In Vitro and In Vivo Nutraceutical Characterization of Two Chickpea Accessions: Differential Effects on Hepatic Lipid Over-Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Chickpea Selection and Preparation of Extracts
2.3. Cell Culture, Treatments, Crystal Violet Assay, and ROS Detection
2.4. Lipid Inclusions Staining
2.5. Cell and Liver Lysates
2.6. Gel Electrophoresis and Western Blotting
2.7. Animals and Experimental Protocol
2.8. Serum Insulin Measurement
2.9. Statistical Analysis
3. Results
3.1. Characterization of the Chickpeas Extracts in FaO Cells
3.2. Effects of Chickpea Extracts on Lipid Accumulation in FaO Cells
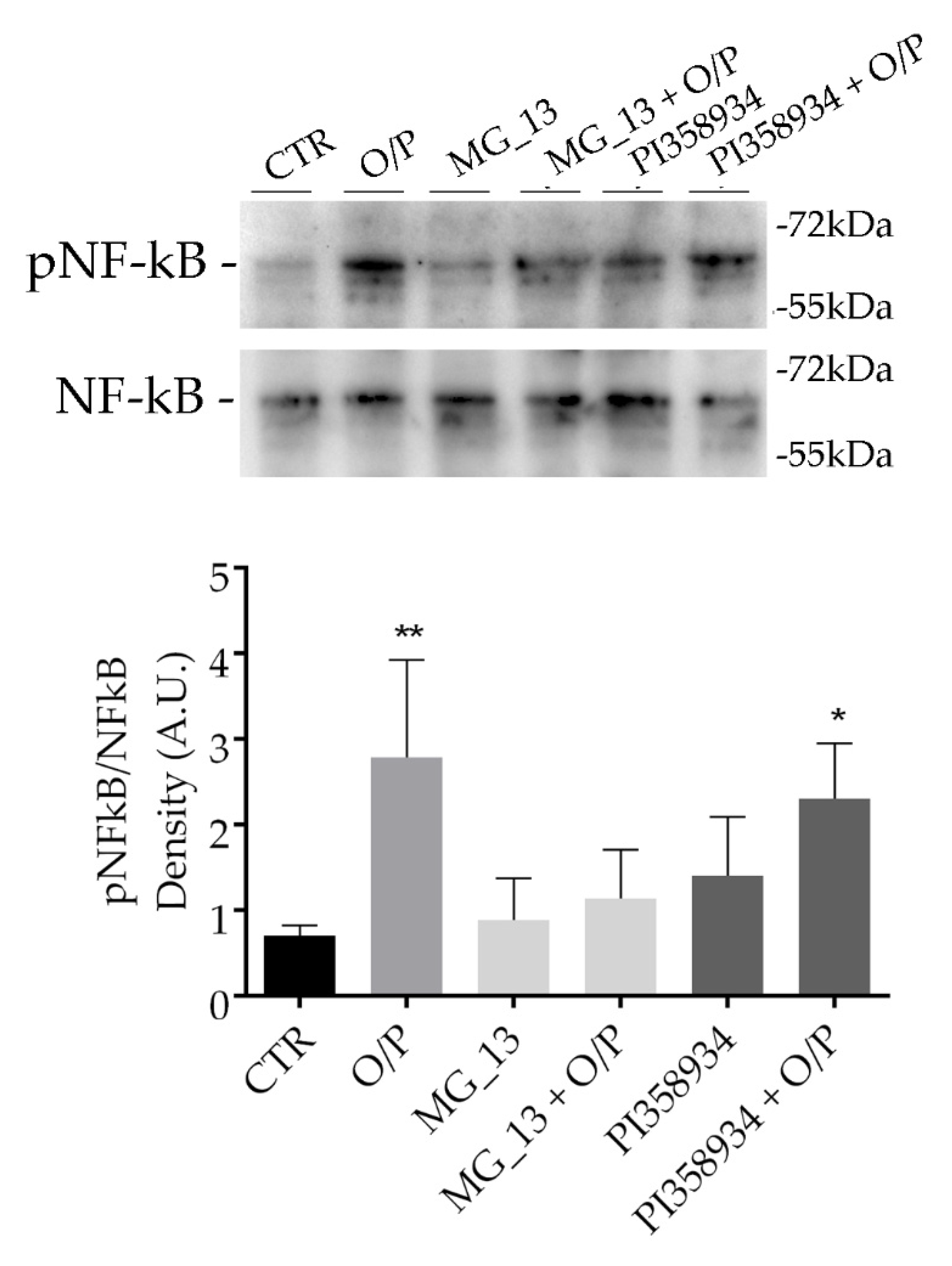
3.3. Effects of Chickpea Extracts on NF-kB Phosphorylation in FaO Cells
3.4. Animal Studies: Body Weight, Food Intake, Food Efficiency Ratio
3.5. Serum Parameters and Liver Function
3.6. Effects of Chickpea Diets on Lipid Accumulation and NF-kB Phosphorylation in Liver
3.7. Effects of Chickpea Diets on ROS Production and Proliferating Cell Nuclear Antigen (PCNA) Expression in Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pejcic, T.; Tosti, T.; Dzamic, Z.; Gasic, U.; Vuksanovic, A.; Dolicanin, Z.; Tesic, Z. The Polyphenols as Potential Agents in Prevention and Therapy of Prostate Diseases. Molecules 2019, 24, 3982. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.; Dumitrascu, D.L. Cardiovascular Risk in Fatty Liver Disease: The Liver-Heart Axis-Literature Review. Front. Med. 2019, 6, 202. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Calamita, G. Phytocompounds modulating Aquaporins: Clinical benefits are anticipated. Food Chem. 2019, 274, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Summo, C.; De Angelis, D.; Ricciardi, L.; Caponio, F.; Lotti, C.; Pavan, S.; Pasqualone, A. Data on the chemical composition, bioactive compounds, fatty acid composition, physico-chemical and functional properties of a global chickpea collection. Data Brief 2019, 27, 104612. [Google Scholar] [CrossRef] [PubMed]
- Rachwa-Rosiak, D.; Nebesny, E.; Budryn, G. Chickpeas-composition, nutritional value, health benefits, application to bread and snacks: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Yuzbashian, E.; Asghari, G.; Sarverzadeh, S.; Azizi, F. Dietary fibre intake in relation to the risk of incident chronic kidney disease. Br. J. Nutr. 2018, 119, 479–485. [Google Scholar] [CrossRef]
- Shinohara, S.; Gu, Y.; Yang, Y.; Furuta, Y.; Tanaka, M.; Yue, X.; Wang, W.; Kitano, M.; Kimura, H. Ethanol extracts of chickpeas alter the total lipid content and expression levels of genes related to fatty acid metabolism in mouse 3T3-L1 adipocytes. Int. J. Mol. Med. 2016, 38, 574–584. [Google Scholar] [CrossRef]
- Valentín-Gamazo, I.G. Chickpea flour ingredient slow glycemic response to pasta in healthy volunteers. Food Chem. 2003, 81, 5. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, L.; Gu, Y.; Zhang, Y.; Tang, J.; Li, F.; Shang, W.; Jiang, B.; Yue, X.; Chen, M. Dietary chickpeas reverse visceral adiposity, dyslipidaemia and insulin resistance in rats induced by a chronic high-fat diet. Br. J. Nutr. 2007, 98, 720–726. [Google Scholar] [CrossRef]
- Matsuzawa-Nagata, N.; Takamura, T.; Ando, H.; Nakamura, S.; Kurita, S.; Misu, H.; Ota, T.; Yokoyama, M.; Honda, M.; Miyamoto, K.; et al. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metab. Clin. Exp. 2008, 57, 1071–1077. [Google Scholar] [CrossRef]
- Charradi, K.; Elkahoui, S.; Limam, F.; Aouani, E. High-fat diet induced an oxidative stress in white adipose tissue and disturbed plasma transition metals in rat: Prevention by grape seed and skin extract. J. Physiol. Sci. Jps 2013, 63, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Lenardo, M.J. The nuclear signaling of NF-kappaB: Current knowledge, new insights, and future future perspectives. Cell Res. 2010, 20, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Buss, H.; Dorrie, A.; Schmitz, M.L.; Hoffmann, E.; Resch, K.; Kracht, M. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-{kappa}B at serine 536 is mediated by multiple protein kinases including I{kappa}B kinase (IKK)-{alpha}, IKK{beta}, IKK{epsilon}, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J. Biol. Chem. 2004, 279, 55633–55643. [Google Scholar] [CrossRef]
- Buss, H.; Handschick, K.; Jurrmann, N.; Pekkonen, P.; Beuerlein, K.; Muller, H.; Wait, R.; Saklatvala, J.; Ojala, P.M.; Schmitz, M.L.; et al. Cyclin-dependent kinase 6 phosphorylates NF-kappaB P65 at serine 536 and contributes to the regulation of inflammatory gene expression. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Zhu, Q.C.; Gao, R.Y.; Wu, W.; Guo, B.M.; Peng, J.Y.; Qin, H.L. Effect of a high-fat diet in development of colonic adenoma in an animal model. World J. Gastroenterol. 2014, 20, 8119–8129. [Google Scholar] [CrossRef]
- Da Costa, R.M.; Neves, K.B.; Mestriner, F.L.; Louzada-Junior, P.; Bruder-Nascimento, T.; Tostes, R.C. TNF-alpha induces vascular insulin resistance via positive modulation of PTEN and decreased Akt/eNOS/NO signaling in high fat diet-fed mice. Cardiovasc. Diabetol. 2016, 15, 119. [Google Scholar] [CrossRef]
- Nestel, P.; Cehun, M.; Chronopoulos, A. Effects of long-term consumption and single meals of chickpeas on plasma glucose, insulin, and triacylglycerol concentrations. Am. J. Clin. Nutr. 2004, 79, 390–395. [Google Scholar] [CrossRef]
- Zulet, M.A.; Macarulla, M.T.; Portillo, M.P.; Noel-Suberville, C.; Higueret, P.; Martinez, J.A. Lipid and glucose utilization in hypercholesterolemic rats fed a diet containing heated chickpea (Cicer aretinum L.): A potential functional food. Int. J. Vitam. Nutr. Res. Int. Z. Fur Vitam.—Und Ernahr. J. Int. De Vitaminol. Et De Nutr. 1999, 69, 403–411. [Google Scholar] [CrossRef]
- De Giovanni, C.; Pavan, S.; Taranto, F.; Di Rienzo, V.; Miazzi, M.M.; Marcotrigiano, A.R.; Mangini, G.; Montemurro, C.; Ricciardi, L.; Lotti, C. Genetic variation of a global germplasm collection of chickpea (Cicer arietinum L.) including Italian accessions at risk of genetic erosion. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2017, 23, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.; Lotti, C.; Marcotrigiano, A.R.; Mazzeo, R.; Bardaro, N.; Bracuto, V.; Ricciardi, F.; Taranto, F.; D’Agostino, N.; Schiavulli, A.; et al. A Distinct Genetic Cluster in Cultivated Chickpea as Revealed by Genome-wide Marker Discovery and Genotyping. Plant Genome 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Summo, C.; De Angelis, D.; Ricciardi, L.; Caponio, F.; Lotti, C.; Pavan, S.; Pasqualone, A. Nutritional, physico-chemical and functional characterization of a global chickpea collection. J. Food Compos. Anal. 2019, 84. [Google Scholar] [CrossRef]
- Baldini, F.; Portincasa, P.; Grasselli, E.; Damonte, G.; Salis, A.; Bonomo, M.; Florio, M.; Serale, N.; Voci, A.; Gena, P.; et al. Aquaporin-9 is involved in the lipid-lowering activity of the nutraceutical silybin on hepatocytes through modulation of autophagy and lipid droplets composition. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158586. [Google Scholar] [CrossRef]
- Grasselli, E.; Cortese, K.; Voci, A.; Vergani, L.; Fabbri, R.; Barmo, C.; Gallo, G.; Canesi, L. Direct effects of Bisphenol A on lipid homeostasis in rat hepatoma cells. Chemosphere 2013, 91, 1123–1129. [Google Scholar] [CrossRef]
- Ranieri, M.; Di Mise, A.; Difonzo, G.; Centrone, M.; Venneri, M.; Pellegrino, T.; Russo, A.; Mastrodonato, M.; Caponio, F.; Valenti, G.; et al. Green olive leaf extract (OLE) provides cytoprotection in renal cells exposed to low doses of cadmium. PLoS ONE 2019, 14, e0214159. [Google Scholar] [CrossRef]
- Tamma, G.; Ranieri, M.; Di Mise, A.; Centrone, M.; Svelto, M.; Valenti, G. Glutathionylation of the aquaporin-2 water channel: A novel post-translational modification modulated by the oxidative stress. J. Biol. Chem. 2014, 289, 27807–27813. [Google Scholar] [CrossRef]
- Liquori, G.E.; Calamita, G.; Cascella, D.; Mastrodonato, M.; Portincasa, P.; Ferri, D. An innovative methodology for the automated morphometric and quantitative estimation of liver steatosis. Histol. Histopathol. 2009, 24, 49–60. [Google Scholar] [CrossRef]
- Ranieri, M.; Tamma, G.; Di Mise, A.; Russo, A.; Centrone, M.; Svelto, M.; Calamita, G.; Valenti, G. Negative feedback from CaSR signaling to aquaporin-2 sensitizes vasopressin to extracellular Ca2. J. Cell Sci. 2015, 128, 2350–2360. [Google Scholar] [CrossRef]
- Vecchione, G.; Grasselli, E.; Voci, A.; Baldini, F.; Grattagliano, I.; Wang, D.Q.; Portincasa, P.; Vergani, L. Silybin counteracts lipid excess and oxidative stress in cultured steatotic hepatic cells. World J. Gastroenterol. 2016, 22, 6016–6026. [Google Scholar] [CrossRef]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. Obesity Management Task Force of the European Association for the Study of, O. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Maric, C.; Hall, J.E. Obesity, metabolic syndrome and diabetic nephropathy. Contrib. Nephrol. 2011, 170, 2835. [Google Scholar] [CrossRef]
- Oosterveer, M.H.; van Dijk, T.H.; Tietge, U.J.; Boer, T.; Havinga, R.; Stellaard, F.; Groen, A.K.; Kuipers, F.; Reijngoud, D.J. High fat feeding induces hepatic fatty acid elongation in mice. PLoS ONE 2009, 4, e6066. [Google Scholar] [CrossRef]
- Vanni, E.; Bugianesi, E.; Kotronen, A.; De Minicis, S.; Yki-Jarvinen, H.; Svegliati-Baroni, G. From the metabolic syndrome to NAFLD or vice versa? Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2010, 42, 320330. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, K.T.; Enos, R.T.; Bader, J.E.; Sougiannis, A.T.; Carson, M.S.; Chatzistamou, I.; Carson, J.A.; Nagarkatti, P.S.; Nagarkatti, M.; Murphy, E.A. Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J. Hepatol. 2019, 11, 619637. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.; Vasconcelos, M.; Gil, A.M.; Pinto, E. Benefits of pulse consumption on metabolism and health: A systematic review of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 1–12. [Google Scholar] [CrossRef]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef]
- Fascella, G.; D’Angiolillo, F.; Mammano, M.M.; Amenta, M.; Romeo, F.V.; Rapisarda, P.; Ballistreri, G. Bioactive compounds and antioxidant activity of four rose hip species from spontaneous Sicilian flora. Food Chem. 2019, 289, 56–64. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Werneburg, N.W.; Canbay, A.; Guicciardi, M.E.; Bronk, S.F.; Rydzewski, R.; Burgart, L.J.; Gores, G.J. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology 2004, 40, 185–194. [Google Scholar] [CrossRef]
- Li, Z.; Berk, M.; McIntyre, T.M.; Gores, G.J.; Feldstein, A.E. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology 2008, 47, 1495–1503. [Google Scholar] [CrossRef]
- Carlsen, H.; Haugen, F.; Zadelaar, S.; Kleemann, R.; Kooistra, T.; Drevon, C.A.; Blomhoff, R. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr. 2009, 4, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Grimble, R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 3), S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K.L. Too much linoleic acid promotes inflammation-doesn’t it? Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]







| Parameters | CTR (12) | HFD (12) | HFD + MG_13 (12) | HFD + PI358934 (9) |
|---|---|---|---|---|
| Initial body weight (g) | 22.78 ± 1.30 | 23.63 ± 1.16 | 22.68 ± 1.02 | 24.19 ± 0.69 * |
| Final body weight (g) | 31.04 ± 1.04 #### | 40.1 ± 3.00 **** | 43.69 ± 6.08 **** | 49.91 ± 4.04 **** #### |
| Weight gain (g/d) | 0.069 ± 0.009 #### | 0.1384 ± 0.019 **** | 0.176 ± 0.036 **** ## | 0.216 ± 0.024 **** #### |
| Food intake (g/d) | 3.176 ± 0.286 | 3.036 ± 0.115 | 3.787 ± 0.698 **** #### | 2.911 ± 0.226 |
| FER (%) | 2.185 ± 0.365 #### | 4.557 ± 0.667 **** | 4.661 ± 1.293 **** | 7.423 ± 1.025 **** #### |
| Parameters | CTR (12) | HFD (12) | HFD + MG_13 (12) | HFD + PI358934 (9) |
|---|---|---|---|---|
| Glycemia (mg/dL) | 87.42 ± 21.46 #### | 214.6 ± 32.84 **** | 184.6 ± 12.89 **** # | 209.4 ± 41.92 **** |
| Insulin (μlU/mL) | 15.75 ± 4.55 | 26.51 ± 8.82 | 23.68 ± 13.41 | 16.05 ± 2.32 |
| Total cholesterol (mg/dL) | 63.00 ± 27.40 | 107.0 ± 9.64 | 186.3 ± 58.07 ** | 221.0 ± 27.87 ** # |
| Triglycerides (mg/dL) | 76.00 ± 39.40 | 128.0 ± 22.72 | 165.3 ± 11.37 * | 181.7 ± 56.36 * |
| Alkaline phosphatases (U/I) | 66.00 ± 10.54 | 65.33 ± 28.31 | 48.00 ± 15.13 | 64.00 ± 7.00 |
| AST (U/I) | 279.0 ± 130.9 # | 582.7 ± 130.6 * | 486.3 ± 89.39 | 703.7 ± 49.92 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Centrone, M.; Gena, P.; Ranieri, M.; Di Mise, A.; D’Agostino, M.; Mastrodonato, M.; Venneri, M.; De Angelis, D.; Pavan, S.; Pasqualone, A.; et al. In Vitro and In Vivo Nutraceutical Characterization of Two Chickpea Accessions: Differential Effects on Hepatic Lipid Over-Accumulation. Antioxidants 2020, 9, 268. https://doi.org/10.3390/antiox9030268
Centrone M, Gena P, Ranieri M, Di Mise A, D’Agostino M, Mastrodonato M, Venneri M, De Angelis D, Pavan S, Pasqualone A, et al. In Vitro and In Vivo Nutraceutical Characterization of Two Chickpea Accessions: Differential Effects on Hepatic Lipid Over-Accumulation. Antioxidants. 2020; 9(3):268. https://doi.org/10.3390/antiox9030268
Chicago/Turabian StyleCentrone, Mariangela, Patrizia Gena, Marianna Ranieri, Annarita Di Mise, Mariagrazia D’Agostino, Maria Mastrodonato, Maria Venneri, Davide De Angelis, Stefano Pavan, Antonella Pasqualone, and et al. 2020. "In Vitro and In Vivo Nutraceutical Characterization of Two Chickpea Accessions: Differential Effects on Hepatic Lipid Over-Accumulation" Antioxidants 9, no. 3: 268. https://doi.org/10.3390/antiox9030268
APA StyleCentrone, M., Gena, P., Ranieri, M., Di Mise, A., D’Agostino, M., Mastrodonato, M., Venneri, M., De Angelis, D., Pavan, S., Pasqualone, A., Summo, C., Fanelli, V., Valenti, G., Calamita, G., & Tamma, G. (2020). In Vitro and In Vivo Nutraceutical Characterization of Two Chickpea Accessions: Differential Effects on Hepatic Lipid Over-Accumulation. Antioxidants, 9(3), 268. https://doi.org/10.3390/antiox9030268














