Reversible Thiol Oxidation Inhibits the Mitochondrial ATP Synthase in Xenopus laevis Oocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Xenopus laevis
2.3. F1-Fo ATP Synthase Assay
2.4. Native Blotting
2.5. In-Gel ATP Hydrolysis Assay
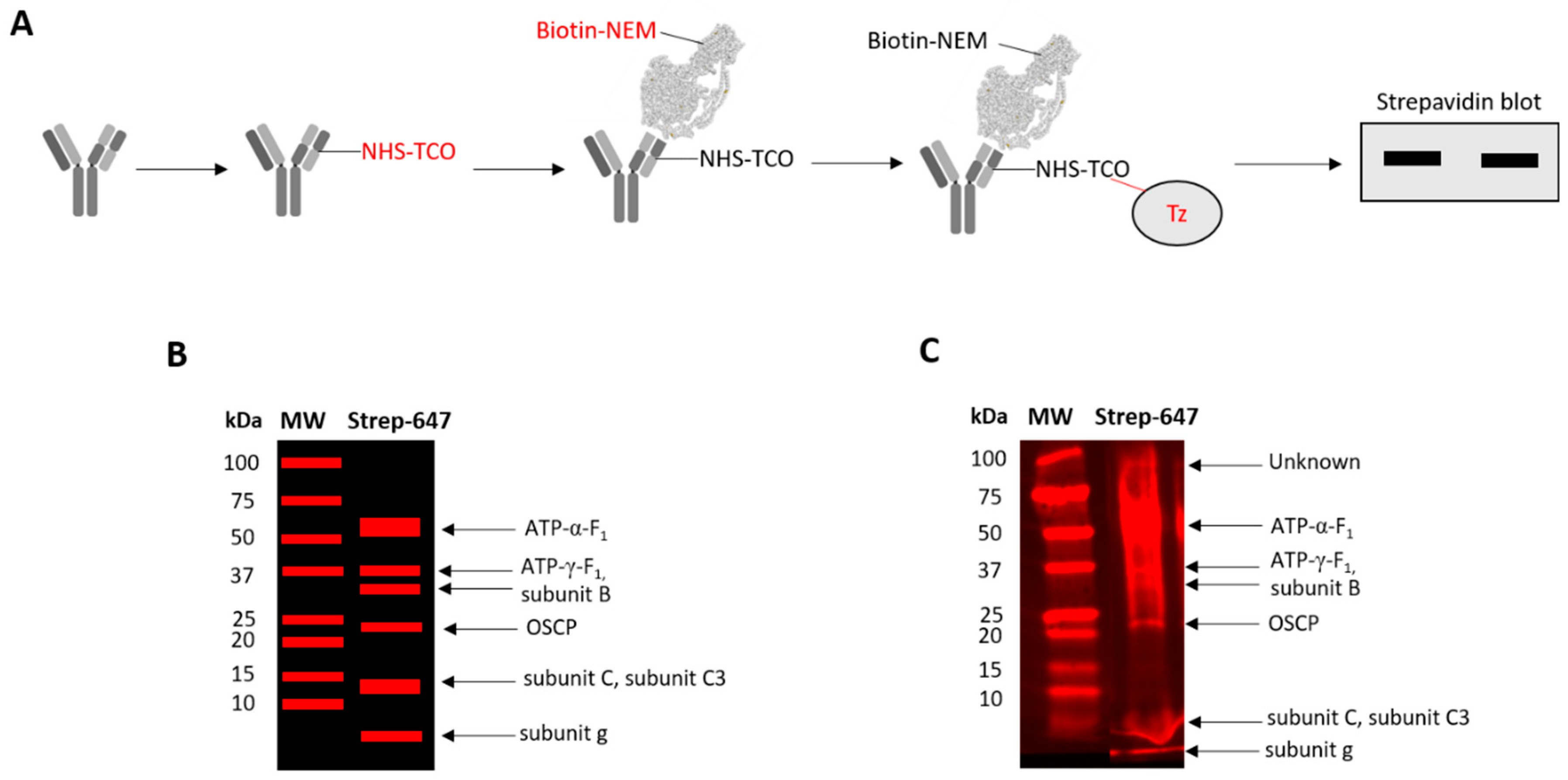
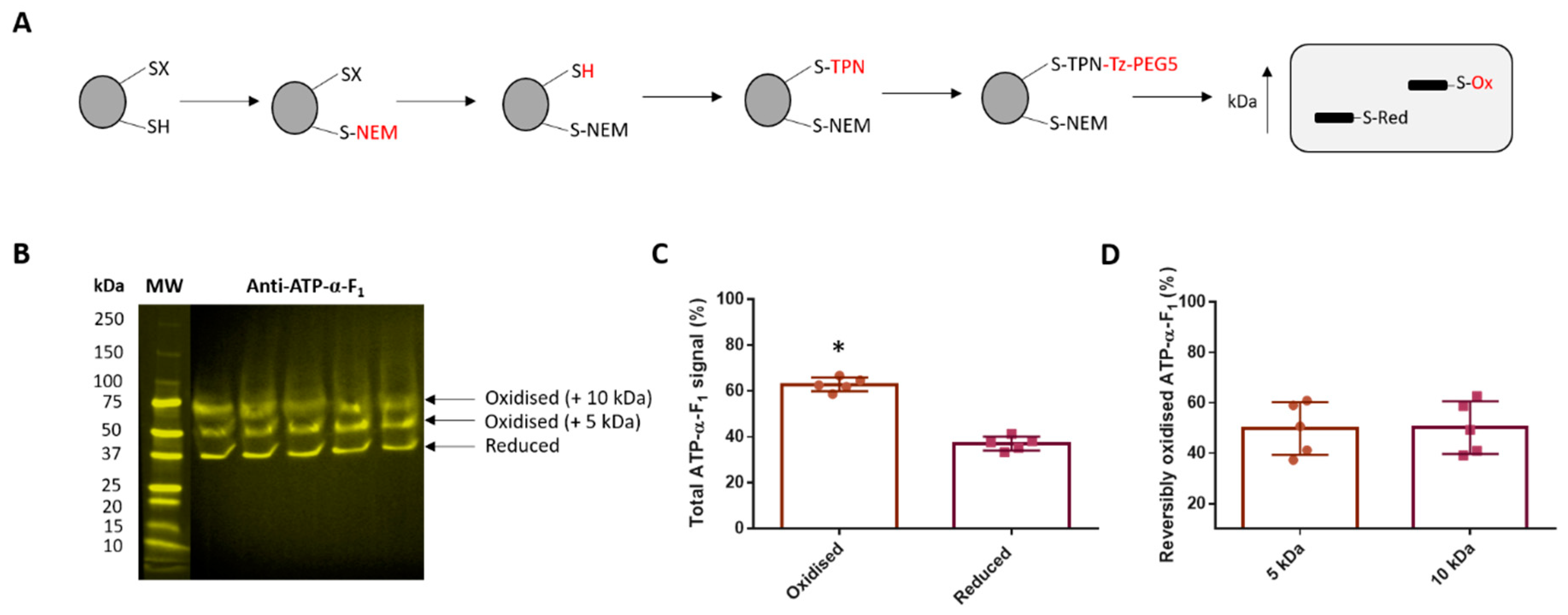
2.6. Catalyst-Free TCO-Tz Immunocapture Coupled to Redox Affinity Blotting
2.7. Catalyst-Free TCO-Tz Click PEGylation
2.8. Redox Mobility Shift Assay
2.9. Statistical Analysis
3. Results
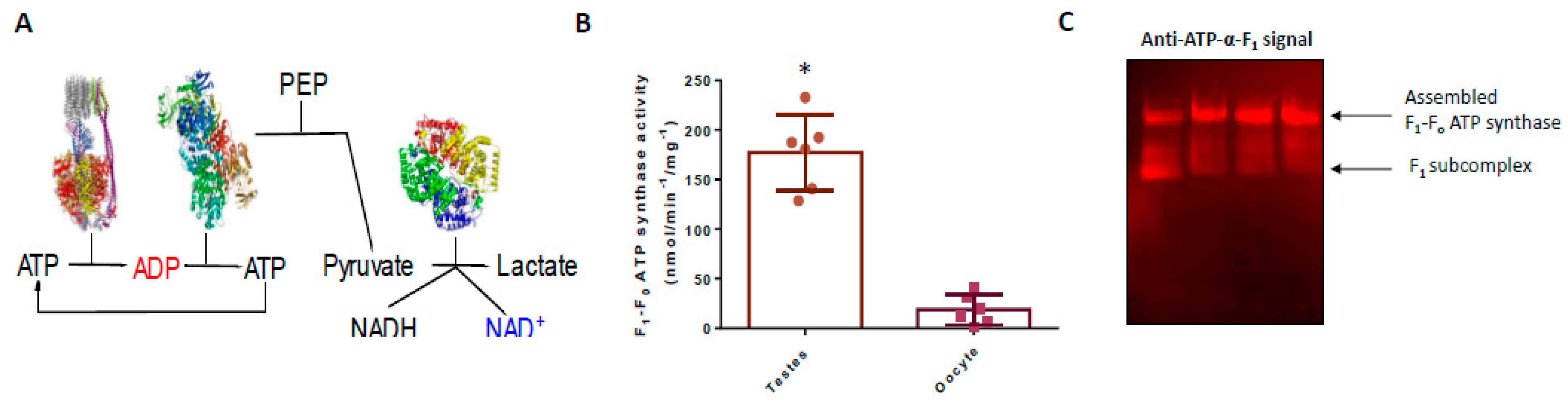
3.1. F1-Fo ATP Synthase Activity is Significantly Greater in Testes Compared to Oocytes
3.2. The F1-Fo ATP Synthase is Assembled in Oocytes
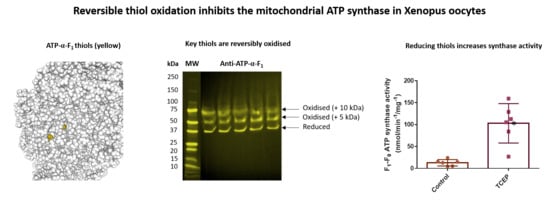
3.3. Several F1-Fo ATP Synthase Subunits are Reversibly Oxidised
3.4. Reversible ATP-α-F1 Oxidation is Significant in Oocytes
3.5. Reversible Thiol Oxidation Inhibits the F1-Fo ATP Synthase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vestre, K. The Making of You; Profile Books Limited: London, UK, 2019. [Google Scholar]
- Nesci, S.; Spinaci, M.; Galeati, G.; Nerozzi, C.; Pagliarani, A.; Algieri, C.; Tamanini, C.; Bucci, D. Sperm function and mitochondrial activity: An insight on boar sperm metabolism. Theriogenology 2020, 144, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Latorre-pellicer, A.; Lechuga-vieco, A.V.; Johnston, I.G.; Jones, N.S.; Enrı, A. Regulation of Mother-to-Offspring Transmission of mtDNA Heteroplasmy Short Article Regulation of Mother-to-Offspring Transmission of mtDNA Heteroplasmy. Cell Metab. 2019, 30, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, H.; Li, H.; Nakagawa, A.; Lin, J.L.J.; William, D.; Mitani, S.; Yuan, H.S.; Kang, B.; Xue, D. Mitochondrial endonuclease G mediates breakdown of paternal mitochondria upon fertilization. Science 2016, 353, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Lane, N. Mitonuclear match: Optimizing fitness and fertility over generations drives ageing within generations. BioEssays 2011, 33, 860–869. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Sawyer, D.; Valentine, J. How super is superoxide? Acc. Chem. Res. 1981, 14, 393–400. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology & Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Cobley, J.N.; Margaritelis, N.V.; Morton, J.P.; Close, G.L.; Nikolaidis, M.G.; Malone, J.K. The basic chemistry of exercise-induced DNA oxidation: Oxidative damage, redox signaling, and their interplay. Front. Physiol. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Ravanat, J.L.; TavernaPorro, M.; Menoni, H.; Angelov, D. Oxidatively generated complex DNA damage: Tandem and clustered lesions. Cancer Lett. 2012, 327, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.F. Separate Sexes and the Mitochondrial Theory of Ageing. J. Theor. Biol. 1996, 180, 135–140. [Google Scholar] [CrossRef] [PubMed]
- De Paula, W.B.M.; Lucas, C.H.; Agip, A.A.; Vizcay-barrena, G.; Allen, J.F.; Allen, J.F. Energy, ageing, fidelity and sex: Oocyte mitochondrial DNA as a protected genetic template. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120263. [Google Scholar] [CrossRef] [PubMed]
- De Paula, W.B.M.; Agip, A.A.; Missirlis, F.; Ashworth, R.; Vizcay-barrena, G.; Lucas, C.H.; Allen, J.F. Female and Male Gamete Mitochondria Are Distinct and Complementary in Transcription, Structure, and Genome Function. Genome Biol. Evol. 2013, 5, 1969–1977. [Google Scholar] [CrossRef]
- Trimarchi, J.R.; Liu, L.; Porterfield, D.M.; Smith, P.J.S.; Keefe, D.L. Oxidative phosphorylation-dependent and -independent oxygen consumption by individual preimplantation mouse embryos. Biol. Reprod. 2000, 62, 1866–1874. [Google Scholar] [CrossRef]
- Motta, P.M.; Nottola, S.A.; Makabe, S.; Heyn, R. Mitochondrial morphology in human fetal and adult female germ cells. Hum. Reprod. 2000, 2 (Suppl. 15), 129–147. [Google Scholar] [CrossRef]
- Houghton, F.D.; Christopher, G.T.; Leese, H.J. Oxygen Consumption and Energy Metabolism of the Early Mouse Embryo. Mol. Reprod. Dev. 1996, 485, 476–485. [Google Scholar] [CrossRef]
- Robb, E.L.; Hall, A.R.; Prime, T.A.; Eaton, S.; Szibor, M.; Viscomi, C.; James, A.M.; Murphy, M.P. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 2018, 293, 9869–9879. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Ferguson, S.J. Bioenergetics 4, 4th ed.; Academic Press: London, UK, 2013. [Google Scholar]
- Han, Y.; Ishibashi, S.; Iglesias-Gonzalez, J.; Chen, Y.; Love, N.R.; Amaya, E. Ca2+-Induced Mitochondrial ROS Regulate the Early Embryonic Cell Cycle. Cell Rep. 2018, 22, 218–231. [Google Scholar] [CrossRef]
- Gibeaux, R.; Acker, R.; Kitaoka, M.; Georgiou, G.; van Kruijsbergen, I.; Ford, B.; Marcotte, E.M.; Nomura, D.K.; Kwon, T.; Veenstra, G.J.C.; et al. Paternal chromosome loss and metabolic crisis contribute to hybrid inviability in Xenopus. Nature 2018, 553, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Noble, A.; Jimenez-fernandez, E.; Moya, M.V.; Guille, M.; Husi, H. Catalyst-free Click PEGylation reveals substantial mitochondrial ATP synthase sub-unit alpha oxidation before and after fertilisation. Redox Biol. 2019, 26, 101258. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T.; Hatefi, Y. Thiols in Oxidative Phosphorylation: Inhibition and Energy-Potentiated Uncoupling by Monothiol and Dithiol Modifiers1. Biochemistry 1984, 23, 2449–2455. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T.; Hatefi, Y. Thiols in oxidative phosphorylation: Thiols in the F0 of ATP synthase essential for ATPase activity. Arch. Biochem. Biophys. 1987, 254, 102–109. [Google Scholar] [CrossRef]
- Nalins, C.M.; Mccarty, R.E. Role of a Disulfide Bond in the y Subunit in Activation of the ATPase of Chloroplast Coupling Factor 1. J. Biol. Chem. 1984, 259, 7275–7280. [Google Scholar]
- Wang, S.B.; Foster, D.B.; Rucker, J.; O’Rourke, B.; Kass, D.A.; van Eyk, J.E. Redox regulation of mitochondrial ATP synthase: Implications for cardiac resynchronization therapy. Circ. Res. 2011, 109, 750–757. [Google Scholar] [CrossRef]
- Wang, S.B.; Murray, C.I.; Chung, H.S.; van Eyk, J.E. Redox Regulation of Mitochondrial ATP Synthase. Trends Cardiovasc. Med. 2013, 23, 18. [Google Scholar] [CrossRef]
- Kaludercic, N.; Giorgio, V. The dual function of reactive oxygen/nitrogen species in bioenergetics and cell death: The role of ATP synthase. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- West, M.B.; Hill, B.G.; Xuan, Y.T.; Bhatnagar, A. Protein glutathiolation by nitric oxide: An intracellular mechanism regulating redox protein modification. FASEB J. 2006, 20, 1715–1717. [Google Scholar] [CrossRef]
- Garcia, J.; Han, D.; Sancheti, H.; Yap, L.P.; Kaplowitz, N.; Cadenas, E. Regulation of mitochondrial glutathione redox status and protein glutathionylation by respiratory substrates. J. Biol. Chem. 2010, 285, 39646–39654. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Hampton, M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008, 45, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Carroll, K.S. Cysteine-Mediated Redox Signaling: Chemistry, Biology, and Tools for Discovery. Chem. Rev. 2013, 133, 4633–4679. [Google Scholar] [CrossRef]
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox Signaling by Reactive Electrophiles and Oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Flohé, L. Basic Principles and Emerging Concepts in the Redox Control of Transcription Factors. Antioxid. Redox Signal. 2011, 15, 2335–2381. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Willmore, W.G. S-glutathionylation reactions in mitochondrial function and disease. Front. Cell Dev. Biol. 2014, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Martínez-reyes, I.; Cuezva, J.M. The H + -ATP synthase: A gate to ROS-mediated cell death or cell survival. BBA Bioenerg. 2014, 1837, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; di Lisa, F.; Fogolari, F.; Lippe, G. From ATP to PTP and Back A Dual Function for the Mitochondrial ATP Synthase. Circ. Res. 2015, 116, 1850–1862. [Google Scholar] [CrossRef]
- Session, A.M.; Uno, Y.; Kwon, T.; Chapman, J.A.; Toyoda, A.; Takahashi, S.; Fukui, A.; Hikosaka, A.; Suzuki, A.; Kondo, M.; et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 2016, 538, 1–15. [Google Scholar] [CrossRef]
- Harland, R.M.; Grainger, R.M. Xenopus research: Metamorphosed by genetics and genomics. Trends Genet. 2011, 27, 507–515. [Google Scholar] [CrossRef]
- Sidlauskaite, E.; Gibson, J.W.; Megson, I.L.; Whitfield, P.D.; Tovmasyan, A.; Batinic-Haberle, I.; Murphy, M.P.; Moult, P.R.; Cobley, J.N. Mitochondrial ROS cause motor deficits induced by synaptic inactivity: Implications for synapse pruning. Redox Biol. 2018, 16, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Sieber, M.H.; Thomsen, M.B.; Spradling, A.C.; Sieber, M.H.; Thomsen, M.B.; Spradling, A.C. Electron Transport Chain Remodeling by GSK3 during Oogenesis Connects Nutrient State to Reproduction. Cell 2016, 164, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Kogo, N.; Tazaki, A.; Kashino, Y.; Morichika, K.; Orii, H.; Mochii, M.; Watanabe, K. Germ-line mitochondria exhibit suppressed respiratory activity to support their accurate transmission to the next generation. Dev. Biol. 2011, 349, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Horb, M.; Wlizla, M.; Abu-daya, A.; Mcnamara, S.; Gajdasik, D.; Igawa, T.; Suzuki, A.; Ogino, H.; Noble, A.; de Ressource, C.; et al. Xenopus Resources: Transgenic, Inbred and Mutant Animals, Training Opportunities, and Web-Based Support. Front. Physiol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, 6–10. [Google Scholar] [CrossRef]
- Grivennikova, V.G.; Kapustin, A.N.; Vinogradov, A.D. Catalytic Activity of NADH-ubiquinone Oxidoreductase (Complex I) in Intact Mitochondria. J. Biol. Chem. 2001, 276, 9038–9044. [Google Scholar] [CrossRef]
- Spinazzi, M.; Casarin, A.; Pertegato, V.; Salviati, L.; Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012, 7, 1235–1246. [Google Scholar] [CrossRef]
- Wittig, I.; Karas, M.; Schägger, H. High Resolution Clear Native Electrophoresis for In-gel Functional Assays and Fluorescence Studies of Membrane Protein Complexes. Mol. Cell. Proteom. 2007, 6, 1215–1225. [Google Scholar] [CrossRef]
- Wittig, I.; Braun, H.P.; Schägger, H. Blue native PAGE. Nat. Protoc. 2006, 1, 418–428. [Google Scholar] [CrossRef]
- Cobley, J.N.; Sakellariou, G.K.; Owens, D.J.; Murray, S.; Waldron, S.; Gregson, W.; Fraser, W.D.; Burniston, J.G.; Iwanejko, L.A.; McArdle, A.; et al. Lifelong training preserves some redox-regulated adaptive responses after an acute exercise stimulus in aged human skeletal muscle. Free Radic. Biol. Med. 2014, 70, 23–32. [Google Scholar] [CrossRef]
- Cobley, J.N.; Bartlett, J.D.; Kayani, A.; Murray, S.W.; Louhelainen, J.; Donovan, T.; Waldron, S.; Gregson, W.; Burniston, J.G.; Morton, J.P.; et al. PGC-1a transcriptional response and mitochondrial adaptation to acute exercise is maintained in skeletal muscle of sedentary elderly males. Biogerontology 2012, 13, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.; Dawid, I. Biogenesis of Mitochondria. Dev. Biol. 1972, 518, 504–518. [Google Scholar] [CrossRef]
- Wühr, M.; Freeman, R.M.; Presler, M.; Horb, M.E.; Peshkin, L.; Gygi, S.P.; Kirschner, M.W. Deep proteomics of the xenopus laevis egg using an mRNA-derived reference database. Curr. Biol. 2014, 24, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Rohou, A.; Schep, D.G.; Bason, J.V.; Montgomery, M.G.; Walker, J.E.; Grigorieff, N. Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM. eLife 2015, 4, e10180. [Google Scholar] [CrossRef]
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe. Justus Liebigs Ann. Chem. 1928, 460, 98–122. [Google Scholar] [CrossRef]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels—Alder Reactivity. J. Am. Chem. Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef]
- Oliveira, B.L.; Guo, Z.; Bernardes, G.J.L. Inverse electron demand Diels-Alder reactions in chemical biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef]
- Burgoyne, J.R.; Oviosu, O.; Eaton, P. The PEG-switch assay: A fast semi-quantitative method to determine protein reversible cysteine oxidation. J. Pharmacol. Toxicol. Methods 2013, 68, 297–301. [Google Scholar] [CrossRef]
- Van Leeuwen, L.A.G.; Hinchy, E.C.; Murphy, M.P.; Robb, E.L.; Cochemé, H.M. Click-PEGylation—A mobility shift approach to assess the redox state of cysteines in candidate proteins. Free Radic. Biol. Med. 2017, 108, 374–382. [Google Scholar] [CrossRef]
- Van Blerkom, J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011, 11, 797–813. [Google Scholar] [CrossRef]
- Shchepinova, M.M.; Cairns, A.G.; Prime, T.A.; Logan, A.; James, A.M.; Hall, A.R.; Vidoni, S.; Arndt, S.; Caldwell, S.T.; Prag, H.A.; et al. MitoNeoD: A Mitochondria-Targeted Superoxide Probe. Cell Chem. Biol. 2017, 3, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Sies, H. The Redox Code, Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef]
- Agathocleous, M.; Love, N.K.; Randlett, O.; Harris, J.J.; Liu, J.; Murray, A.J.; Harris, W.A. Metabolic differentiation in the embryonic retina. Nat. Cell Biol. 2012, 14, 859–864. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Sakellariou, G.K.; Husi, H.; Mcdonagh, B. Proteomic strategies to unravel age-related redox signalling defects in skeletal muscle. Free Radic. Biol. Med. 2019, 132, 24–32. [Google Scholar] [CrossRef]
- Leichert, L.I.; Dick, T.P. Incidence and physiological relevance of protein thiol switches. Biol. Chem. 2015, 396, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Jedrychowski, M.P.; Schweppe, D.K.; Gray, N.S.; Gygi, S.P.; Chouchani, E.T.; Xiao, H.; Jedrychowski, M.P.; Schweppe, D.K.; Huttlin, E.L.; et al. A Quantitative Tissue-Specific Landscape of Protein Redox Regulation during Aging Article A Quantitative Tissue-Specific Landscape of Protein Redox Regulation during Aging. Cell 2020, 180, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lippe, G.; Salas, F.D.; Sorgatofj, M.C. ATP Synthase Complex from Beef Heart Mitochondria role of the thiol group. J. Biol. Chem. 1988, 263, 18627–18634. [Google Scholar] [PubMed]
- Watt, I.N.; Montgomery, M.G.; Runswick, M.J.; Leslie, A.G.W.; Walker, J.E. Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc. Natl. Acad. Sci. USA 2010, 107, 16823–16827. [Google Scholar] [CrossRef]
- Walker, J.E. Keilin Memorial Lecture Keilin Memorial Lecture The ATP synthase: The understood, the uncertain and the unknown. Biochem. Soc. Trans. 2013, 41, 1–16. [Google Scholar] [CrossRef]
- Colina-tenorio, L.; Dautant, A.; Miranda-astudillo, H.; Giraud, M.; González-halphen, D.; González-halphen, D. The Peripheral Stalk of Rotary ATPases. Front. Physiol. 2018, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Arselin, G.; Giraud, M.; Dautant, A.; Vaillier, J.; Brethes, D.; Coulary-Salin, B.; Velours, J. The GxxxG motif of the transmembrane domain of subunit e is involved in the dimerization/oligomerization of the yeast ATP synthase complex in the mitochondrial membrane. Eur. J. Biochem. 2003, 1884, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Arselin, G.; Vaillier, J.; Salin, B.; Schaeffer, J.; Giraud, M.; Dautant, A.; Brethes, D.; Velours, J. The Modulation in Subunits e and g Amounts of Yeast ATP Synthase Modifies Mitochondrial Cristae Morphology. J. Biol. Chem. 2004, 279, 40392–40399. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.M.; Strauss, M.; Daum, B.; Kief, J.H.; Osiewacz, H.D.; Rycovska, A. Macromolecular organization of ATP synthase and complex I in whole mitochondria. Proc. Natl. Acad. Sci. USA 2011, 108, 14121–14126. [Google Scholar] [CrossRef]
- Teixeira, F.K.; Sanchez, C.G.; Hurd, T.R.; Seifert, J.R.K.; Czech, B.; Preall, J.B.; Hannon, G.J.; Lehmann, R. ATP synthase promotes germ cell differentiation independent of oxidative phosphorylation. Nat. Cell Biol. 2015, 17, 689–696. [Google Scholar] [CrossRef]
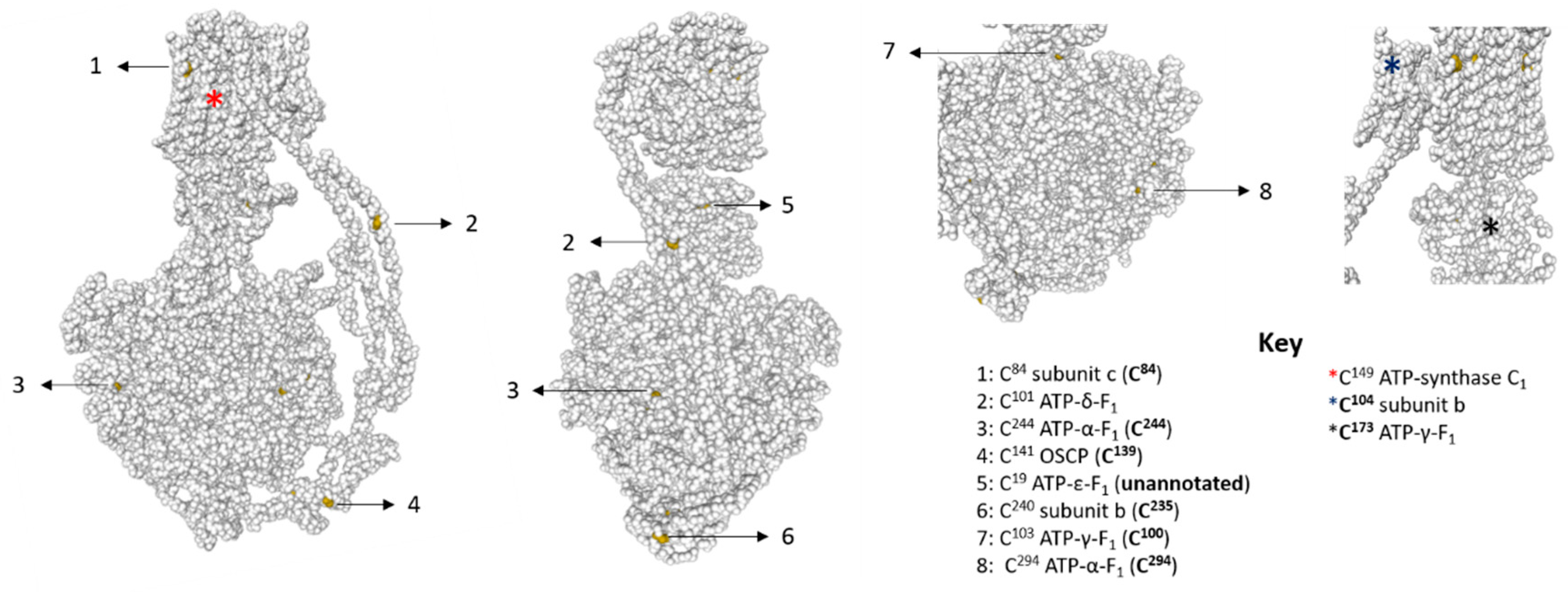

| ATP Synthase Subunit | Uniprot ID | Domain | Molecular Weight (kDa) | Cysteine Residues |
|---|---|---|---|---|
| Subunit a | P00849 | Fo | 25 | None |
| Subunit ACL | P03931 | Fo | 6.5 | None |
| C domain-containing protein (subunit c) | A0A1L8HIH0 | Fo | 16.9 | 34 *, 49 *, 84, 149 |
| Coupling factor 6 | Q6PG55 | Fo | 12.3 | None |
| Subunit C3 (subunit c3) | Q8AVE1 | Fo | 14.7 | 4 *, 131 |
| Subunit f | A0A1L8EX92 | Fo | 10.4 | None |
| Subunit g (subunit g) | Q66L24 | Fo | 11 | 96 |
| Subunit alpha (ATP-α-F1) | Q68EY5 | F1 | 60 | 244, 294 |
| Subunit beta (ATP-β-F1) | A0A1L8HHY6 | F1 | 56.4 | 9 *@, 20 *, 31 * |
| Subunit gamma (ATP-γ-F1) | Q6INB6 | F1 | 32.4 | 100, 173 # |
| Subunit delta (ATP-δ-F1) | Q66KY9 | F1 | 16.9 | None |
| Oligomycin sensitivity conferring protein (OSCP) | Q3KQC0 | F1 | 22.8 | 139 |
| Subunit b (subunit b) | Q9IAJ7 | F1 | 28.2 | 26 *, 104, 235 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobley, J.; Noble, A.; Bessell, R.; Guille, M.; Husi, H. Reversible Thiol Oxidation Inhibits the Mitochondrial ATP Synthase in Xenopus laevis Oocytes. Antioxidants 2020, 9, 215. https://doi.org/10.3390/antiox9030215
Cobley J, Noble A, Bessell R, Guille M, Husi H. Reversible Thiol Oxidation Inhibits the Mitochondrial ATP Synthase in Xenopus laevis Oocytes. Antioxidants. 2020; 9(3):215. https://doi.org/10.3390/antiox9030215
Chicago/Turabian StyleCobley, James, Anna Noble, Rachel Bessell, Matthew Guille, and Holger Husi. 2020. "Reversible Thiol Oxidation Inhibits the Mitochondrial ATP Synthase in Xenopus laevis Oocytes" Antioxidants 9, no. 3: 215. https://doi.org/10.3390/antiox9030215
APA StyleCobley, J., Noble, A., Bessell, R., Guille, M., & Husi, H. (2020). Reversible Thiol Oxidation Inhibits the Mitochondrial ATP Synthase in Xenopus laevis Oocytes. Antioxidants, 9(3), 215. https://doi.org/10.3390/antiox9030215