyal, A.K.; Das, A.P.; Sen, A. Antioxidant and pharmaceutical potential of Clerodendrum L.: An overview. Int. J. Green Pharm. 2014, 8, 210–216. [Google Scholar]
- Zhou, J.; Zheng, X.; Yang, Q.; Liang, Z.; Li, D.; Yang, X.; Xu, J. Optimization of Ultrasonic-Assisted Extraction and Radical-Scavenging Capacity of Phenols and Flavonoids from Clerodendrum cyrtophyllum Turcz Leaves. PLoS ONE 2013, 8, e68392. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, L.; Wang, M. Antioxidant and Nitric Oxide Release Inhibition Activities of Methanolic Extract from Clerodendrum cyrtophyllum Turcz. Hort. Environ. Biotechnol. 2011, 52, 309–314. [Google Scholar] [CrossRef]
- Lackmann, C.; Martinez, M.; Rainieri, S.; Barranco, A.; Hollert, H.; Spirhanzlova, P.; Velki, M.; Seiler, T. Chemosphere Novel procedures for whole organism detection and quantification of fluorescence as a measurement for oxidative stress in zebrafish (Danio Rerio) larvae. Chemosphere 2018, 197, 200–209. [Google Scholar] [CrossRef]
- Wang, C.Z.; Yuan, H.H.; Bao, X.L.; Lan, M.B. In vitro antioxidant and cytotoxic properties of ethanol extract of Alpinia oxyphylla fruits. Pharm. Biol. 2013, 51, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Brizola, V.R.A.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Olivari, F.A.; Hernández, P.P.; Allende, M.L. Acute copper exposure induces oxidative stress and cell death in lateral line hair cells of zebrafish larvae. Brain Res. 2008, 1244, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ko, C.I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.S.; Jeon, Y.J. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar] [CrossRef]
- Bio-Rad. Real-Time qPCR Data Analysis. In Real-Time PCR Applications Guid; Bio-Rad Laboratories, Inc.: Hercules, CA, USA, 2006; pp. 34–44. [Google Scholar]
- Hernandez, P.P.; Undurraga, C.; Gallardo, V.E.; Mackenzie, N.; Allende, M.L.; Reyes, A.E. Sublethal concentrations of waterborne copper induce cellular stress and cell death in zebrafish embryos and larvae. Biol. Res. 2011, 44, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Cruz, F.F.; Leite, C.E.; Kist, L.W.; de Oliveira, G.M.; Bogo, M.R.; Bonan, C.D.; Campos, M.M.; Morrone, F.B. Effects of caffeine on behavioral and inflammatory changes elicited by copper in zebrafish larvae: Role of adenosine receptors. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 194, 28–36. [Google Scholar] [CrossRef]
- Leite, C.E.; de Oliveira Maboni, L.; Cruz, F.F.; Rosemberg, D.B.; Zimmermann, F.F.; Pereira, T.C.B.; Bogo, M.R.; Bonan, C.D.; Campos, M.M.; Morrone, F.B.; et al. Involvement of purinergic system in inflammation and toxicity induced by copper in zebrafish larvae. Toxicol. Appl. Pharmacol. 2013, 272, 681–689. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K. Therapeutic and Nutraceutical Potential of Bioactive Compounds Extracted from Fruit Residues. Crit. Rev. Food Sci. Nutr. 2015, 55, 319–337. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manay, A.; Abdollahi, M. From in vitro Experiments to in vivo and Clinical Studies; Pros and cons. Curr. Drug Discov Technol. 2015, 12, 218–224. [Google Scholar] [CrossRef]
- Yang, L.L.; Wang, G.Q.; Yang, L.M.; Huang, Z.B.; Zhang, W.Q.; Yu, L.Z. Endotoxin molecule lipopolysaccharide-induced zebrafish inflammation model: A novel screening method for anti-inflammatory drugs. Molecules 2014, 19, 2390–2409. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Q.; Ji, H.; Zheng, Z.; Yu, X.; Sun, L.; Liu, X. Copper induces apoptosis in BA/F3β cells: Bax, reactive oxygen species, and NFκB are involved. J. Cell. Physiol. 2000, 184, 161–170. [Google Scholar] [CrossRef]
- Moskalev, A.A.; Smit-McBride, Z.; Fraifeld, V.E. Gadd45 proteins: Relevance to aging, longevity and age-related pathologies. Ageing Res. Rev. 2012, 11, 51–66. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zhang, Y.H.; Jiang, Y.; Li, L.L.; Chen, Q.; He, G.Q.; Tan, X.D.; Li, C.Q. Gadd45b is a novel mediator of neuronal apoptosis in ischemic stroke. Int. J. Biol. Sci. 2015, 11, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Choo, X.Y.; Alukaidey, L.; White, A.R.; Grubman, A. Neuroinflammation and copper in Alzheimer’s disease. Int. J. Alzheimers. Dis. 2013. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.F.; Malik, A.B. NF-kB activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 622–645. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF-kB Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, a001651. [Google Scholar] [CrossRef]
- Lawrence, T.; Gilroy, D.W.; Colville-Nash, P.R.; Willoughby, D.A. Possible new role for NF-κB in the resolution of inflammation. Nat. Med. 2001, 7, 1291–1297. [Google Scholar] [CrossRef]
- D’Alencon, C.A.; Pena, O.A.; Wittmann, C.; Gallardo, V.E.; Jones, R.A.; Allende, M.L. A high-throughput chemically induced inflammation assay in zebrafish. BMC Biol. 2010, 8, 151. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Alsahli, M.; Rahmani, A. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harizi, H.; Gualde, N. Pivotal role of PGE2 and IL-10 in the cross-regulation of dendritic cell-derived inflammatory mediators. Cell. Mol. Immunol. 2006, 3, 271–277. [Google Scholar] [PubMed]
- Zhang, J.M.; An, J. Cytokines, Inflammation and Pain. Int. Anesth. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, J.; van Boxel-Dezaire, A.; Methorst, D.; Brunt, T.; De Kloet, E.R.; Nagelkerken, L. Differential regulation of interleukin-10 (IL-10) and IL-12 by glucocorticoids in vitro. Blood 1998, 91, 4255–4264. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Function | GenBank Accession No. | Forward and Reverse Primer Sequences (5′-3′) |
|---|---|---|---|
| β-actin | Housekeeping gene | AF057040 | Fwd: CCCCATTGAGCACGGTATTG Rev: ATACATGGCAGGGGTGTTGA |
| Elongation factor 1 alpha (efl1-α) | Housekeeping gene | L23807.1 | Fwd: CCAAGGAAGTCAGCGCATAC Rev: CCTCCTTGCGCTCAATCTTC |
| Interleukin-1 (il-1ß) | Pro-inflammatory cytokine | NM_212844.2 | Fwd: AAAGTGCGCTTCAGCATGTC Rev: GCTGGTCGTATCCGTTTGGA |
| Interleukin -8 il-8 (cxcl8b.1) | cytokine | NM_001327985.1 | Fwd: GCCTTCATGCTTCTGATCTGC Rev: AATCACCCACGTCTCGGTAGGA |
| Cyclooxygenase -2 (ptgs2a or cox2) | Catalyze the formation of prostaglandin, thromboxane | NM_153657.1 | Fwd: ACAGATGCGCTACCAGTCTT Rev: CCCATGAGGCCTTTGAGAGA |
| Phospholipase A2 pla2 (pla2g4aa) | Provide precursors for generation of eicosanoids | NM_131295.2 | Fwd: TCATGTCTCCTGGGCTGTTT Rev: CCAGCTCCTCCTCCATAGTG |
| Tumor necrosis factor (tnf-α) | Pro-inflammatory cytokine | AB183467 | Fwd: CACAAAGGCTGCCATTCACT Rev: GATTGATGGTGTGGCTCAGGT |
| nf-ƙb (nkap) | A ubiquitous transcription factor | NM_001003414.1 | Fwd: GGTCGGACAGAGATCACGGATT Rev: TGCTGTTCTTCACGTCCTCT |
| Interleukin -10 (il-10) | Anti-inflammatory cytokine | AY887900.1 | Fwd: AGTCATCCTTTCTGCTCTGCT Rev: AAAGCCCTCCACAAATGAGC |
| c3a (c3a.1) | Complement 3a | NM_131242.1 | Fwd: GTACGAGGCGAACAACTGGA Rev: CATCATACGCCGCAGCTTTC |
| mpo | Myeloperoxidase | AF349034.1 | Fwd: GTGGTCGTGTCGGTTCTCTT Rev: GCAGATTATGCGGGCCATTG |
| sod (sod1) | Superoxide dismutases | NM_131294.1 | Fwd: ATGGTGAACAAGGCCGTTTG Rev: AAAGCATGGACGTGGAAACC |
| gpx4b | glutathione peroxidase | BC095133.1 | Fwd: TGAGAAGGGTTTACGCATCCTG Rev: TGTTGTTCCCCAGTGTTCCT |
| hsp70 | Protect cell from oxidative stress | AF210640.1 | Fwd: CAACGTGCTGATCTTTGACC Rev: TCCTCTTGGCTCGTTCACAT |
| gadd45bb | Growth arrest and DNA-damage-inducible | NM_001012386.2 | Fwd: CGCTTCAGATCCACTTCACG Rev: TCCCACTTCCTTCAGCTTGA |
| EE | Quercetin | |
|---|---|---|
| Emax (%) | 94.24 | 93.82 |
| IC50 (µg/mL) | 16.45 ± 1.11 | 3.93 ± 1.09 |
| 95% Confidence Interval | 11.63–23.27 | 2.98–5.17 |
| R2 | 0.9958 | 0.98995 |
| Number of Points Analyzed | 7 | 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.H.; Le, H.D.; Nguyen Thi Kim, T.; Pham The, H.; Nguyen, T.M.; Cornet, V.; Lambert, J.; Kestemont, P. Anti–Inflammatory and Antioxidant Properties of the Ethanol Extract of Clerodendrum Cyrtophyllum Turcz in Copper Sulfate-Induced Inflammation in Zebrafish. Antioxidants 2020, 9, 192. https://doi.org/10.3390/antiox9030192
Nguyen TH, Le HD, Nguyen Thi Kim T, Pham The H, Nguyen TM, Cornet V, Lambert J, Kestemont P. Anti–Inflammatory and Antioxidant Properties of the Ethanol Extract of Clerodendrum Cyrtophyllum Turcz in Copper Sulfate-Induced Inflammation in Zebrafish. Antioxidants. 2020; 9(3):192. https://doi.org/10.3390/antiox9030192
Chicago/Turabian StyleNguyen, Thu Hang, Hong Diep Le, Thanh Nguyen Thi Kim, Hai Pham The, Thi Mai Nguyen, Valérie Cornet, Jérôme Lambert, and Patrick Kestemont. 2020. "Anti–Inflammatory and Antioxidant Properties of the Ethanol Extract of Clerodendrum Cyrtophyllum Turcz in Copper Sulfate-Induced Inflammation in Zebrafish" Antioxidants 9, no. 3: 192. https://doi.org/10.3390/antiox9030192
APA StyleNguyen, T. H., Le, H. D., Nguyen Thi Kim, T., Pham The, H., Nguyen, T. M., Cornet, V., Lambert, J., & Kestemont, P. (2020). Anti–Inflammatory and Antioxidant Properties of the Ethanol Extract of Clerodendrum Cyrtophyllum Turcz in Copper Sulfate-Induced Inflammation in Zebrafish. Antioxidants, 9(3), 192. https://doi.org/10.3390/antiox9030192




