Effect of the Enrichment of Corn Oil With alpha- or gamma-Tocopherol on Its In Vitro Digestion Studied by 1H NMR and SPME-GC/MS; Formation of Hydroperoxy-, Hydroxy-, Keto-Dienes and Keto-E-epoxy-E-Monoenes in the More alpha-Tocopherol Enriched Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Subject of Study
2.2. Digestion Experiments
2.3. Digestate Lipid Extraction
2.4. Study by 1H NMR of Oil Samples and Lipid Extracts of Digestates
2.4.1. Operating Conditions
2.4.2. Identification of the Components
2.4.3. Quantifications Made from 1H NMR Spectral Data
- (A)
- Estimation of the Molar Percentage of the Different Kinds of Glycerides in the Digestates
- (B)
- Estimation of the Percentage of Fatty Acids Plus Acyl Groups that have Linoleic Structure in Relation to the Total of all Types of Fatty Acids and Acyl Groups in Digestates
- (C)
- Estimation of the Concentration of Specific Compounds (SC) in Oil Samples and in the Digestates
2.5. Study by SPME-GC/MS of the Headspace of Digestates and of the Mixture of the Digestive Juices Submitted to Digestion Conditions with the Corn Oil
2.6. Statistical Analysis
3. Results
3.1. Extent and Pattern of Lipolysis Produced by the In Vitro Digestion in the Several Samples
3.2. Bioaccessibility of Oil Main Components
3.3. Study of the Occurrence of Oxidation Reactions during In Vitro Digestion of Corn Oil and of Corn Oil Samples Enriched in Tocopherols
3.3.1. Information Provided by 1H NMR Spectroscopy about the Occurrence of Oxidation Reactions during In Vitro Digestion
- Evaluation of the Concentration of Acyl Groups and Fatty Acids Having Linoleic Structure in the Corn Oil, in Its Digestate and in Those of Corn Oil Samples Enriched in Tocopherols
- Study of the Formation of Oxidation Compounds during in vitro Digestion of Corn Oil and of Corn Oil Samples Enriched in Tocopherols
3.3.2. Information Provided by SPME-GC/MS about the Occurrence of Oxidation Reactions during In Vitro Digestion
3.4. Bioaccessibility of Gamma- and Alpha-Tocopherols in the Different Digestates. Influence of the Enrichment with Alpha-Tocopherol on the Bioaccessibility of Gamma–Tocopherol Naturally Present in Corn Oil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halliwell, B.; Zhao, K.; Whiteman, M. The gastrointestinal tract: A major site of antioxidant action? Free Radic Res. 2000, 33, 819–830. [Google Scholar] [CrossRef]
- Gorelik, S.; Lapidot, T.; Shaham, I.; Granit, R.; Ligumsky, M.; Kohen, R.; Kanner, J. Lipid peroxidation and coupled vitamin oxidation in simulated and human gastric fluid inhibited by dietary polyphenols: Health implications. J. Agric. Food Chem. 2005, 53, 3397–3402. [Google Scholar] [CrossRef]
- Goicoechea, E.; Brandon, E.D.; Blokland, M.H.; Guillén, M.D. Fate in digestion in vitro of several food components, including some toxic compounds coming from omega-3 and omega-6 lipids. Food Chem. Toxicol. 2011, 49, 115–124. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Guillén, M.D.; Goicoechea, E. Toxic oxygenated α,β-unsaturated aldehydes and their study in foods: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 119–136. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Domenichiello, A.F.; Yuan, Z.X.; Sapio, M.R.; Keyes, G.S.; Mishra, S.K.; Davis, J.M. A systems approach for discovering linoleic acid derivatives that potentially mediate pain and itch. Sci. Signal. 2017, 10, eaal5241. [Google Scholar] [CrossRef] [Green Version]
- Martin-Rubio, A.S.; Sopelana, P.; Ibargoitia, M.L.; Guillén, M.D. Prooxidant effect of α-tocopherol on soybean oil. Global monitoring of its oxidation process under accelerated storage conditions by 1H nuclear magnetic resonance. Food Chem. 2018, 245, 312–323. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Guillén, M.D. A thorough insight into the complex effect of gamma-tocopherol on the oxidation process of soybean oil by means of 1H Nuclear Magnetic Resonance. Comparison with alpha-tocopherol. Food Res. Int. 2018, 114, 230–239. [Google Scholar] [CrossRef]
- Martínez-Yusta, A.; Guillén, M.D. Enrichment of sunflower oil with γ-Tocopherol. Study by 1H NMR of its effect under accelerated storage conditions. Eur. J. Lipid Sci. Technol. 2019, 121, 1800457. [Google Scholar] [CrossRef] [Green Version]
- Larsson, K.; Cavonius, L.; Alminger, M.; Undeland, I. Oxidation of cod liver oil during gastrointestinal in vitro digestion. J. Agric. Food Chem. 2012, 60, 7556–7564. [Google Scholar] [CrossRef]
- Tarvainen, M.; Phuphusit, A.; Suomela, J.P.; Kuksis, A.; Kallio, H. Effects of antioxidants on rapeseed oil oxidation in an artificial digestion model analyzed by UHPLC–ESI–MS. J. Agric. Food Chem. 2012, 60, 3564–3579. [Google Scholar] [CrossRef]
- Van Hecke, T.; Wouters, A.; Rombouts, C.; Izzati, T.; Berardo, A.; Vossen, E.; Claeys, E.; Van Camp, J.; Raes, K.; Vanhaecke, L.; et al. Reducing compounds equivocally influence oxidation during digestion of a high-fat beef product, which promotes cytotoxicity in colorectal carcinoma cell lines. J. Agric. Food Chem. 2016, 64, 1600–1609. [Google Scholar] [CrossRef]
- Raes, K.; Doolaege, E.H.; Deman, S.; Vossen, E.; De Smet, S. Effect of carnosic acid, quercetin and α-tocopherol on lipid and protein oxidation in an in vitro simulated gastric digestion model. Int. J. Food Sci. Nutr. 2015, 66, 216–221. [Google Scholar] [CrossRef]
- Tirosh, O.; Shpaizer, A.; Kanner, J. Lipid peroxidation in a stomach medium is affected by dietary oils (olive/fish) and antioxidants: The Mediterranean versus western diet. J. Agric. Food Chem. 2015, 63, 7016–7023. [Google Scholar] [CrossRef]
- Tullberg, C.; Vegarud, G.; Undeland, I. Oxidation of marine oils during in vitro gastrointestinal digestion with human digestive fluids–Role of oil origin, added tocopherols and lipolytic activity. Food Chem. 2019, 270, 527–537. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Effect of adding alpha-tocopherol on the oxidation advance during in vitro gastrointestinal digestion of sunflower and flaxseed oils. Food Res. Int. 2019, 125, 108558. [Google Scholar] [CrossRef]
- Goicoechea, E.; Van Twillert, K.; Duits, M.; Brandon, E.D.; Kootstra, P.R.; Blokland, M.H.; Guillén, M.D. Use of an in vitro digestion model to study the bioaccessibility of 4-hydroxy-2-nonenal and related aldehydes present in oxidized oils rich in omega-6 acyl groups. J. Agric. Food Chem. 2008, 56, 8475–8483. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Rapid simultaneous determination by proton NMR of unsaturation and composition of acyl groups in vegetable oils. Eur. J. Lipid Sci. Technol. 2003, 105, 688–696. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Study by 1H NMR spectroscopy of the evolution of extra virgin olive oil composition submitted to frying temperature in an industrial fryer for a prolonged period of time. Food Chem. 2012, 134, 162–172. [Google Scholar] [CrossRef]
- Versantvoort, C.H.M.; Oomen, A.G.; Van de Kamp, E.; Rompelberg, C.J.; Sips, A.J. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. A study by 1H NMR on the influence of some factors affecting lipid in vitro digestion. Food Chem. 2016, 211, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. 1H NMR and SPME-GC/MS study of hydrolysis, oxidation and other reactions occurring during in vitro digestion of non-oxidized and oxidized sunflower oil. Formation of hydroxy-octadecadienoates. Food Res. Int. 2017, 91, 171–182. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Monitoring by 1H nuclear magnetic resonance of the changes in the composition of virgin linseed oil heated at frying temperature. Comp. Evol. Other Edible Oils Food Control. 2012, 28, 59–68. [Google Scholar]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. A method based on 1H NMR spectral data useful to evaluate the hydrolysis level in complex lipid mixtures. Food Res. Int. 2014, 66, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Guillén, M.D.; Cabo, N.; Ibargoitia, M.L.; Ruiz, A. Study of both sunflower oil and its headspace throughout the oxidation process. Occurrence in the headspace of toxic oxygenated aldehydes. J. Agric. Food Chem. 2005, 53, 1093–1101. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Guillén, M.D. Influence of minor components on lipid bioaccessibility and oxidation during in vitro digestion of soybean oil. J. Sci. Food Agric. 2019, 99, 4793–4800. [Google Scholar] [CrossRef]
- Tafazoli, S.; Wright, J.S.; O’Brien, P.J. Prooxidant and antioxidant activity of vitamin E analogues and troglitazone. Chem. Res. Toxicol. 2005, 18, 1567–1574. [Google Scholar] [CrossRef]
- Heaney, R.P. Factors influencing the measurement of bioavailability, taking calcium as a model. J. Nutr. 2001, 131, 1344S–1348S. [Google Scholar] [CrossRef] [Green Version]
- Guillén, M.D.; Ruiz, A. Oxidation process of oils with high content of linoleic acyl groups and formation of toxic hydroperoxy- and hydroxyalkenals. A study by 1H nuclear magnetic resonance. J. Sci. Food Agric. 2005, 85, 2413–2420. [Google Scholar] [CrossRef]
- Guillén, M.D.; Goicoechea, E. Detection of primary and secondary oxidation products by Fourier transform infrared spectroscopy (FTIR) and 1H nuclear magnetic resonance (NMR) in sunflower oil during storage. J. Agric. Food Chem. 2007, 55, 10729–10736. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Formation of hydroperoxy-and hydroxyalkenals during thermal oxidative degradation of sesame oil monitored by proton NMR. Eur. J. Lipid Sci. Technol. 2004, 106, 680–687. [Google Scholar] [CrossRef]
- Cohn, J.S. Oxidized fat in the diet, postprandial lipaemia and cardiovascular disease. Curr. Opin. Lipidol. 2002, 13, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, A.; Sawa, T.; Akaike, T.; Maeda, H. Dietary lipid peroxidation products and DNA damage in colon carcinogenesis. Eur. J. Lipid. Sci. Technol. 2002, 104, 439–447. [Google Scholar] [CrossRef]
- Kanner, J. Dietary advanced lipid oxidation end products are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Guillén, M.D. Monitoring of minor compounds in corn oil oxidation by direct immersion-solid phase microextraction-gas chromatography/mass spectrometry. New oil oxidation markers. Food Chem. 2019, 290, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Granado-Lorencio, F.; Olmedilla-Alonso, B.; Herrero-Barbudo, C.; Blanco-Navarro, I.; Pérez-Sacristán, B.; Blázquez-García, S. in vitro bioaccessibility of carotenoids and tocopherols from fruits and vegetables. Food Chem. 2007, 102, 641–648. [Google Scholar] [CrossRef]
- Kenmogne-Domguia, H.B.; Moisan, S.; Viau, M.; Genot, C.; Meynier, A. The initial characteristics of marine oil emulsions and the composition of the media inflect lipid oxidation during in vitro gastrointestinal digestion. Food Chem. 2014, 152, 146–154. [Google Scholar] [CrossRef]


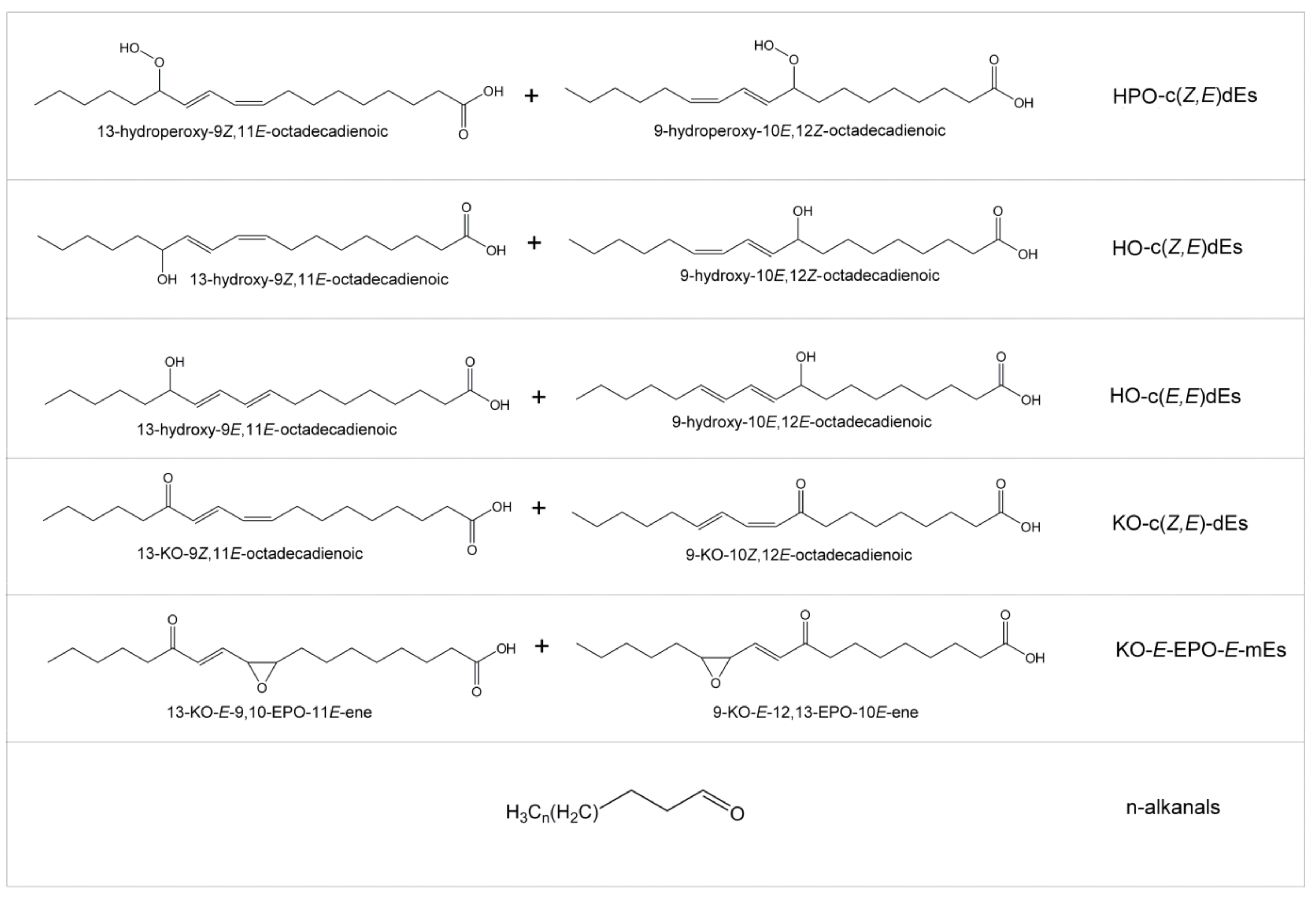
| Sample | Lipolysis Extent | ||||||
|---|---|---|---|---|---|---|---|
| TG (%) | 1,2-DG (%) | 1,3-DG (%) | 2-MG (%) | 1-MG (%) | Gol (%) | BOMC | |
| C | 99.8 ±0.2a | 1.1±0.1a | - | - | - | - | - |
| DC | 22.3±5.9b | 14.0±1.6b | 1.8±1.0a | 26.6±5.6a | 4.4±1.1a | 30.8±1.8a | 0.67±0.07a |
| DC0.2γT | 22.2±4.4b | 14.7±1.0b | 1.7±0.2a | 26.0±1.6a | 4.1±0.7a | 31.3±1.6a | 0.67±0.04a |
| DC2γT | 20.7±3.4b | 14.1±2.2b | 1.4±0.5a | 28.2±2.7a | 5.3±0.4a | 30.3±2.6a | 0.69±0.02a |
| DC5γT | 17.0±7.8b | 14.3±0.6b | 1.7±0.9a | 30.2±6.0a | 5.1±2.3a | 31.7±0.2a | 0.72±0.08a |
| DC0.2αT | 20.1±4.3b | 13.9±0.7b | 1.5±0.5a | 28.0±2.9a | 5.0±0.5a | 31.4±1.9a | 0.70±0.05a |
| DC2αT | 21.8±1.8b | 14.1±1.1b | 1.9±0.2a | 28.5±2.9a | 5.3±0.4a | 28.5±0.6a | 0.68±0.02a |
| DC5αT | 24.8±2.0b | 14.9±0.9b | 1.5±0.7a | 24.7±2.9a | 4.3±0.2a | 29.9±1.1a | 0.64±0.03a |
| Samples | Linoleic FA+AG (%) | Concentration of Some Oxidation Compounds (mmol/mol(AG + FA)) | |||||
|---|---|---|---|---|---|---|---|
| HPO-c(Z,E)dEs | HOc(Z,E) dEs | HO-c(E,E) dEs | KO-c(Z,E) dEs | KO-E-EPO-E-mEs | n- Alkanals | ||
| C | 49.2±0.5a | - | - | - | - | - | - |
| DC | 41.3±0.0b | 1.8 ±0.31a | - | - | - | - | - |
| DC0.2γT | 43.3± 0.0c | 1.54±0.27a | - | - | - | - | - |
| DC2γT | 43.1±0.2c | - | - | - | - | - | - |
| DC5γT | 44.4± 0.3c | - | - | - | - | - | - |
| DC0.2αT | 40.5±0.6b | 2.77±0.25b | - | - | - | - | - |
| DC2αT | 40.8± 0.0b | 2.21±0.31a,b | 1.22±0.07a | 2.27±0.00a | 0.53±0.03a | 0.71±0.00a | - |
| DC5αT | 40.6±1.2b | 2.06±0.38a,b | 2.78±0.19b | 2.43±0.71a | 1.09±0.14b | 1.12±0.16b | 0.27±0.09 |
| Compound (Molecular Weight) | Bp | CDJ | DC | DC0.2γT | DC2γT | DC5γT | DC0.2αT | DC2αT | DC5αT |
|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | |||||||||
| Alkanals | |||||||||
| Pentanal (86)* | 44 | 17.8 ± 0.9 | 41.4 ± 1.8 | 36.0 ± 0.5 | 48.4 ± 2.9 | 22.3 ± 1.8 | 41.4 ± 5.4 | 52.4 ± 7.3 | 83.9 ± 6.4 |
| Hexanal (100)* | 44 | 12.9 ± 3.2 | 76.0 ± 3.1 | 79.1 ± 11.8 | 72.4 ± 4.9 | 45.6 ± 4.9 | 184.4 ± 12.3 | 480.6 ± 75.3 | 604.0 ± 56.0 |
| Heptanal (114)* | 70 | 0.7 ± 0.0 | 3.0 ± 1.2 | 4.4 ± 0.7 | 3.5 ± 0.5 | 2.4 ± 0.4 | 6.8 ± 0.1 | 51.2 ± 11.7 | 164.3 ± 2.1 |
| Octanal (128)* | 41 | - | - | - | - | - | - | 34.0 ± 6.4 | 46.4 ± 5.1 |
| Nonanal (142)* | 57 | 3.3 ± 0.2 | 11.4 ± 0.3 | 11.3 ± 0.5 | 17.0 ± 1.7 | 17.1 ± 1.1 | 12.4 ± 1.2 | 42.6 ± 2.4 | 69.7 ± 11.4 |
| Decanal (156)* | 41 | - | - | - | - | - | - | - | 4.6 ± 0.5 |
| Total | 34.7 ± 2.1 | 131.8 ± 0.1 | 130.8 ± 12.47 | 141.3 ± 10.1 | 87.5 ± 8.3 | 245.1 ± 5.9 | 660.8 ± 103.1 | 972.9 ± 64.4 | |
| (E)-2-alkenals | |||||||||
| (E)-2-Butenal (70)* | 70 | 19.5 ± 2.8 | 20.6 ± 3.4 | 20.8 ± 5.8 | 16.1 ± 2.3 | 15.2 ± 2.6 | 18.2 ± 3.3 | 20.7 ± 2.3 | 23.0± 7.8 |
| (E)-2-Pentenal (84) | 55 | - | - | - | - | - | - | 1.3 ± 0.0 | 5.1 ± 1.1 |
| (Z)-4-Heptenal (112) | 41 | - | - | - | - | - | - | 3.9 ± 0.7 | 5.4 ± 0.5 |
| (E)-2-Heptenal (112)* | 41 | 2.3 ± 0.3 | 47.4 ± 8.1 | 40.2 ± 6.4 | 16.5 ± 8.9 | 8.8 ± 3.0 | 64.5 ± 8.7 | 186.3 ± 28.3 | 275.0 ± 47.7 |
| (E)-2-Octenal (126)* | 70 | - | - | - | - | - | 42.3 ± 2.2 | 186.3 ± 26.2 | 346.3 ± 58.9 |
| (E)-2-Nonenal (140)* | 55 | - | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.4 ± 0.1 | 1.8 ± 0.1 | 1.3 ± 0.1 | 2.8 ± 0.8 | 4.7 ± 0.4 |
| (E)-2-Decenal (154)* | 70 | - | - | - | - | - | 4.1 ± 0.6 | 6.7 ± 1.0 | 10.8 ± 1.8 |
| (E)-2-Undecenal (168)* | 70 | - | - | - | - | - | 0.2 ± 0.0 | 1.4 ± 0.3 | 3.0 ± 0.4 |
| Total | 21.8 ± 3.1 | 69.2 ± 4.9 | 62.08 ± 12.28 | 38.9 ± 4.4 | 25.8 ± 5.4 | 130.6 ± 8.3 | 409.3 ± 58.1 | 673.3 ± 102.0 | |
| 2,4-Alkadienals | |||||||||
| (Z,E)-2,4-Heptadienal (110) | 81 | - | - | - | - | - | 3.8 ± 0.1 | 24.0 ± 4.9 | 38.6 ± 1.6 |
| (E,E)-2,4-Heptadienal (110)* | 81 | - | - | - | - | - | 3.5 ± 0.5 | 24.4 ± 3.0 | 36.4 ± 10.5 |
| (Z,E)-2,4-Octadienal (124) | 81 | - | - | - | - | - | - | - | 1.9 ± 1.0 |
| (E,E)-2,4-Octadienal (124) | 81 | - | - | - | - | - | 0.8 ± 0.0 | 3.4 ± 0.1 | 6.1 ± 0.8 |
| (E,E)-2,4-Nonadienal (138) | 81 | - | 1.0 ± 0.1 | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.4 ± 0.1 | 1.2 ± 0.2 | 1.9 ± 0.2 | 2.4 ± 0.5 |
| (Z,E)-2,4-Decadienal (152) | 81 | - | 0.6 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.3 ± 0.0 | 2.6 ± 0.8 | 19.3 ± 3.1 | 38.4 ± 10.1 |
| (E,E)-2,4-Decadienal (152)* | 81 | - | 0.8 ± 0.1 | 0.5 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.1 | 3.3 ± 0.3 | 15.8 ± 0.5 | 29.0 ± 13.2 |
| Total | - | 2.4 ± 0.2 | 1.6 ± 0.3 | 1.3 ± 0.1 | 1.0 ± 0.2 | 15.3 ± 1.6 | 88.7 ± 11.7 | 152.8 ± 37.6 | |
| Oxigenated α,β-unsaturated | |||||||||
| 4,5-epoxy-(E)-2-heptenal (126) | 68 | - | - | - | - | - | - | 0.9 ± 0.2 | 3.1 ± 1.0 |
| 4,5-epoxy-2-decenal (isomer) (168) | 68 | - | - | - | - | - | 0.4 ± 0.1 | 2.7 ± 0.2 | 4.3 ± 2.2 |
| 4,5-epoxy-(E)-2-decenal (168)* | 68 | - | - | - | - | - | 1.1 ± 0.2 | 10.9 ± 0.4 | 31.8 ± 0.0 |
| Total | - | - | - | - | - | 1.5 ± 0.3 | 15.0 ± 0.1 | 41.1 ± 3.2 | |
| Furanones | |||||||||
| 5-butyl-5H-furan-2-one (140) | 84 | - | - | - | - | - | - | 0.9 ± 0.0 | 1.6 ± 0.1 |
| 5-pentyl-2(3H)-furanone (154) (or isomer) | 98 | - | - | - | - | - | - | 0.5± 0.0 | 0.9± 0.2 |
| 5-pentyl-2(5H)-furanone (154) | 84 | - | - | - | - | - | - | 2.7 ± 0.3 | 4.4 ± 0.9 |
| Total | - | - | - | - | - | - | 4.1 ± 0.4 | 6.9± 1.2 | |
| Furan derivatives | |||||||||
| Furan, 2-ethyl (96) | 81 | - | - | - | - | - | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.6 ± 0.0 |
| Furan, 2-propyl (110) | 81 | - | - | - | - | - | 0.2 ± 0.0 | 0.6 ± 0.0 | 0.9 ± 0.1 |
| Furan, 2-butyl (124) | 81 | - | 0.5 ± 0.0 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.0 | 2.5 ± 0.5 | 5.4 ± 0.1 |
| Furan, 2-pentyl (138)* | 81 | 4.7 ± 1.4 | 21.5 ± 1.5 | 19.8 ± 0.8 | 19.4 ± 0.5 | 17.6 ± 1.1 | 43.5 ± 2.1 | 186.8 ± 42.8 | 308.3 ± 21.4 |
| Furan, 2-heptyl (166) | 81 | - | - | - | - | - | - | 0.7 ± 0.2 | 8.3 ± 1.7 |
| Total | 4.7 ± 1.4 | 22.4 ± 1.5 | 20.4 ± 0.6 | 20.1 ± 0.4 | 18.1 ± 1.0 | 44.5 ± 2.2 | 190.8 ± 43.5 | 316.0 ± 21.5 |
| Samples | BγT | B´γT | BαT | B´αT |
|---|---|---|---|---|
| DC | 0.33 ± 0.00 | 0.67 ± 0.00 | ||
| DC0.2γT | 0.89 ± 0.18 | 0.65 ± 0.14 | ||
| DC2γT | 8.55 ± 0.00 | 0.70 ± 0.01 | ||
| DC5γT | 35.65 ± 1.12 | 0.82 ± 0.03 | ||
| DC0.2αT | 0.33 ± 0.03 | 0.67 ± 0.01 | - | - |
| DC2αT | 0.46 ± 0.02 | 0.94 ± 0.00 | 6.36 ± 0.00 | 0.58 ± 0.00 |
| DC5αT | 0.47 ± 0.04 | 0.96 ± 0.01 | 12.91 ± 0.15 | 0.32 ± 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberdi-Cedeño, J.; Ibargoitia, M.L.; Guillén, M.D. Effect of the Enrichment of Corn Oil With alpha- or gamma-Tocopherol on Its In Vitro Digestion Studied by 1H NMR and SPME-GC/MS; Formation of Hydroperoxy-, Hydroxy-, Keto-Dienes and Keto-E-epoxy-E-Monoenes in the More alpha-Tocopherol Enriched Samples. Antioxidants 2020, 9, 246. https://doi.org/10.3390/antiox9030246
Alberdi-Cedeño J, Ibargoitia ML, Guillén MD. Effect of the Enrichment of Corn Oil With alpha- or gamma-Tocopherol on Its In Vitro Digestion Studied by 1H NMR and SPME-GC/MS; Formation of Hydroperoxy-, Hydroxy-, Keto-Dienes and Keto-E-epoxy-E-Monoenes in the More alpha-Tocopherol Enriched Samples. Antioxidants. 2020; 9(3):246. https://doi.org/10.3390/antiox9030246
Chicago/Turabian StyleAlberdi-Cedeño, Jon, María L. Ibargoitia, and María D. Guillén. 2020. "Effect of the Enrichment of Corn Oil With alpha- or gamma-Tocopherol on Its In Vitro Digestion Studied by 1H NMR and SPME-GC/MS; Formation of Hydroperoxy-, Hydroxy-, Keto-Dienes and Keto-E-epoxy-E-Monoenes in the More alpha-Tocopherol Enriched Samples" Antioxidants 9, no. 3: 246. https://doi.org/10.3390/antiox9030246
APA StyleAlberdi-Cedeño, J., Ibargoitia, M. L., & Guillén, M. D. (2020). Effect of the Enrichment of Corn Oil With alpha- or gamma-Tocopherol on Its In Vitro Digestion Studied by 1H NMR and SPME-GC/MS; Formation of Hydroperoxy-, Hydroxy-, Keto-Dienes and Keto-E-epoxy-E-Monoenes in the More alpha-Tocopherol Enriched Samples. Antioxidants, 9(3), 246. https://doi.org/10.3390/antiox9030246




