Potential Benefits of Nrf2/Keap1 Targeting in Pancreatic Islet Cell Transplantation
Abstract
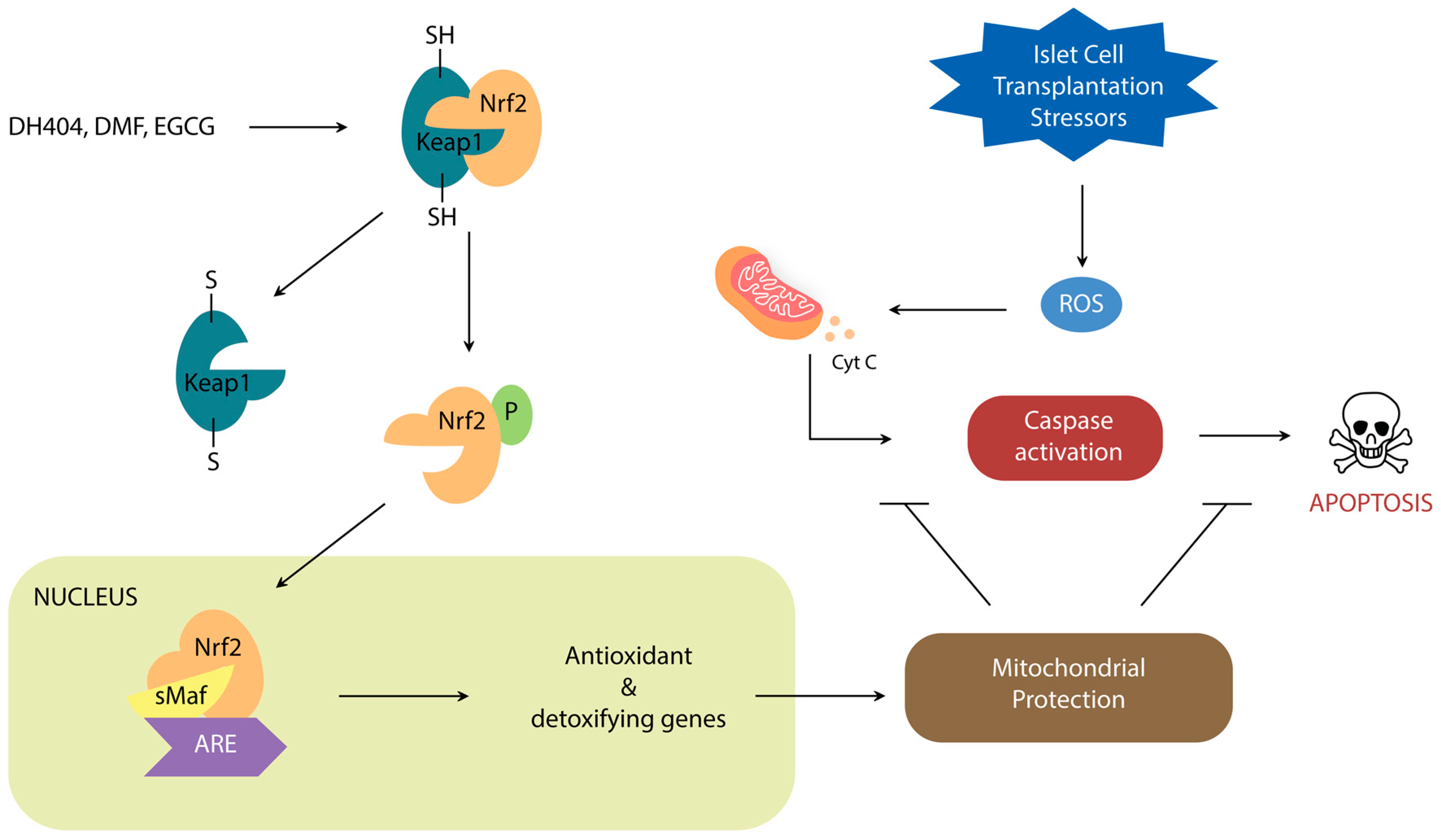
:1. Introduction
2. Nrf2/Keap1 Signaling Pathway
Nrf2 Activators
3. Nrf2 Roles in Steps of Islet Transplantation
3.1. Nrf2/Keap1: A Target for Pre-Transplant Protection of Islet Cells
3.1.1. Isolation: Metabolic Challenges
3.1.2. Preservation: Dynamic Temperature Stress on Islet Cells
3.1.3. Digestion and Isolation: Chemical and Mechanical Stress on Islet Cells
3.2. Nrf2/Keap1: A Target for Post-Transplant Protection of Islet Grafts
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rabinovitch, A.; Suarez-Pinzon, W.L. Cytokines and their roles in pancreatic islet β-cell destruction and insulin-dependent diabetes mellitus. Biochem. Pharmacol. 1998, 55, 1139–1149. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Mandrup-Poulsen, T. A choice of death–the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 2001, 44, 2115–2133. [Google Scholar] [CrossRef]
- Rewers, M.; Ludvigsson, J. Environmental risk factors for type 1 diabetes. Lancet 2016, 387, 2340–2348. [Google Scholar] [CrossRef] [Green Version]
- Goulden, M.R. The pain of chronic pancreatitis: A persistent clinical challenge. Br. J. Pain 2013, 7, 8–22. [Google Scholar] [CrossRef] [Green Version]
- Cdc.gov/Diabetes. Available online: https://www.cdc.gov/diabetes/atlas/countydata/atlas.html (accessed on 30 January 2020).
- Yadav, D.; Lowenfels, A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1252–1561. [Google Scholar] [CrossRef] [Green Version]
- Raimondi, S.; Lowenfels, A.B.; Morselli-Labate, A.M.; Maisonneuve, P.; Pezzilli, R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 349–358. [Google Scholar] [CrossRef]
- Choudhary, P.; Rickels, M.R.; Senior, P.A.; Vantyghem, M.C.; Maffi, P.; Kay, T.W.; Keymeulen, B.; Inagaki, N.; Saudek, F.; Lehmann, R.; et al. Evidence-informed clinical practice recommendations for treatment of type 1 diabetes complicated by problematic hypoglycemia. Diabetes Care 2015, 38, 1016–1029. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.M.; Kim, K.W. Is islet transplantation a realistic approach to curing diabetes? Korean J. Intern. Med. 2017, 32, 62. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.G.; Vatamaniuk, M.Z. Two tales of antioxidant enzymes on β cells and diabetes. Antioxid. Redox. Signal. 2011, 14, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Lenzen, S.; Drinkgern, J.; Tiedge, M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 1996, 20, 463–466. [Google Scholar] [CrossRef]
- Acharya, J.D.; Ghaskadbi, S.S. Islets and their antioxidant defense. Islets 2010, 2, 225–235. [Google Scholar] [CrossRef]
- Barton, F.B.; Rickels, M.R.; Alejandro, R.; Hering, B.J.; Wease, S.; Naziruddin, B.; Oberholzer, J.; Odorico, J.S.; Garfinkel, M.R.; Levy, M.; et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 2012, 35, 1436–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellin, M.D.; Barton, F.B.; Heitman, A.; Harmon, J.V.; Kandaswamy, R.; Balamurugan, A.N.; Sutherland, D.E.; Alejandro, R.; Hering, B.J. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am. J. Transplant. 2012, 12, 1576–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, T.B.; Kirchner, V.; Bellin, M.D. Beta-cell replacement therapy: Current outcomes and future landscape. Curr. Opin. Organ Transplant. 2015, 20, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Kojayan, G.G.; Alexander, M.; Imagawa, D.K.; Lakey, J.R. Systematic review of islet cryopreservation. Islets 2018, 10, 40–49. [Google Scholar] [CrossRef]
- Al-Adra, D.P.; Gill, R.S.; Imes, S.; O’Gorman, D.; Kin, T.; Axford, S.J.; Shi, X.; Senior, P.A.; Shapiro, A.J. Single-donor islet transplantation and long-term insulin independence in select patients with type 1 diabetes mellitus. Transplantation 2014, 98, 1007–1012. [Google Scholar] [CrossRef]
- Özmen, L.; Ekdahl, K.N.; Elgue, G.; Larsson, R.; Korsgren, O.; Nilsson, B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: Possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes 2002, 51, 1779–1784. [Google Scholar] [CrossRef] [Green Version]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Itoh, K.; Igarashi, K.; Hayashi, N.; Nishizawa, M.; Yamamoto, M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol. Cell. Biol. 1995, 15, 4184–4193. [Google Scholar] [CrossRef] [Green Version]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, A.; Kang, M.I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, H.; Katsuoka, F.; Motohashi, H.; Engel, J.D.; Yamamoto, M. Embryonic lethality and fetal liver apoptosis in mice lacking all three small Maf proteins. Mol. Cell. Biol. 2012, 32, 808–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirotsu, Y.; Katsuoka, F.; Funayama, R.; Nagashima, T.; Nishida, Y.; Nakayama, K.; Douglas Engel, J.; Yamamoto, M. Nrf2–MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucleic Acids Res. 2012, 40, 10228–10239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [Green Version]
- Sigfrid, L.A.; Cunningham, J.M.; Beeharry, N.; Lortz, S.; Tiedge, M.; Lenzen, S.; Carlsson, C.; Green, I.C. Cytokines and nitric oxide inhibit the enzyme activity of catalase but not its protein or mRNA expression in insulin-producing cells. J. Mol. Endocrinol. 2003, 31, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Yagishita, Y.; Fukutomi, T.; Sugawara, A.; Kawamura, H.; Takahashi, T.; Pi, J.; Uruno, A.; Yamamoto, M. Nrf2 protects pancreatic β-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes 2014, 63, 605–618. [Google Scholar] [CrossRef] [Green Version]
- Aminzadeh, M.A.; Reisman, S.A.; Vaziri, N.D.; Khazaeli, M.; Yuan, J.; Meyer, C.J. The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores Nrf2 activity and attenuates oxidative stress, inflammation, and fibrosis in rats with chronic kidney disease. Xenobiotica 2014, 44, 570–578. [Google Scholar] [CrossRef] [Green Version]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Nishino, H.; Nishino, A.; Takayasu, J.; Hasegawa, T.; Iwashima, A.; Hirabayashi, K.; Iwata, S.; Shibata, S. Inhibition of the tumor-promoting action of 12-O-tetradecanoylphorbol-13-acetate by some oleanane-type triterpenoid compounds. Cancer Res. 1988, 48, 5210–5215. [Google Scholar]
- Huang, M.T.; Ho, C.T.; Wang, Z.Y.; Ferraro, T.; Lou, Y.R.; Stauber, K.; Ma, W.; Georgiadis, C.; Laskin, J.D.; Conney, A.H. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994, 54, 701–708. [Google Scholar]
- Yates, M.S.; Kwak, M.K.; Egner, P.A.; Groopman, J.D.; Bodreddigari, S.; Sutter, T.R.; Baumgartner, K.J.; Roebuck, B.D.; Liby, K.T.; Yore, M.M.; et al. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-, 12-dioxooleana-1, 9 (11)-dien-28-oyl] imidazole. Cancer Res. 2006, 66, 2488–2494. [Google Scholar] [CrossRef] [Green Version]
- Dinkova-Kostova, A.T.; Liby, K.T.; Stephenson, K.K.; Holtzclaw, W.D.; Gao, X.; Suh, N.; Williams, C.; Risingsong, R.; Honda, T.; Gribble, G.W.; et al. Extremely potent triterpenoid inducers of the phase 2 response: Correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. USA 2005, 102, 4584–4589. [Google Scholar] [CrossRef] [Green Version]
- Robles, L.; Vaziri, N.D.; Li, S.; Masuda, Y.; Takasu, C.; Takasu, M.; Vo, K.; Farzaneh, S.H.; Stamos, M.J.; Ichii, H. The Synthetic Triterpenoid RTA dh404 (CDDO-dhTFEA) Ameliorates Acute Pancreatitis. Pancreas 2016, 45, 720. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, T.; Li, J.; Meyer, C.J.; Janicki, J.S.; Hannink, M.; Cui, T. Dihydro-CDDO-trifluoroethyl amide (dh404), a novel Nrf2 activator, suppresses oxidative stress in cardiomyocytes. PLoS ONE 2009, 4, e8391. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Wu, W.; Song, H.; Wang, F.; Li, H.; Chen, L.; Lai, Y.; Janicki, J.S.; Ward, K.W.; Meyer, C.J.; et al. Targeting Nrf2 by dihydro-CDDO-trifluoroethyl amide enhances autophagic clearance and viability of β-cells in a setting of oxidative stress. FEBS Lett. 2014, 588, 2115–2124. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.N. Autophagy as a second level protective process in conferring resistance to environmentally-induced oxidative stress. Autophagy 2008, 4, 254–256. [Google Scholar] [CrossRef] [Green Version]
- Held, K.D.; Epp, E.R.; Clark, E.P.; Biaglow, J.E. Effect of dimethyl fumarate on the radiation sensitivity of mammalian cells in vitro. Radiat Res. 1988, 115, 495–502. [Google Scholar] [CrossRef]
- Saidu, N.E.; Kavian, N.; Leroy, K.; Jacob, C.; Nicco, C.; Batteux, F.; Alexandre, J. Dimethyl fumarate, a two-edged drug: Current status and future directions. Med. Res. Rev. 2019, 39, 1923–1952. [Google Scholar] [CrossRef]
- Robles, L.; Vaziri, N.D.; Li, S.; Takasu, C.; Masuda, Y.; Vo, K.; Farzaneh, S.H.; Stamos, M.J.; Ichii, H. Dimethyl fumarate ameliorates acute pancreatitis in rodent. Pancreas 2015, 44, 441. [Google Scholar] [CrossRef] [Green Version]
- Robles, L.; Vaziri, N.D.; Li, S.; Masuda, Y.; Takasu, C.; Takasu, M.; Vo, K.; Farzaneh, S.H.; Stamos, M.J.; Ichii, H. Dimethyl fumarate protects pancreatic islet cells and non-endocrine tissue in L-arginine-induced chronic pancreatitis. PLoS ONE 2014, 9, e107111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.X.; Zhao, J.H.; Ping, F.M.; Liu, Z.J.; Gu, J.X.; Lu, X.Q. Effect of dimethyl fumarate on rats with chronic pancreatitis. Asian Pac. J. Trop Biomed. 2016, 9, 261–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultheis, J.; Beckmann, D.; Mulac, D.; Müller, L.; Esselen, M.; Düfer, M. Nrf2 Activation Protects Mouse Beta Cells from Glucolipotoxicity by Restoring Mitochondrial Function and Physiological Redox Balance. Oxid. Med. Cell. Longev. 2019, 2019, 7518510. [Google Scholar] [CrossRef]
- Loewe, R.; Holnthoner, W.; Gröger, M.; Pillinger, M.; Gruber, F.; Mechtcheriakova, D.; Hofer, E.; Wolff, K.; Petzelbauer, P. Dimethylfumarate inhibits TNF-induced nuclear entry of NF-κB/p65 in human endothelial cells. J. Immunol 2002, 168, 4781–4787. [Google Scholar] [CrossRef]
- Lehmann, J.C.; Listopad, J.J.; Rentzsch, C.U.; Igney, F.H.; von Bonin, A.; Hennekes, H.H.; Asadullah, K.; Docke, W.D. Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1. J. Investig. Dermatol. 2007, 127, 835–845. [Google Scholar] [CrossRef] [Green Version]
- Linker, R.A.; Lee, D.H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011, 134, 678–692. [Google Scholar] [CrossRef] [Green Version]
- Scannevin, R.H.; Chollate, S.; Jung, M.Y.; Shackett, M.; Patel, H.; Bista, P.; Zeng, W.; Ryan, S.; Yamamoto, M.; Lukashev, M.; et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J. Pharmacol. Exp. Ther. 2012, 341, 274–284. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, M.; Suzuki, K.; Toda, M.; Okubo, S.; Hara, Y.; Shimamura, T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antivir. Res. 1993, 21, 289–299. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, Z.Y. Tea and cancer. J. Natl. Cancer Inst. 1993, 85, 1038–1049. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Mukhtar, H. Tea antioxidants in cancer chemoprevention. J. Cell. Biochem. 1997, 67, 59–67. [Google Scholar] [CrossRef]
- Broadhurst, C.L.; Polansky, M.M.; Anderson, R.A. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J. Agric. Food Chem. 2000, 48, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Saito, Y.; Mori, H.; Takasu, C.; Komatsu, M.; Hanaoka, J.; Yamada, S.; Asanoma, M.; Ikemoto, T.; Imura, S.; Morine, Y.; et al. Beneficial effects of green tea catechin on massive hepatectomy model in rats. J. Gastroenterol. 2014, 49, 692–701. [Google Scholar] [CrossRef]
- Han, M.K. Epigallocatechin gallate, a constituent of green tea, suppresses cytokine-induced pancreatic β-cell damage. Exp. Mol. Med. 2003, 35, 136–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, E.K.; Hur, H.; Han, M.K. Epigallocatechin gallate prevents autoimmune diabetes induced by multiple low doses of streptozotocin in mice. Arch. Pharm. Res. 2003, 26, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Jeon, H.J.; Park, J.; Chang, M.S. Epigallocatechin-3-gallate prevents oxidative stress-induced cellular senescence in human mesenchymal stem cells via Nrf2. Int. J. Mol. Med. 2016, 38, 1075–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreadi, C.K.; Howells, L.M.; Atherfold, P.A.; Manson, M.M. Involvement of Nrf2, p38, B-Raf, and nuclear factor-κB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol. Pharm. 2006, 69, 1033–1040. [Google Scholar] [CrossRef]
- Xu, C.; Li, C.Y.; Kong, A.N. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 2005, 28, 249. [Google Scholar] [CrossRef]
- Hong, J.; Lu, H.; Meng, X.; Ryu, J.H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar]
- Lablanche, S.; Cottet-Rousselle, C.; Argaud, L.; Laporte, C.; Lamarche, F.; Richard, M.J.; Berney, T.; Benhamou, P.Y.; Fontaine, E. Respective effects of oxygen and energy substrate deprivation on beta cell viability. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 629–639. [Google Scholar] [CrossRef]
- Li, S.; Vaziri, N.D.; Masuda, Y.; Hajighasemi-Ossareh, M.; Robles, L.; Le, A.; Vo, K.; Chan, J.Y.; Foster, C.E.; Stamos, M.J.; et al. Pharmacological activation of Nrf2 pathway improves pancreatic islet isolation and transplantation. Cell Transplant. 2015, 24, 2273–2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, Y.; Vaziri, N.D.; Li, S.; Le, A.; Hajighasemi-Ossareh, M.; Robles, L.; Foster, C.E.; Stamos, M.J.; Al-Abodullah, I.; Ricordi, C.; et al. The effect of Nrf2 pathway activation on human pancreatic islet cells. PLoS ONE 2015, 10, e0131012. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Jang, H.J.; Oh, M.Y.; Lee, J.H.; Kang, K.S. Tetrahydrocurcumin enhances islet cell function and attenuates apoptosis in mouse islets. Transplant. Proc. 2018, 50, 2847–2853. [Google Scholar] [CrossRef]
- Luo, D.D.; Chen, J.F.; Liu, J.J.; Xie, J.H.; Zhang, Z.B.; Gu, J.Y.; Zhuo, J.Y.; Huang, S.; Su, Z.R.; Sun, Z.H. Tetrahydrocurcumin and octahydrocurcumin, the primary and final hydrogenated metabolites of curcumin, possess superior hepatic-protective effect against acetaminophen-induced liver injury: Role of CYP2E1 and Keap1-Nrf2 pathway. Food Chem. Toxicol. 2019, 123, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janjic, D.; Andereggen, E.; Deng, S.; Bartley, C.; Buhler, L.; Morel, P.; Wollheim, C.B. Improved insulin secretion of cryopreserved human islets by antioxidant treatment. Pancreas 1996, 13, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Kanitkar, M.; Bhonde, R.R. Curcumin treatment enhances islet recovery by induction of heat shock response proteins, Hsp70 and heme oxygenase-1, during cryopreservation. Life Sci. 2008, 82, 182–189. [Google Scholar] [CrossRef]
- Brandhorst, H.; Iken, M.; Scott, I.I.I.W.E.; Papas, K.K.; Theisinger, B.; Johnson, P.R.; Korsgren, O.; Brandhorst, D. Quality of isolated pig islets is improved using perfluorohexyloctane for pancreas storage in a split lobe model. Cell Transplant. 2013, 22, 1477–1484. [Google Scholar] [CrossRef] [Green Version]
- Brandhorst, H.; Theisinger, B.; Guenther, B.; Johnson, P.R.; Brandhorst, D. Pancreatic L-glutamine administration protects pig islets from cold ischemic injury and increases resistance toward inflammatory mediators. Cell Transplant. 2016, 25, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Avila, J.; Barbaro, B.; Gangemi, A.; Romagnoli, T.; Kuechle, J.; Hansen, M.; Shapiro, J.; Testa, G.; Sankary, H.; Benedetti, E.; et al. Intra-ductal glutamine administration reduces oxidative injury during human pancreatic islet isolation. Am. J. Transplant. 2005, 5, 2830–2837. [Google Scholar] [CrossRef]
- Avila, J.G.; Tsujimura, T.; Oberholzer, J.; Churchill, T.; Salehi, P.; Shapiro, A.J.; Lakey, J.R. Improvement of pancreatic islet isolation outcomes using glutamine perfusion during isolation procedure. Cell Transplant. 2003, 12, 877–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amores-Sánchez, M.I.; Medina, M.Á. Glutamine, as a precursor of glutathione, and oxidative stress. Mol. Genet. Metab. 1999, 67, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayin, V.I.; LeBoeuf, S.E.; Singh, S.X.; Davidson, S.M.; Biancur, D.; Guzelhan, B.S.; Alvarez, S.W.; Wu, W.L.; Karakousi, T.R.; Zavitsanou, A.M.; et al. Activation of the NRF2 antioxidant program generates an imbalance in central carbon metabolism in cancer. Elife 2017, 6, e28083. [Google Scholar] [CrossRef] [PubMed]
- Kenmochi, T.; Miyamoto, M.; Une, S.; Nakagawa, Y.; Moldovan, S.; Navarro, R.A.; Benhamou, P.Y.; Brunicardi, F.C.; Mullen, Y. Improved quality and yield of islets isolated from human pancreata using a two-step digestion method. Pancreas 2000, 20, 184–190. [Google Scholar] [CrossRef]
- Ito, T.; Omori, K.; Rawson, J.; Todorov, I.; Asari, S.; Kuroda, A.; Shintaku, J.; Itakura, S.; Ferreri, K.; Kandeel, F.; et al. Improvement of canine islet yield by donor pancreas infusion with a p38MAPK inhibitor. Transplantation 2008, 86, 321. [Google Scholar] [CrossRef] [Green Version]
- Naidu, S.; Vijayan, V.; Santoso, S.; Kietzmann, T.; Immenschuh, S. Inhibition and genetic deficiency of p38 MAPK up-regulates heme oxygenase-1 gene expression via Nrf2. J. Immunol. 2009, 182, 7048–7057. [Google Scholar] [CrossRef] [Green Version]
- Naziruddin, B.; Iwahashi, S.; Kanak, M.A.; Takita, M.; Itoh, T.; Levy, M.F. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am. J. Transplant. 2014, 14, 428–437. [Google Scholar] [CrossRef]
- Vajkoczy, P.; Menger, M.D.; Simpson, E.; Messmer, K. Angiogenesis and vascularization of murine pancreatic islet isografts. Transplantation 1995, 60, 123–127. [Google Scholar] [CrossRef]
- Muthyala, S.; Safley, S.; Gordan, K.; Barber, G.; Weber, C.; Sambanis, A. The effect of hypoxia on free and encapsulated adult porcine islets—an in vitro study. Xenotransplant 2017, 24. [Google Scholar] [CrossRef] [Green Version]
- Eich, T.; Eriksson, O.; Lundgren, T. Visualization of early engraftment in clinical islet transplantation by positron-emission tomography. N. Engl. J. Med. 2007, 356, 2754–2755. [Google Scholar] [CrossRef]
- Eich, T.; Eriksson, O.; Sundin, A.; Estrada, S.; Brandhorst, D.; Brandhorst, H.; Langstrom, B.; Nilsson, B.; Korsgren, O.; Lundgren, T. Positron emission tomography: A real-time tool to quantify early islet engraftment in a preclinical large animal model. Transplantation 2007, 84, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Saudek, F.; Jirák, D.; Girman, P.; Herynek, V.; Dezortová, M.; Kríž, J.; Peregrin, J.; Berková, Z.; Zacharovová, K.; Hájek, M. Magnetic resonance imaging of pancreatic islets transplanted into the liver in humans. Transplantation 2010, 90, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Jirak, D.; Kriz, J.; Strzelecki, M.; Yang, J.; Hasilo, C.; White, D.J.; Foster, P.J. Monitoring the survival of islet transplants by MRI using a novel technique for their automated detection and quantification. MAGMA 2009, 22, 257–265. [Google Scholar] [CrossRef]
- Kosinova, L.; Patikova, A.; Jirak, D.; Galisova, A.; Vojtiskova, A.; Saudek, F.; Kriz, J. A novel model for in vivo quantification of immediate liver perfusion impairment after pancreatic islet transplantation. Islets 2019, 11, 129–140. [Google Scholar] [CrossRef]
- Wada, Y.; Takata, A.; Ikemoto, T.; Morine, Y.; Imura, S.; Iwahashi, S.; Saito, Y.; Shimada, M. The protective effect of epigallocatechin 3-gallate on mouse pancreatic islets via the Nrf2 pathway. Surg. Today 2019, 49, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Takasu, C.; Vaziri, N.D.; Li, S.; Robles, L.; Vo, K.; Takasu, M.; Pham, C.; Farzaneh, S.H.; Shimada, M.; Stamos, M.J.; et al. Treatment with dimethyl fumarate ameliorates liver ischemia/reperfusion injury. World J. Gastroenterol. 2017, 23, 4508. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Kang, N.I.; Lee, H.K.; Jang, K.Y.; Park, J.W.; Park, B.H. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem. Pharmacol. 2008, 75, 2214–2223. [Google Scholar] [CrossRef]
- Takasu, C.; Vaziri, N.D.; Li, S.; Robles, L.; Vo, K.; Takasu, M.; Pham, C.; Liu, S.; Farzaneh, S.H.; Foster, C.E., 3rd; et al. Treatment with dimethyl fumarate attenuates calcineurin inhibitor-induced nephrotoxicity. Transplantation 2015, 99, 1144. [Google Scholar] [CrossRef]
- Saidu, N.E.; Noé, G.; Cerles, O.; Cabel, L.; Kavian-Tessler, N.; Chouzenoux, S.; Bahuaud, M.; Chéreau, C.; Nicco, C.; Leroy, K.; et al. Dimethyl fumarate controls the NRF2/DJ-1 axis in cancer cells: Therapeutic applications. Mol. Cancer Ther. 2017, 16, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Saidu, N.E.; Bretagne, M.; Mansuet, A.L.; Just, P.A.; Leroy, K.; Cerles, O.; Chouzenoux, S.; Nicco, C.; Damotte, D.; Alifano, M.; et al. Dimethyl fumarate is highly cytotoxic in KRAS mutated cancer cells but spares non-tumorigenic cells. Oncotarget 2018, 9, 9088. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and inhibitors of NRF2: A review of their potential for clinical development. Oxid. Med. Cell. Longev. 2019, 9372182. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Sireesh, D.; Rajaguru, P.; Paulmurugan, R.; Ramkumar, K.M. The emerging role of redox-sensitive Nrf2–Keap1 pathway in diabetes. Pharm. Res. 2015, 91, 104–114. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarrin Lopez, A.; Lau, H.; Li, S.; Ichii, H. Potential Benefits of Nrf2/Keap1 Targeting in Pancreatic Islet Cell Transplantation. Antioxidants 2020, 9, 321. https://doi.org/10.3390/antiox9040321
Jarrin Lopez A, Lau H, Li S, Ichii H. Potential Benefits of Nrf2/Keap1 Targeting in Pancreatic Islet Cell Transplantation. Antioxidants. 2020; 9(4):321. https://doi.org/10.3390/antiox9040321
Chicago/Turabian StyleJarrin Lopez, Alberto, Hien Lau, Shiri Li, and Hirohito Ichii. 2020. "Potential Benefits of Nrf2/Keap1 Targeting in Pancreatic Islet Cell Transplantation" Antioxidants 9, no. 4: 321. https://doi.org/10.3390/antiox9040321
APA StyleJarrin Lopez, A., Lau, H., Li, S., & Ichii, H. (2020). Potential Benefits of Nrf2/Keap1 Targeting in Pancreatic Islet Cell Transplantation. Antioxidants, 9(4), 321. https://doi.org/10.3390/antiox9040321



