AKR1B1-Induced Epithelial–Mesenchymal Transition Mediated by RAGE-Oxidative Stress in Diabetic Cataract Lens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Reagents
2.3. Immunohistochemical Procedures
2.4. Immunofluorescence Staining
2.5. Statistical Analyses
3. Results
3.1. Demographic Characteristics
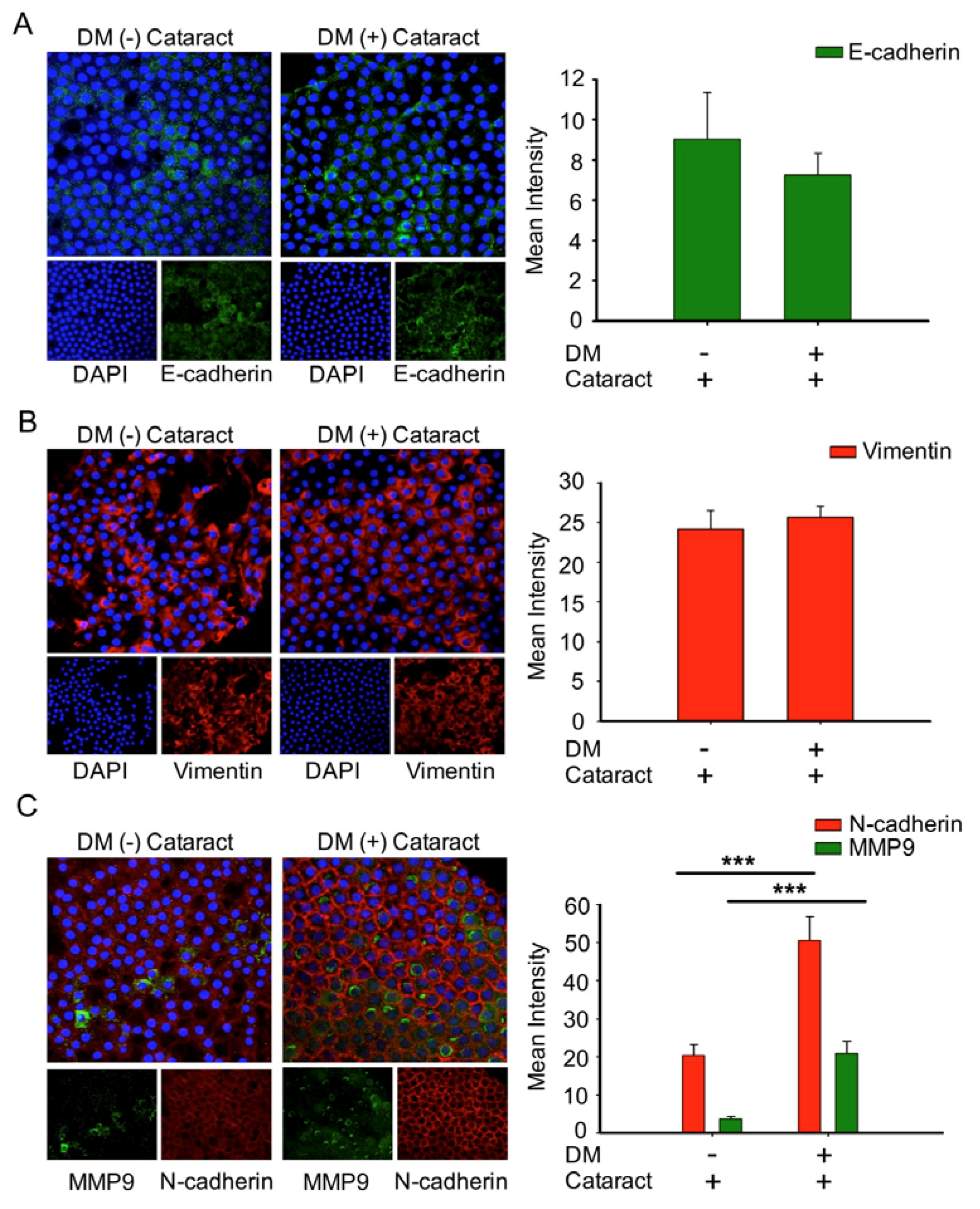
3.2. N-Cadherin May Contribute to the Formation of Diabetic Cataracts
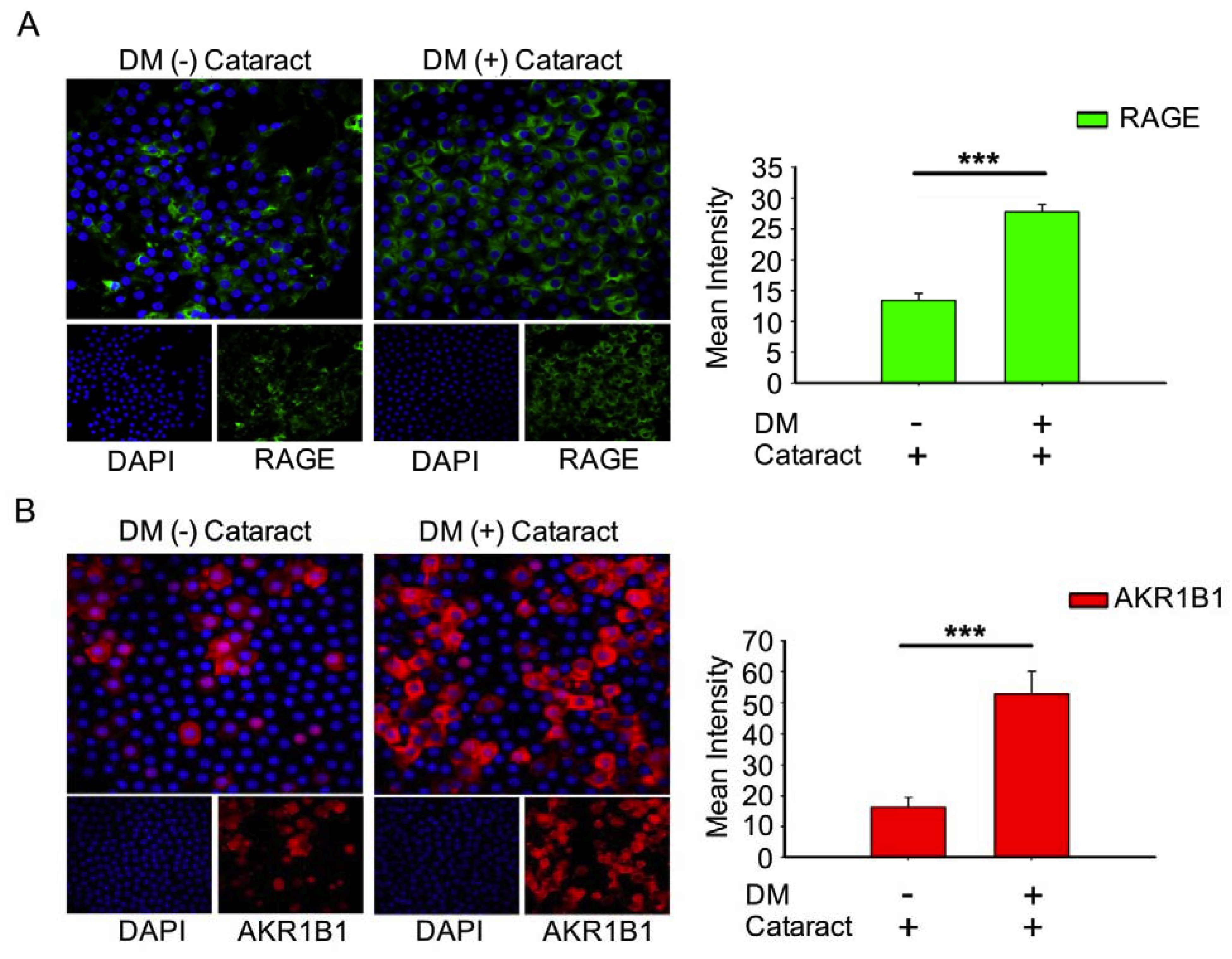
3.3. Activated AKR1B1, enhancedRAGE Production Were Involved in DM (+) Cataract Pathogenesis of LECs
3.4. Inhibition of AMP-Activated Protein Kinase (AMPK) Increased ROS Production and Acetylation of SOD2 in the LECs of DM (+) Patients
3.5. EMT Was Associated with Cataracts, the Mesenchymal Cell Marker N-Cadherin, and MMP9 in DM (+) Cataract Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boyle, J.P.; Thompson, T.J.; Gregg, E.W.; Barker, L.E.; Williamson, D.F. Projection of the year 2050 burden of diabetes in the us adult population: Dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul. Health Metr. 2010, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Pollreisz, A.; Schmidt-Erfurth, U. Diabetic cataract-pathogenesis, epidemiology and treatment. J. Ophthalmol. 2010, 2010, 608751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albulescu, R.; Zolog, I. Specific aspects in diabetic cataract. Oftalmologia 2011, 55, 47–52. [Google Scholar] [PubMed]
- Wernecke, L.; Keckeis, S.; Reichhart, N.; Strauss, O.; Salchow, D.J. Epithelial-mesenchymal transdifferentiation in pediatric lens epithelial cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5785–5794. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, C.T.; Nagaraj, R.H. Age-rage interaction in the tgfbeta2-mediated epithelial to mesenchymal transition of human lens epithelial cells. Glycoconj. J. 2016, 33, 631–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guglielmotto, M.; Aragno, M.; Tamagno, E.; Vercellinatto, I.; Visentin, S.; Medana, C.; Catalano, M.G.; Smith, M.A.; Perry, G.; Danni, O.; et al. Ages/rage complex upregulates bace1 via nf-kappab pathway activation. Neurobiol. Aging 2012, 33, 196.e13–196.e27. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Adeli, K. Dietary fructose and the metabolic syndrome. Curr. Opin. Gastroenterol. 2008, 24, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, E.M.; Marklund, S.L.; Behndig, A. Enhanced diabetes-induced cataract in copper-zinc superoxide dismutase-null mice. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2913–2918. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Y.; Wu, T.T.; Ho, C.Y.; Yeh, T.C.; Sun, G.C.; Kung, Y.H.; Wong, T.Y.; Tseng, C.J.; Cheng, P.W. Dapagliflozin prevents nox- and sglt2-dependent oxidative stress in lens cells exposed to fructose-induced diabetes mellitus. Int. J. Mol. Sci. 2019, 20, 4357. [Google Scholar] [CrossRef] [Green Version]
- Kandarakis, S.A.; Piperi, C.; Topouzis, F.; Papavassiliou, A.G. Emerging role of advanced glycation-end products (ages) in the pathobiology of eye diseases. Prog. Retin. Eye Res. 2014, 42, 85–102. [Google Scholar] [CrossRef]
- Sadi, G.; Eryilmaz, N.; Tutuncuoglu, E.; Cingir, S.; Guray, T. Changes in expression profiles of antioxidant enzymes in diabetic rat kidneys. Diabetes Metab. Res. Rev. 2012, 28, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.; Shieh, B.; Chang, K.C.; Pal, A.; Lenhart, P.; Ammar, D.; Ruzycki, P.; Palla, S.; Reddy, G.B.; Petrash, J.M. Aldose reductase expression as a risk factor for cataract. Chem. Biol. Interact. 2015, 234, 247–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zablocki, G.J.; Ruzycki, P.A.; Overturf, M.A.; Palla, S.; Reddy, G.B.; Petrash, J.M. Aldose reductase-mediated induction of epithelium-to-mesenchymal transition (emt) in lens. Chem. Biol. Interact. 2011, 191, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, S.D.; Kinoshita, J.H. The absence of cataracts in mice with congenital hyperglycemia. Exp. Eye Res. 1974, 19, 577–582. [Google Scholar] [CrossRef]
- Reddy, G.B.; Satyanarayana, A.; Balakrishna, N.; Ayyagari, R.; Padma, M.; Viswanath, K.; Petrash, J.M. Erythrocyte aldose reductase activity and sorbitol levels in diabetic retinopathy. Mol. Vis. 2008, 14, 593–601. [Google Scholar]
- Li, W.C.; Kuszak, J.R.; Dunn, K.; Wang, R.R.; Ma, W.; Wang, G.M.; Spector, A.; Leib, M.; Cotliar, A.M.; Weiss, M.; et al. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J. Cell Biol. 1995, 130, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Chylack, L.T., Jr.; Wolfe, J.K.; Singer, D.M.; Leske, M.C.; Bullimore, M.A.; Bailey, I.L.; Friend, J.; McCarthy, D.; Wu, S.Y. The lens opacities classification system iii. The longitudinal study of cataract study group. Arch. Ophthalmol. 1993, 111, 831–836. [Google Scholar] [CrossRef]
- Verdaasdonk, J.S.; Lawrimore, J.; Bloom, K. Determining absolute protein numbers by quantitative fluorescence microscopy. Methods Cell Biol. 2014, 123, 347–365. [Google Scholar]
- De Iongh, R.U.; Wederell, E.; Lovicu, F.J.; McAvoy, J.W. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: A model for cataract formation. Cells Tissues Organs 2005, 179, 43–55. [Google Scholar] [CrossRef]
- Du, L.; Hao, M.; Li, C.; Wu, W.; Wang, W.; Ma, Z.; Yang, T.; Zhang, N.; Isaac, A.T.; Zhu, X.; et al. Quercetin inhibited epithelial mesenchymal transition in diabetic rats, high-glucose-cultured lens, and sra01/04 cells through transforming growth factor-beta2/phosphoinositide 3-kinase/akt pathway. Mol. Cell Endocrinol. 2017, 452, 44–56. [Google Scholar] [CrossRef]
- Balasubbu, S.; Sundaresan, P.; Rajendran, A.; Ramasamy, K.; Govindarajan, G.; Perumalsamy, N.; Hejtmancik, J.F. Association analysis of nine candidate gene polymorphisms in indian patients with type 2 diabetic retinopathy. BMC Med. Genet. 2010, 11, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taskoparan, B.; Seza, E.G.; Demirkol, S.; Tuncer, S.; Stefek, M.; Gure, A.O.; Banerjee, S. Opposing roles of the aldo-keto reductases akr1b1 and akr1b10 in colorectal cancer. Cell. Oncol. 2017, 40, 563–578. [Google Scholar] [CrossRef] [Green Version]
- Misra, P.; Chakrabarti, R. The role of amp kinase in diabetes. Indian J. Med. Res. 2007, 125, 389–398. [Google Scholar] [PubMed]
- Kubota, S.; Ozawa, Y.; Kurihara, T.; Sasaki, M.; Yuki, K.; Miyake, S.; Noda, K.; Ishida, S.; Tsubota, K. Roles of amp-activated protein kinase in diabetes-induced retinal inflammation. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9142–9148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahjoub, S.; Masrour-Roudsari, J. Role of oxidative stress in pathogenesis of metabolic syndrome. Caspian J. Intern. Med. 2012, 3, 386–396. [Google Scholar]
- NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar]
- Kim, Y.K.; Kim, S.G.; Kim, J.Y.; Heo, Y.K.; Park, J.C.; Oh, J.S. Histologic evaluation of a retrieved endosseous implant: A case report. Int. J. Periodontics Restor. Dent. 2013, 33, e32–e36. [Google Scholar] [CrossRef] [Green Version]
- Kaur, N.; Vanita, V. Association of aldose reductase gene (akr1b1) polymorphism with diabetic retinopathy. Diabetes Res. Clin. Pract. 2016, 121, 41–48. [Google Scholar] [CrossRef]
- Wang, Y.; Luk, A.O.; Ng, M.C.; Pang, C.C.; Lam, V.; Lee, S.C.; Lam, D.S.; Choy, K.W.; Ma, R.C.; So, W.Y.; et al. Additive effect of aldose reductase z-4 microsatellite polymorphism and glycaemic control on cataract development in type 2 diabetes. J. Diabetes Complicat. 2014, 28, 147–151. [Google Scholar] [CrossRef]
- Tan, A.G.; Kifley, A.; Holliday, E.G.; Klein, B.E.K.; Iyengar, S.K.; Lee, K.E.; Jun, G.R.; Cumming, R.G.; Zhao, W.; Wong, T.Y.; et al. Aldose reductase polymorphisms, fasting blood glucose, and age-related cortical cataract. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4755–4762. [Google Scholar] [CrossRef]
- Stitt, A.W. Advanced glycation: An important pathological event in diabetic and age related ocular disease. Br. J. Ophthalmol. 2001, 85, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V. Aldose reductase: New insights for an old enzyme. Biomol. Concepts 2011, 2, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wautier, M.P.; Chappey, O.; Corda, S.; Stern, D.M.; Schmidt, A.M.; Wautier, J.L. Activation of nadph oxidase by age links oxidant stress to altered gene expression via rage. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E685–E694. [Google Scholar] [CrossRef] [PubMed]
- Hashim, Z.; Zarina, S. Advanced glycation end products in diabetic and non-diabetic human subjects suffering from cataract. Age 2011, 33, 377–384. [Google Scholar] [CrossRef]
- Kumamoto, Y.; Takamura, Y.; Kubo, E.; Tsuzuki, S.; Akagi, Y. Epithelial cell density in cataractous lenses of patients with diabetes: Association with erythrocyte aldose reductase. Exp. Eye Res. 2007, 85, 393–399. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Liang, B.; Xu, J.; Xie, Z.; Liu, C.; Viollet, B.; Yan, D.; Zou, M.H. Ampkalpha2 deletion causes aberrant expression and activation of nad(p)h oxidase and consequent endothelial dysfunction in vivo: Role of 26s proteasomes. Circ. Res. 2010, 106, 1117–1128. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Min, Z.; Liu, X.; Wang, C.; Wang, Z.; Shen, J.; Tang, W.; Zhang, X.; Liu, D.; Xu, X. Tolrestat acts atypically as a competitive inhibitor of the thermostable aldo-keto reductase tm1743 from thermotoga maritima. FEBS Lett. 2019, 594, 564–580. [Google Scholar] [CrossRef]
- Mamuya, F.A.; Duncan, M.K. Av integrins and tgf-beta-induced emt: A circle of regulation. J. Cell. Mol. Med. 2012, 16, 445–455. [Google Scholar] [CrossRef]
- Korol, A.; Pino, G.; Dwivedi, D.; Robertson, J.V.; Deschamps, P.A.; West-Mays, J.A. Matrix metalloproteinase-9-null mice are resistant to tgf-beta-induced anterior subcapsular cataract formation. Am. J. Pathol. 2014, 184, 2001–2012. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-T.; Chen, Y.-Y.; Chang, H.-Y.; Kung, Y.-H.; Tseng, C.-J.; Cheng, P.-W. AKR1B1-Induced Epithelial–Mesenchymal Transition Mediated by RAGE-Oxidative Stress in Diabetic Cataract Lens. Antioxidants 2020, 9, 273. https://doi.org/10.3390/antiox9040273
Wu T-T, Chen Y-Y, Chang H-Y, Kung Y-H, Tseng C-J, Cheng P-W. AKR1B1-Induced Epithelial–Mesenchymal Transition Mediated by RAGE-Oxidative Stress in Diabetic Cataract Lens. Antioxidants. 2020; 9(4):273. https://doi.org/10.3390/antiox9040273
Chicago/Turabian StyleWu, Tsung-Tien, Ying-Ying Chen, Hui-Yu Chang, Ya-Hsin Kung, Ching-Jiunn Tseng, and Pei-Wen Cheng. 2020. "AKR1B1-Induced Epithelial–Mesenchymal Transition Mediated by RAGE-Oxidative Stress in Diabetic Cataract Lens" Antioxidants 9, no. 4: 273. https://doi.org/10.3390/antiox9040273
APA StyleWu, T.-T., Chen, Y.-Y., Chang, H.-Y., Kung, Y.-H., Tseng, C.-J., & Cheng, P.-W. (2020). AKR1B1-Induced Epithelial–Mesenchymal Transition Mediated by RAGE-Oxidative Stress in Diabetic Cataract Lens. Antioxidants, 9(4), 273. https://doi.org/10.3390/antiox9040273





