The Aqueous Extract from Ceratonia siliqua Leaves Protects against 6-Hydroxydopamine in Zebrafish: Understanding the Underlying Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction
2.3. Total Phenolic Content
2.4. Flavonoids Content
2.5. Condensed Tannins Content
2.6. UHPLC Semiquantitative Analysis
2.7. Antioxidant Activity Assays
2.8. In Vitro Inhibition Assay of Acetylcholinesterase
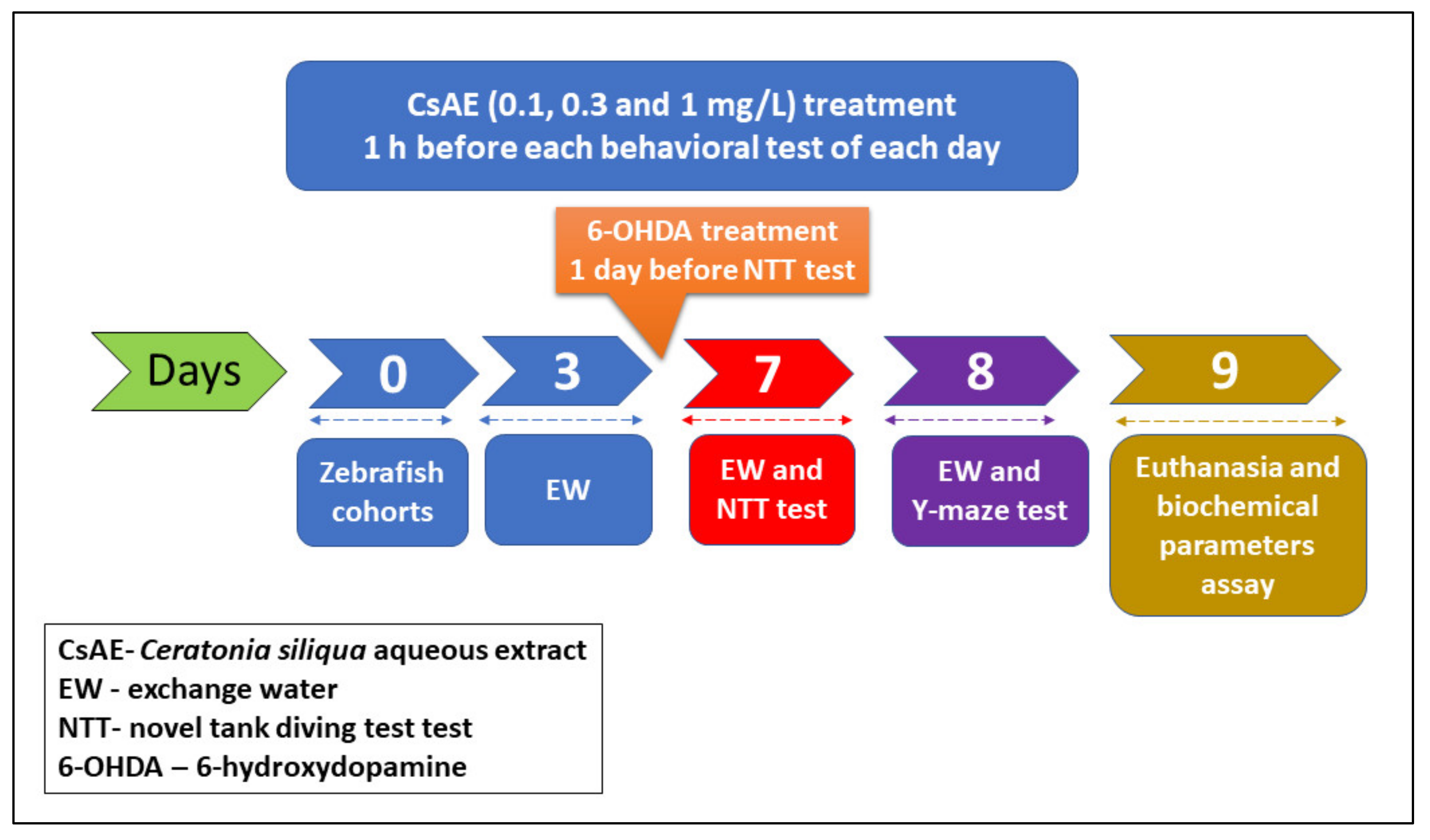
2.9. Animals
2.10. Behavioral Analysis
2.10.1. Novel Tank Diving Test (NTT)
2.10.2. Y-Maze Test
2.11. Biochemical Parameters Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Content
3.2. Flavonoids Content
3.3. Condensed Tannins Content
3.4. UHPLC Analysis
3.5. Determination of Antioxidant Activity
3.6. Acetylcholinesterase Inhibitory Assay
3.7. Effects on Anxiety-Like Behavior in NTT Test and on Spatial Memory in Y-Maze Test
3.8. Effects on the Brain AChE Activity
3.9. Effects on the Brain SOD, CAT, and GPX Specific Activities, and the MDA Level
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, E.; Park, H.R.; Ji, S.T.; Lee, Y.; Lee, J. Baicalein attenuates astroglial activation in the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-induced Parkinson’s disease model by downregulating the activations of nuclear factor-κB, ERK, and JNK. J. Neurosci. Res. 2013, 92, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Daviaud, N.; Garbayo, E.; Lautram, N.; Franconi, F.; Lemaire, L.; Perez-Pinzon, M.; Montero-Menei, C.N. Modeling nigrostriatal degeneration in organotypic cultures, a new ex vivo model of Parkinson’s disease. Neuroscience 2013, 256, 10–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaki, G.S.; Papavassiliou, A.G. Oxidative Stress-Induced Signaling Pathways Implicated in the Pathogenesis of Parkinson’s Disease. NeuroMolecular Med. 2014, 16, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.S.; Salinas, K.; Garduno, A.C.; Johansson, J.U.; Wang, Q.; Manning-Bog, A.; Andreasson, K.I. Anti-Inflammatory and Neuroprotective Effects of PGE2 EP4 Signaling in Models of Parkinson’s Disease. J. Neuroimm. Pharmacol. 2016, 12, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Manchado, A.B.; Villadiego, J.; Suárez-Luna, N.; Bermejo-Navas, A.; Garrido-Gil, P.; Labandeira-Garcia, J.L.; Echevarría, M.; López-Barneo, J.; Toledo-Aral, J.J.; Echevarría, M. Neuroprotective and reparative effects of carotid body grafts in a chronic MPTP model of Parkinson’s disease. Neurobiol. Aging 2013, 34, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Prediger, R.D.; Matheus, F.C.; Schwarzbold, M.L.; Lima, M.M.S.; Vital, M.A. Anxiety in Parkinson’s disease: A critical review of experimental and clinical studies. Neuropharmacology 2012, 62, 115–124. [Google Scholar] [CrossRef]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010, 9, 1200–1213. [Google Scholar] [CrossRef]
- Erro, R.; Santangelo, G.; Picillo, M.; Vitale, C.; Amboni, M.; Longo, K.; Costagliola, A.; Pellecchia, M.T.; Allocca, R.; Rosa, A.; et al. Link between non-motor symptoms and cognitive dysfunctions in de novo, drug-naive PD patients. J. Neurol. 2012, 259, 1808–1813. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, D.A.; Schrag, A. Psychosis, apathy, depression and anxiety in Parkinson’s disease. Neurobiol. Dis. 2012, 46, 581–589. [Google Scholar] [CrossRef]
- Sekeroglu, N.; Deniz, F.S.S.; Orhan, I.E.; Gulpinar, A.R.; Kartal, M.; Sener, B.; Şekeroǧlu, N. In vitro prospective effects of various traditional herbal coffees consumed in Anatolia linked to neurodegeneration. Food Res. Int. 2012, 45, 197–203. [Google Scholar] [CrossRef]
- Khan, M.M.; Ahmad, A.; Ishrat, T.; Khan, M.B.; Hoda, N.; Khuwaja, G.; Raza, S.S.; Khan, A.; Javed, H.; Vaibhav, K.; et al. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson’s disease. Brain Res. 2010, 1328, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Z.; Cheang, L.C.-V.; Lin, Z.; Lee, S.M.-Y. Eriocaulon buergerianum extract protects PC12 cells and neurons in zebrafish against 6-hydroxydopamine-induced damage. Chin. Med. 2011, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Li, C.; Chen, S.; Li, Z.; Jia, X.; Wang, K.; Bao, J.; Liang, Y.; Wang, X.; Chen, M.; et al. Berberine protects against 6-OHDA-induced neurotoxicity in PC12 cells and zebrafish through hormetic mechanisms involving PI3K/AKT/Bcl-2 and Nrf2/HO-1 pathways. Redox Biol. 2017, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panula, P.; Chen, Y.-C.; Priyadarshini, M.; Kudo, H.; Semenova, S.; Sundvik, M.; Sallinen, V. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 2010, 40, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.; Ebrahimie, E.; Lardelli, M. Using the zebrafish model for Alzheimer’s disease research. Front. Genet. 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Richetti, S.; Blank, M.; Capiotti, K.; Piato, A.; Bogo, M.; Vianna, M.R.M.; Bonan, C.D. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav. Brain Res. 2011, 217, 10–15. [Google Scholar] [CrossRef]
- Santana, S.; Rico, E.P.; Burgos, J. Can zebrafish be used as animal model to study Alzheimer’s disease? Am. J. Neurodegener. Dis. 2012, 1, 32–48. [Google Scholar]
- Gulcin, I.; Bursal, E.; Şehitoğlu, M.H.; Bílsel, M.; Goren, A.C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem. Toxicol. 2010, 48, 2227–2238. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 674, 137–147. [Google Scholar] [CrossRef]
- Kamdem, J.P.; Olalekan, E.O.; Hassan, W.; Kade, I.J.; Yetunde, O.; Boligon, A.; Athayde, M.L.; Souza, D.O.; Rocha, J.B.T. Trichilia catigua (Catuaba) bark extract exerts neuroprotection against oxidative stress induced by different neurotoxic agents in rat hippocampal slices. Ind. Crop. Prod. 2013, 50, 625–632. [Google Scholar] [CrossRef]
- Roberts, R.A.; Smith, R.A.; Safe, S.; Szabó, C.; Tjalkens, R.B.; Robertson, F.M. Toxicological and pathophysiological roles of reactive oxygen and nitrogen species. Toxicology 2010, 276, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.-M.; Zhou, Z.-Y.; Razmovski-Naumovski, V.; Cui, G.-Z.; Zhang, L.-Q.; Sa, F.; Hoi, P.-M.; Chan, K.; Lee, S.M.-Y. Danshensu protects against 6-hydroxydopamine-induced damage of PC12 cells in vitro and dopaminergic neurons in zebrafish. Neurosci. Lett. 2013, 543, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Darley-Usmar, V.; Zhang, J. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol. 2013, 2, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Almanasrah, M.; Roseiro, L.B.; Lukasik, R.; Carvalheiro, F.; Brazinha, C.; Crespo, J.G.; Kallioinen, M.; Mänttäri, M.; Duarte, L.C.; Roseiro, M.L.D.B.W. Selective recovery of phenolic compounds and carbohydrates from carob kibbles using water-based extraction. Ind. Crop. Prod. 2015, 70, 443–450. [Google Scholar] [CrossRef] [Green Version]
- El Hajaji, H.; Lachkar, N.; Alaoui, K.; Cherrah, Y.; Farah, A.; Ennabili, A.; El Bali, B.; Lachkar, M. Antioxidant properties and total phenolic content of three varieties of carob tree leaves from Morocco. Rec. Nat. Prod. 2010, 4, 193. [Google Scholar]
- Roseiro, L.B.; Duarte, L.C.; Oliveira, D.; Roque, R.; Bernardo-Gil, M.G.; Martins, A.; Sepúlveda, C.; Almeida, J.; Meireles, M.; Gírio, F.M.; et al. Supercritical, ultrasound and conventional extracts from carob (Ceratonia siliqua L.) biomass: Effect on the phenolic profile and antiproliferative activity. Ind. Crop. Prod. 2013, 47, 132–138. [Google Scholar] [CrossRef]
- Kasrati, A.; Jamali, C.A.; Fadli, M.; Bekkouche, K.; Hassani, L.; Wohlmuth, H.; Leach, D.; Abbad, A. Antioxidative activity and synergistic effect of Thymus saturejoides Coss. essential oils with cefixime against selected food-borne bacteria. Ind. Crop. Prod. 2014, 61, 338–344. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Podgórski, R. Influence of different extraction methods on the quantification of selected flavonoids and phenolic acids from Tilia cordata inflorescence. Ind. Crop. Prod. 2015, 76, 509–514. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Aligiannis, N.; Halabalaki, M.; Skaltsounis, A.-L.; Glowniak, K.; Kalpoutzakis, E. Influence of extraction procedures on phenolic content and antioxidant activity of Cretan barberry herb. Food Chem. 2013, 138, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Jhon, D.-Y. The antioxidant, angiotensin converting enzyme inhibition activity, and phenolic compounds of bamboo shoot extracts. LWT 2010, 43, 655–659. [Google Scholar] [CrossRef]
- Chonpathompikunlert, P.; Wattanathorn, J.; Muchimapura, S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer’s disease. Food Chem. Toxicol. 2010, 48, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Escapa, A.L.; Fernandes, E.; Fajardo, A.; Aligué, R.; Alberício, F.; Neng, N.; Nogueira, J.M.F.; Romano, A. In vitro cytotoxic effects and apoptosis induction by a methanol leaf extract of carob tree (Ceratonia siliqua L.). J. Med. Plants Res. 2011, 5, 1987–1996. [Google Scholar]
- Obeidat, B.; Alrababah, M.; Alhamad, M.; Gharaibeh, M.; Abu Ishmais, M. Effects of feeding carob pods (Ceratonia siliqua L.) on nursing performance of Awassi ewes and their lambs. Small Rumin. Res. 2012, 105, 9–15. [Google Scholar] [CrossRef]
- Tetik, N.; Yuksel, E. Ultrasound-assisted extraction of d-pinitol from carob pods using Response Surface Methodology. Ultrason. Sonochem. 2014, 21, 860–865. [Google Scholar] [CrossRef]
- Osório, J.; Osório, J.; Gonçalves, S.; David, M.M.; Correia, M.J.; Romano, A. Carob trees (Ceratonia siliqua L.) regenerated in vitro can acclimatize successfully to match the field performance of seed-derived plants. Trees 2012, 26, 1837–1846. [Google Scholar] [CrossRef]
- Vekiari, S.A.; Ouzounidou, G.; Öztürk, M.; Görk, G. Variation of quality characteristics in Greek and Turkish carob pods during fruit development. Procedia Soc. Behav. Sci. 2011, 19, 750–755. [Google Scholar] [CrossRef] [Green Version]
- ElBatal, H.; Hasib, A.; Ouatmane, A.; Dehbi, F.; Jaouad, A.; Boulli, A. Sugar composition and yield of syrup production from the pulp of Moroccan carob pods (Ceratonia siliqua L.). Arab. J. Chem. 2016, 9, S955–S959. [Google Scholar] [CrossRef] [Green Version]
- Hsouna, A.B.; Saoudi, M.; Trigui, M.; Jamoussi, K.; Boudawara, T.; Jaoua, S.; Feki, A. El Characterization of bioactive compounds and ameliorative effects of Ceratonia siliqua leaf extract against CCl4 induced hepatic oxidative damage and renal failure in rats. Food Chem. Toxicol. 2011, 49, 3183–3191. [Google Scholar] [CrossRef]
- Papagiannopoulos, M.; Wollseifen, H.R.; Mellenthin, A.; Haber, B.; Galensa, R. Identification and Quantification of Polyphenols in Carob Fruits (Ceratonia siliqua L.) and Derived Products by HPLC-UV-ESI/MS n. J. Agric. Food Chem. 2004, 52, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.K.E.; El-Manfaloty, M.M.; Ali, H.M. Assessment of proximate chemical composition, nutritional status, fatty acid composition and phenolic compounds of carob (Ceratonia siliqua L.). Food Public Heal. 2013, 3, 304–308. [Google Scholar]
- Sidina, M.M.; El Hansali, M.; Wahid, N.; Ouatmane, A.; Boulli, A.; Haddioui, A. Fruit and seed diversity of domesticated carob (Ceratonia siliqua L.) in Morocco. Sci. Hortic. 2009, 123, 110–116. [Google Scholar] [CrossRef]
- El Hajaji, H.; Lachkar, N.; Alaoui, K.; Cherrah, Y.; Farah, A.; Ennabili, A.; El Bali, B.; Lachkar, M. Antioxidant activity, phytochemical screening, and total phenolic content of extracts from three genders of carob tree barks growing in Morocco. Arab. J. Chem. 2011, 4, 321–324. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.-L.; Chen, S.-G.; Xie, Y.-Q.; Chen, F.; Zhao, Y.-Y.; Luo, C.-X.; Gao, Y.-Q. Total phenolic, flavonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Li, H.-B.; Wong, C.-C.; Cheng, K.-W.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Kusirisin, W.; Srichairatanakool, S.; Lerttrakarnnon, P.; Lailerd, N.; Suttajit, M.; Jaikang, C.; Chaiyasut, C. Antioxidative activity, polyphenolic content and anti-glycation effect of some Thai medicinal plants traditionally used in diabetic patients. Med. Chem. 2009, 5, 139–147. [Google Scholar] [CrossRef]
- Velázquez, M.E.; Tournier, H.; De Buschiazzo, P.M.; Saavedra, G.; Schinella, G. Antioxidant activity of Paraguayan plant extracts. Fitoterapia 2003, 74, 91–97. [Google Scholar] [CrossRef]
- Chahmi, N.; Anissi, J.; Jennan, S.; Farah, A.; Sendide, K.; Hassouni, M. El Antioxidant activities and total phenol content of Inula viscosa extracts selected from three regions of Morocco. Asian Pac. J. Trop. Biomed. 2015, 5, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Meghashri, S.; Kumar, H.V.; Gopal, S. Antioxidant properties of a novel flavonoid from leaves of Leucas aspera. Food Chem. 2010, 122, 105–110. [Google Scholar] [CrossRef]
- Chaiyana, W.; Okonogi, S. Inhibition of cholinesterase by essential oil from food plant. Phytomedicine 2012, 19, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-L.; Souders, C.L.; Denslow, N.D.; Martyniuk, C.J. Quercetin, a natural product supplement, impairs mitochondrial bioenergetics and locomotor behavior in larval zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 2017, 327, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.-W.; Wen, Z.-H.; Huang, S.-Y.; Hung, H.-C.; Chen, C.-H.; Yang, S.-N.; Chen, N.-F.; Wang, H.-M.; Hsiao, C.-D.; Chen, W.-F. Effects of 6-Hydroxydopamine Exposure on Motor Activity and Biochemical Expression in Zebrafish (Danio Rerio) Larvae. Zebrafish 2014, 11, 227–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cachat, J.; Canavello, P.R.; Elkhayat, S.I.; Bartels, B.K.; Hart, P.; Elegante, M.F.; Beeson, E.C.; Laffoon, A.L.; Haymore, W.A.; Tien, D.H.; et al. Video-Aided Analysis of Zebrafish Locomotion and Anxiety-Related Behavioral Responses. Viral Vector Approaches Neurobiol. Brain Dis. 2010, 51, 1–14. [Google Scholar]
- Dumitru, G.; El-Nashar, H.A.; Mostafa, N.M.; Eldahshan, O.A.; Boiangiu, R.S.; Todirascu-Ciornea, E.; Hritcu, L.; Singab, A.N.B. Agathisflavone isolated from Schinus polygamus (Cav.) Cabrera leaves prevents scopolamine-induced memory impairment and brain oxidative stress in zebrafish (Danio rerio). Phytomedicine 2019, 58, 152889. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.L.A.; Lima, L.M.; Abrante, I.A.; De Araújo, J.I.F.; Batista, F.L.A.; Abrante, I.A.; Magalhães, F.E.A.; De Lima, D.R.; Lima, M.D.C.L.; Prado, B.S.D.; et al. Antinociceptive activity of ethanolic extract of Azadirachta indica A. Juss (Neem, Meliaceae) fruit through opioid, glutamatergic and acid-sensitive ion pathways in adult zebrafish (Danio rerio). Biomed. Pharmacother. 2018, 108, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Krohn, R.; Hermanson, G.; Mallia, A.; Gartner, F.; Provenzano, M.; Fujimoto, E.; Goeke, N.; Olson, B.; Klenk, D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Uysal, S.; Acqua, S.D.; Aktumsek, A.; Karatas, S. Chemical and biological approaches on nine fruit tree leaves collected from the Mediterranean region of Turkey. J. Funct. Foods 2016, 22, 518–532. [Google Scholar] [CrossRef]
- Alali, F.; Tawaha, K.; El-Elimat, T.; Syouf, M.; El-Fayad, M.; Abulaila, K.; Nielsen, S.J.; Wheaton, W.D.; Iii, J.O.F.; Oberlies, N.H. Antioxidant activity and total phenolic content of aqueous and methanolic extracts of Jordanian plants: An ICBG project. Nat. Prod. Res. 2007, 21, 1121–1131. [Google Scholar] [CrossRef] [Green Version]
- Ben Othmen, K.; Elfalleh, W.; Beltrán, J.M.G.; Esteban, M. Ángeles; Haddad, M. An in vitro study of the effect of carob (Ceratonia siliqua L.) leaf extracts on gilthead seabream (Sparus aurata L.) leucocyte activities. Antioxidant, cytotoxic and bactericidal properties. Fish Shellfish Immunol. 2020, 99, 35–43. [Google Scholar] [CrossRef]
- Aboura, I.; Nani, A.; Belarbi, M.; Murtaza, B.; Fluckiger, A.; Dumont, A.; Benammar, C.; Tounsi, M.; Ghiringhelli, F.; Rialland, M.; et al. Protective effects of polyphenol-rich infusions from carob (Ceratonia siliqua) leaves and cladodes of Opuntia ficus-indica against inflammation associated with diet-induced obesity and DSS-induced colitis in Swiss mice. Biomed. Pharmacother. 2017, 96, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Daglia, M.; Nabavi, S.; Loizzo, M.; Sobarzo-Sánchez, E.; Nabavi, S. Flavonoids and dementia: An update. Curr. Med. Chem. 2015, 22, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Sassi, A.; Bouhlel, I.; Mustapha, N.; Mokdad-Bzeouich, I.; Chaabane, F.; Ghedira, K.; Chekir-Ghedira, L. Assessment in vitro of the genotoxicity, antigenotoxicity and antioxidant of Ceratonia siliqua L. extracts in murine leukaemia cells L1210 by comet assay. Regul. Toxicol. Pharmacol. 2016, 77, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Vaya, J.; Mahmood, S. Flavonoid content in leaf extracts of the fig (Ficus carica L.), carob (Ceratonia siliqua L.) and pistachio (Pistacia lentiscus L.). BioFactors 2006, 28, 169–175. [Google Scholar] [CrossRef]
- Gong, Y.-S.; Guo, J.; Hu, K.; Gao, Y.-Q.; Xie, B.-J.; Sun, Z.; Yang, E.-N.; Hou, F.-L. Ameliorative effect of lotus seedpod proanthocyanidins on cognitive impairment and brain aging induced by d-galactose. Exp. Gerontol. 2016, 74, 21–28. [Google Scholar] [CrossRef]
- Rusu, M.E.; Fizeșan, I.; Pop, A.; Gheldiu, A.-M.; Mocan, A.; Crișan, G.; Vlase, L.; Loghin, F.; Popa, D.S.; Tomuță, I.; et al. Enhanced Recovery of Antioxidant Compounds from Hazelnut (Corylus avellana L.) Involucre Based on Extraction Optimization: Phytochemical Profile and Biological Activities. Antioxidants 2019, 8, 460. [Google Scholar]
- Eldahshan, O.A. Eldahshan Isolation and Structure Elucidation of Phenolic Compounds of Carob Leaves Grown in Egypt. Curr. Res. J. Biol. Sci. 2011, 3, 52–55. [Google Scholar]
- Goulas, V.; Georgiou, E. Utilization of Carob Fruit as Sources of Phenolic Compounds with Antioxidant Potential: Extraction Optimization and Application in Food Models. Foods 2019, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Corsi, L.; Avallone, R.; Cosenza, F.; Farina, F.; Baraldi, C.; Baraldi, M. Antiproliferative effects of Ceratonia siliqua L. on mouse hepatocellular carcinoma cell line. Fitoterapia 2002, 73, 674–684. [Google Scholar] [CrossRef]
- Končić, M.Z.; Kremer, D.; Karlovic, K.; Kosalec, I. Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat. Food Chem. Toxicol. 2010, 48, 2176–2180. [Google Scholar] [CrossRef]
- Loizzo, M.; Said, A.; Tundis, R.; Hawas, U.; Rashed, K.; Menichini, F.; Frega, N.G.; Menichini, F. Antioxidant and Antiproliferative Activity of Diospyros lotus L. Extract and Isolated Compounds. Plant Foods Hum. Nutr. 2009, 64, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Fernandes, E.; Escapa, A.L.; López-Avilés, S.; Fajardo, A.; Aligué, R.; Albericio, F.; Romano, A. Antioxidant activity andin vitroinhibition of tumor cell growth by leaf extracts from the carob tree (Ceratonia siliqua). Pharm. Biol. 2009, 47, 721–728. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Ouchemoukh, S.; Meziant, N.; Idiri, Y.; Hernanz, D.; Stinco, C.M.; Rodríguez-Pulido, F.J.; Heredia, F.J.; Madani, K.; Luis, J. Bioactive metabolites involved in the antioxidant, anticancer and anticalpain activities of Ficus carica L., Ceratonia siliqua L. and Quercus ilex L. extracts. Ind. Crop. Prod. 2017, 95, 6–17. [Google Scholar] [CrossRef]
- Benchikh, Y.; Louaileche, H. Effects of extraction conditions on the recovery of phenolic compounds and in vitro antioxidant activity of carob (Ceratonia siliqua L.) pulp. Acta Bot. Gallica 2014, 161, 175–181. [Google Scholar] [CrossRef]
- Mbaebie, B.; Edeoga, H.; Afolayan, A.J. Phytochemical analysis and antioxidants activities of aqueous stem bark extract of Schotia latifolia Jacq. Asian Pac. J. Trop. Biomed. 2012, 2, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Skotti, E.; Anastasaki, E.; Kanellou, G.; Polissiou, M.; Tarantilis, P. Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants. Ind. Crop. Prod. 2014, 53, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Tagne, R.S.; Telefo, P.; Nyemb, J.N.; Yemele, D.M.; Njina, S.N.; Goka, S.M.C.; Lienou, L.L.; Kamdje, A.H.N.; Moundipa, P.F.; Farooq, A.D. Anticancer and antioxidant activities of methanol extracts and fractions of some Cameroonian medicinal plants. Asian Pac. J. Trop. Med. 2014, 7, S442–S447. [Google Scholar] [CrossRef] [Green Version]
- Shahwar, D.; Raza, M.A. Antioxidant potential of phenolic extracts of Mimusops elengi. Asian Pac. J. Trop. Biomed. 2012, 2, 547–550. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, S.; Gomes, D.; Costa, P.; Romano, A. The phenolic content and antioxidant activity of infusions from Mediterranean medicinal plants. Ind. Crop. Prod. 2013, 43, 465–471. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem. Toxicol. 2006, 44, 198–206. [Google Scholar] [CrossRef]
- Kumaran, D.; Udayabanu, M.; Kumar, M.; Aneja, R.; Katyal, A. Involvement of angiotensin converting enzyme in cerebral hypoperfusion induced anterograde memory impairment and cholinergic dysfunction in rats. Neuroscience 2008, 155, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Terpinc, P.; Ceh, B.; Ulrih, N.P.; Abramovič, H. Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Ind. Crop. Prod. 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Hinneburg, I.; Dorman, H.D.; Hiltunen, R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006, 97, 122–129. [Google Scholar] [CrossRef]
- Sabir, S.M.; Rocha, J.; Rocha, J.B.T. Water-extractable phytochemicals from Phyllanthus niruri exhibit distinct in vitro antioxidant and in vivo hepatoprotective activity against paracetamol-induced liver damage in mice. Food Chem. 2008, 111, 845–851. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Cai, P.; Luo, C.; Qian, Z.; Dai, Y.; Feng, H. Characterizing iron deposition in Parkinson’s disease using susceptibility-weighted imaging: An in vivo MR study. Brain Res. 2010, 1330, 124–130. [Google Scholar] [CrossRef]
- Zheng, H.; Gal, S.; Weiner, L.M.; Bar-Am, O.; Warshawsky, A.; Fridkin, M.; Youdim, M.B. Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases: In vitro studies on antioxidant activity, prevention of lipid peroxide formation and monoamine oxidase inhibition. J. Neurochem. 2005, 95, 68–78. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols and antioxidant activity of hydro-alcoholic extracts of propolis from Algarve, South of Portugal. Food Chem. Toxicol. 2010, 48, 3418–3423. [Google Scholar] [CrossRef]
- Abidar, S.; Yildiz, O.; Degirmenci, A.; Amakran, A.; El Maadoudi, M.; Nhiri, M. Glucose-mediated protein glycation: Contribution of methanolic extract of Ceratonia siliqua L. in protection and in vitro potential inhibition of acetylcholinesterase. J. Food Biochem. 2019, 43, e13009. [Google Scholar] [CrossRef]
- Benković, M.; Belščak-Cvitanović, A.; Bauman, I.; Komes, D.; Srečec, S. Flow properties and chemical composition of carob ( Ceratonia siliqua L.) flours as related to particle size and seed presence. Food Res. Int. 2017, 100, 211–218. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B.; Martínez-Villaluenga, C.; Aguirre, L.; Silvan, J.M.; Dueñas, M.; De Luis, D.; Lasa, A. In vitro approach for evaluation of carob by-products as source bioactive ingredients with potential to attenuate metabolic syndrome (MetS). Heliyon 2019, 5, e01175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzoubi, K.H.; Alibbini, S.; Khabour, O.F.; El-Elimat, T.; Al-Zubi, M.; Alali, F. Carob (Ceratonia siliqua L.) Prevents Short-Term Memory Deficit Induced by Chronic Stress in Rats. J. Mol. Neurosci. 2018, 66, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Ammari, M.; Othman, H.; Rtibi, K.; Sakly, M.; Abdelmelek, H. The Effects of Carob (Ceratonia siliqua L.) on Emotional Behavior Impairment and Metabolic Disorders Induced by Estrogen Deficiency in Rats. J. Med. Food 2020. [Google Scholar] [CrossRef] [PubMed]
- Scherer, E.B.; Da Cunha, M.J.; Matté, C.; Schmitz, F.; Netto, C.A.; Wyse, A.T.S. Methylphenidate affects memory, brain-derived neurotrophic factor immunocontent and brain acetylcholinesterase activity in the rat. Neurobiol. Learn. Mem. 2010, 94, 247–253. [Google Scholar] [CrossRef]
- Pegan, K.; Matkovic, U.; Mars, T.; Mis, K.; Pirkmajer, S.; Brecelj, J.; Grubic, Z. Acetylcholinesterase is involved in apoptosis in the precursors of human muscle regeneration. Chem. Interactions 2010, 187, 96–100. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crop. Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Ingkaninan, K.; Temkitthawon, P.; Chuenchom, K.; Yuyaem, T.; Thongnoi, W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J. Ethnopharmacol. 2003, 89, 261–264. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Albin, R.L. The cholinergic system and Parkinson disease. Behav. Brain Res. 2010, 221, 564–573. [Google Scholar] [CrossRef] [Green Version]
- Chou, K.L.; Lenhart, A.; Koeppe, R.A.; Bohnen, N.I. Abnormal MoCA and normal range MMSE scores in Parkinson disease without dementia: Cognitive and neurochemical correlates. Park. Relat. Disord. 2014, 20, 1076–1080. [Google Scholar] [CrossRef] [Green Version]
- Custódio, L.; Patarra, J.; Albericio, F.; Neng, N.; Nogueira, J.; Romano, A. In vitro antioxidant and inhibitory activity of water decoctions of carob tree (Ceratonia siliqua L.) on cholinesterases, α-amylase and α-glucosidase. Nat. Prod. Res. 2015, 29, 1–5. [Google Scholar] [CrossRef]
- Syed, F.; Awasthi, K.K.; Chandravanshi, L.P.; Verma, R.; Rajawat, N.K.; Khanna, V.K.; John, P.J.; Soni, I. Bifenthrin-induced neurotoxicity in rats: Involvement of oxidative stress. Toxicol. Res. 2018, 7, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabavi, S.M.; Nabavi, S.F.; Eslami, S.; Moghaddam, A.H. In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem. 2012, 132, 931–935. [Google Scholar] [CrossRef]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; Mccord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Yoo, S.; Yoon, H.-G.; Park, J.; Lee, Y.-H.; Kim, S.; Oh, K.-T.; Lee, J.; Cho, H.-Y.; Jun, W. In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem. Toxicol. 2010, 48, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, H.; Yilmaz, O. Antioxidant Capacity and Radical Scavenging Activity of Silybum marianum and Ceratonia siliqua. Ekoloji 2012, 21, 9–16. [Google Scholar] [CrossRef]
- Sebai, H.; Souli, A.; Chehimi, L.; Rtibi, K.; Amri, M.; El-Benna, J.; Sakly, M. In vitro and in vivo antioxidant properties of Tunisian carob (Ceratonia siliqua L.). J. Med. Plants Res. 2013, 7, 85–90. [Google Scholar]
- Rtibi, K.; Jabri, M.A.; Selmi, S.; Souli, A.; Sebai, H.; El-Benna, J.; Amri, M.; Marzouki, L. Gastroprotective effect of carob (Ceratonia siliqua L.) against ethanol-induced oxidative stress in rat. BMC Complement. Altern. Med. 2015, 15, 292. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M. Biochemical studies on nephroprotective effect of carob (Ceratonia siliqua L.) growing in Egypt. Nat. Sci. 2010, 8, 41–47. [Google Scholar]






| Sample | Compound (w/w in the Extract) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| CsAE | 4.54 | 17.79 | 3.59 | 4.84 | 7.41 | 23.78 | 7.50 |
| Antioxidant Activity Assays | IC50 (mg/mL) | Coefficient and Regression Equation | Maximal Inhibition Percentages (%) | |||
|---|---|---|---|---|---|---|
| 1,1-Diphenyl-2-picrylhydrazyl (DPPH) scavenging power | CsAE | Ascorbic acid | CsAE | Ascorbic acid | CsAE | Ascorbic acid (1 mg/mL) |
| 0.116 ± 0.002 | 0.106 ± 0.0005 | y = −10.929x + 97.105 R2 = 0.9948 | y = 15.48x−12.535 R2 = 0.9915 | 84.65 ± 0.01 | 92.14 ± 0.04 | |
| Ferric reducing antioxidant power (FRAP) | CsAE (EC50) | BHT (EC50) | CsAE | BHT | CsAE | BHT |
| 0.123 ± 0.003 | 0.118 ± 0.00 | y = −0.101x + 0.915 R2 = 0.9999 | y = 0.311x − 0.017 R2 = 0.9958 | - | - | |
| Iron chelating activity | CsAE | EDTA | CsAE | EDTA | CsAE | EDTA (1 mg/mL) |
| 0.971 ± 0.006 | 0.117 ± 0.0005 | y = −12.356x + 62.71 R2 = 0.9939 | y = 15.927x − 12.002 R2 = 0.9914 | 51.5 ± 0.02 | 94.96 ± 0.003 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abidar, S.; Boiangiu, R.S.; Dumitru, G.; Todirascu-Ciornea, E.; Amakran, A.; Cioanca, O.; Hritcu, L.; Nhiri, M. The Aqueous Extract from Ceratonia siliqua Leaves Protects against 6-Hydroxydopamine in Zebrafish: Understanding the Underlying Mechanism. Antioxidants 2020, 9, 304. https://doi.org/10.3390/antiox9040304
Abidar S, Boiangiu RS, Dumitru G, Todirascu-Ciornea E, Amakran A, Cioanca O, Hritcu L, Nhiri M. The Aqueous Extract from Ceratonia siliqua Leaves Protects against 6-Hydroxydopamine in Zebrafish: Understanding the Underlying Mechanism. Antioxidants. 2020; 9(4):304. https://doi.org/10.3390/antiox9040304
Chicago/Turabian StyleAbidar, Sara, Razvan Stefan Boiangiu, Gabriela Dumitru, Elena Todirascu-Ciornea, Amina Amakran, Oana Cioanca, Lucian Hritcu, and Mohamed Nhiri. 2020. "The Aqueous Extract from Ceratonia siliqua Leaves Protects against 6-Hydroxydopamine in Zebrafish: Understanding the Underlying Mechanism" Antioxidants 9, no. 4: 304. https://doi.org/10.3390/antiox9040304








