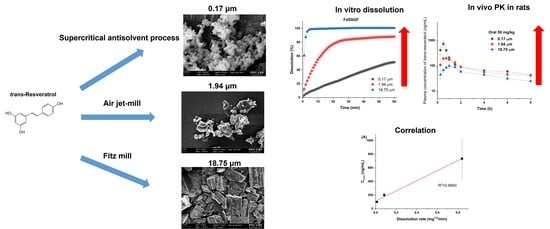
Pure Trans-Resveratrol Nanoparticles Prepared by a Supercritical Antisolvent Process Using Alcohol and Dichloromethane Mixtures: Effect of Particle Size on Dissolution and Bioavailability in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Study of Trans-Resveratrol in the Alcohol and Dichloromethane Mixtures
2.3. Preparation of Pure Trans-Resveratrol Nanoparticles Using the SAS Process
2.4. Particle Size Reduction of Trans-Resveratrol Using Fitz Mill or Air Jet-Mill
2.5. Analysis of Trans-Resveratrol Purity and Residual Solvent
2.6. Solid-State Characterizations
2.6.1. Scanning Electron Microscopy
2.6.2. Particle Size Measurements
2.6.3. Specific Surface Area Measurements
2.6.4. Differential Scanning Calorimetry (DSC) Analysis
2.6.5. Powder X-Ray Diffraction (PXRD) Analysis
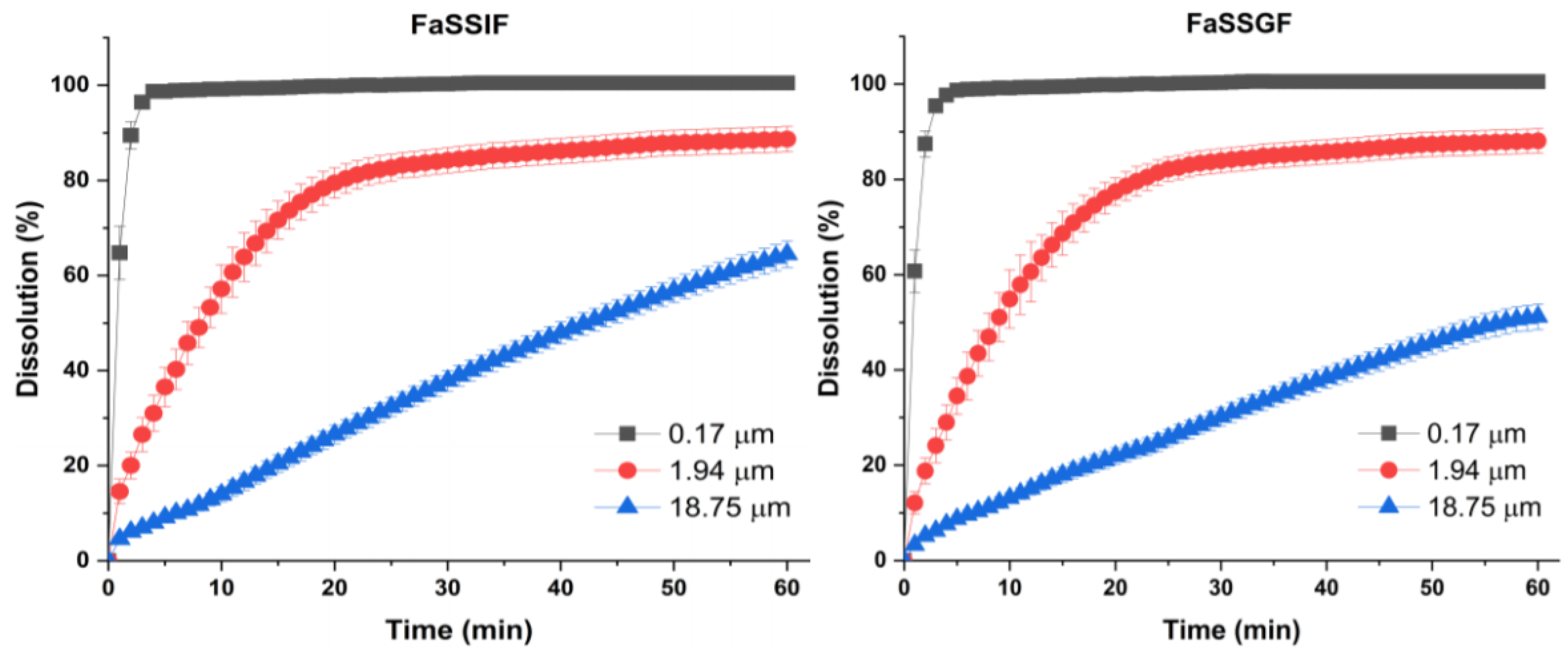
2.7. Dissolution Study
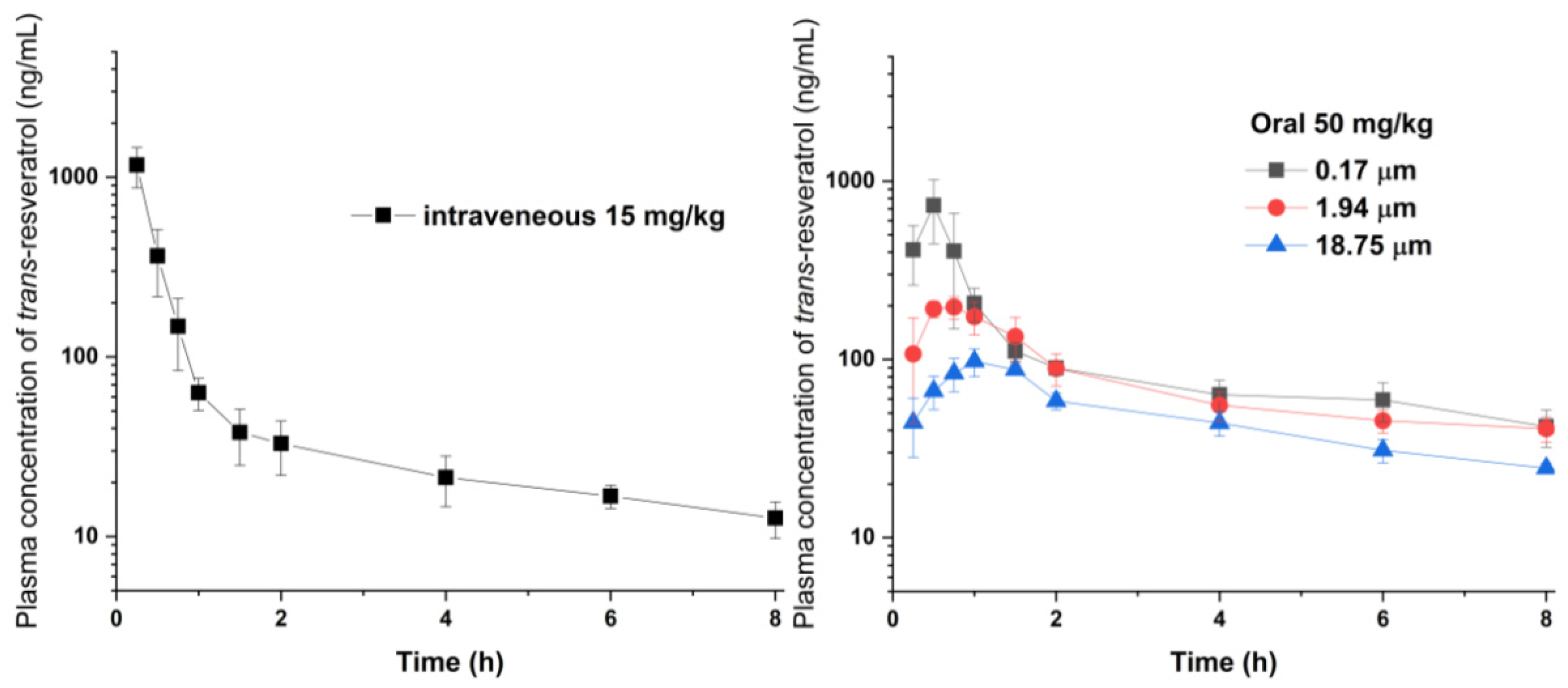
2.8. Pharmacokinetic Study in Rats
2.9. Data Analysis
3. Results and Discussion
3.1. Pure Trans-Resveratrol Nanoparticles Prepared by the SAS Process
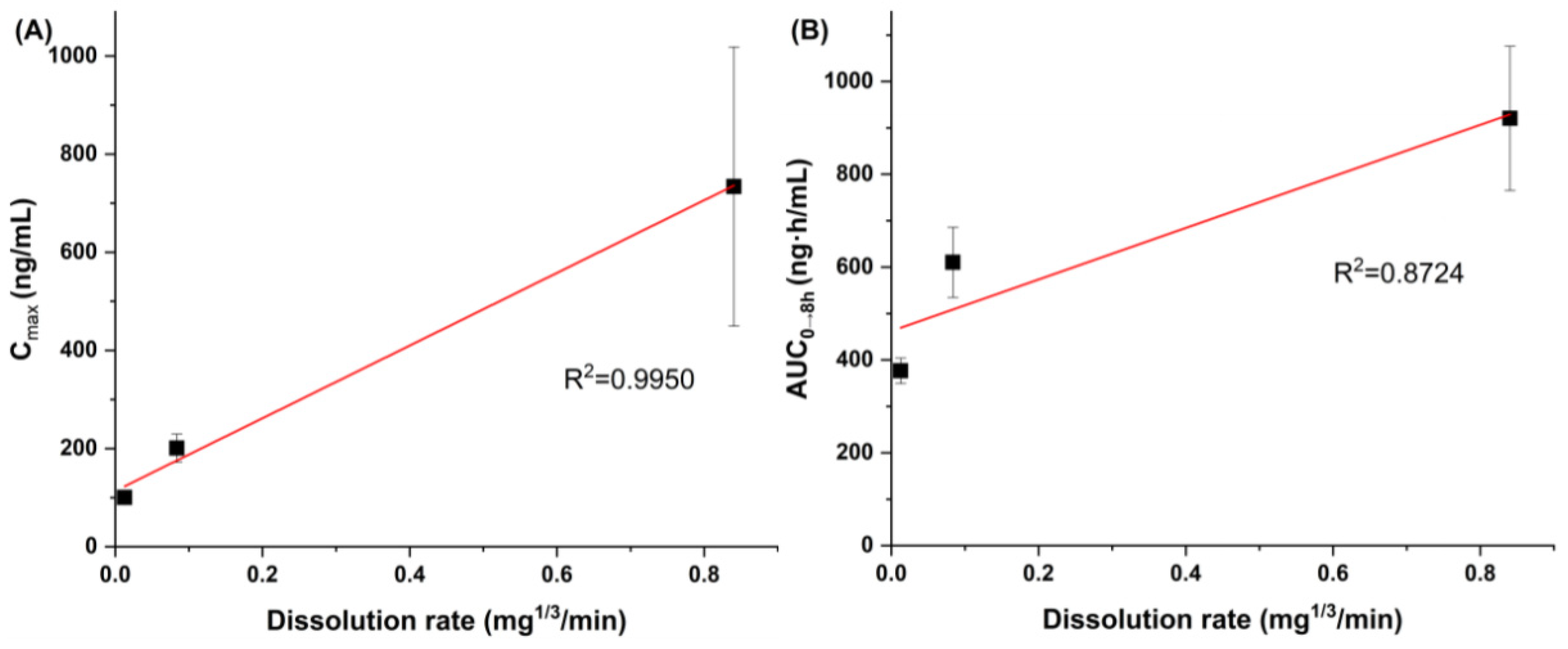
3.2. Effect of Particle Size on Dissolution and Oral Bioavailability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Health effects of resveratrol: Results from human intervention trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef]
- Na, J.-I.; Shin, J.-W.; Choi, H.-R.; Kwon, S.-H.; Park, K.-C. Resveratrol as a multifunctional topical hypopigmenting agent. Int. J. Mol. Sci. 2019, 20, 956. [Google Scholar] [CrossRef]
- Fonseca, J.; Moradi, F.; Valente, A.J.F.; Stuart, J.A. Oxygen and glucose levels in cell culture media determine resveratrol’s effects on growth, hydrogen peroxide production, and mitochondrial dynamics. Antioxidants 2018, 7, 157. [Google Scholar] [CrossRef]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to improve oral bioavailability and beneficial effects of resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, M.; Ye, J.-H.; Zheng, X.-Q.; Lu, J.-L.; Liang, Y.-R. Photo-induced chemical reaction of trans-resveratrol. Food Chem. 2015, 171, 137–143. [Google Scholar] [CrossRef]
- Marier, J.F.; Vachon, P.; Gritsas, A.; Zhang, J.; Moreau, J.P.; Ducharme, M.P. Metabolism and disposition of resveratrol in rats: Extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J. Pharmacol. Exp. Ther. 2002, 302, 369–373. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. Npj Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Ha, E.-S.; Sim, W.-Y.; Lee, S.-K.; Jeong, J.-S.; Kim, J.-S.; Baek, I.-H.; Choi, D.H.; Park, H.; Hwang, S.-J.; Kim, M.-S. Preparation and evaluation of resveratrol-loaded composite nanoparticles using a supercritical fluid technology for enhanced oral and skin delivery. Antioxidants 2019, 8, 554. [Google Scholar] [CrossRef]
- Mamadou, G.; Charrueau, C.; Dairou, J.; Nzouzi, N.L.; Eto, B.; Ponchel, G. Increased intestinal permeation and modulation of presystemic metabolism of resveratrol formulated into self-emulsifying drug delivery systems. Int. J. Pharm. 2017, 521, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to improve resveratrol systemic and topical bioavailability: An update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef]
- Aguiar, G.P.S.; Boschetto, D.L.; Chaves, L.M.P.C.; Arcari, B.D.; Piato, A.L.; Oliveira, J.V.; Lanza, M. Trans-resveratrol micronization by SEDS technique. Ind. Crops Prod. 2016, 89, 350–355. [Google Scholar] [CrossRef]
- Zu, Y.; Zhang, Y.; Wang, W.; Zhao, X.; Han, X.; Wang, K.; Ge, Y. Preparation and in vitro/in vivo evaluation of resveratrol-loaded carboxymethyl chitosan nanoparticles. Drug Deliv. 2016, 23, 981–991. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Matencio, A.; Caldera, F.; Argenziano, M.; Cavalli, R.; Dianzani, C.; Zanetti, M.; López-Nicolás, J.M.; Trotta, F. Comparative evaluation of solubility, cytotoxicity and photostability studies of resveratrol and oxyresveratrol loaded nanosponges. Pharmaceutics 2019, 11, 545. [Google Scholar] [CrossRef]
- Kuk, D.-H.; Ha, E.-S.; Ha, D.-H.; Sim, W.-Y.; Lee, S.-K.; Jeong, J.-S.; Kim, J.-S.; Baek, I.-H.; Park, H.; Choi, D.H.; et al. Development of a resveratrol nanosuspension using the antisolvent precipitation method without solvent removal, based on a quality by design (QbD) approach. Pharmaceutics 2019, 11, 688. [Google Scholar] [CrossRef]
- He, H.; Zhang, Q.; Wang, J.-R.; Mei, X. Structure, physicochemical properties and pharmacokinetics of resveratrol and piperine coparticles. Cryst. Eng. Comm. 2017, 19, 6154–6163. [Google Scholar] [CrossRef]
- Shimojo, A.A.M.; Fernandes, A.R.V.; Ferreira, N.R.E.; Sanchez-Lopez, E.; Santana, M.H.A.; Souto, E.B. Evaluation of the influence of process parameters on the properties of resveratrol-loaded NLC using 22 full factorial design. Antioxidants 2019, 8, 272. [Google Scholar] [CrossRef]
- Aguiar, G.P.S.; Arcari, B.D.; Chaves, L.M.P.C.; Magro, C.D.; Boschetto, D.L.; Piato, A.L.; Lanza, M.; Oliveira, J.V. Micronization of trans-resveratrol by supercritical fluid: Dissolution, solubility and in vitro antioxidant activity. Ind. Crops Prod. 2018, 112, 1–5. [Google Scholar] [CrossRef]
- Amiot, M.J.; Romier, B.; Dao, T.M.; Fanciullino, R.; Ciccolini, J.; Burcelin, R.; Pechere, L.; Emond, C.; Savouret, J.F.; Seree, E. Optimization of trans-resveratrol bioavailability for human therapy. Biochimie 2013, 95, 1233–1238. [Google Scholar] [CrossRef]
- Calvo-Castro, L.A.; Schiborr, C.; David, F.; Ehrt, H.; Voggel, J.; Sus, N.; Behnam, D.; Bosy-Westphal, A.; Frank, J. The oral bioavailability of trans-resveratrol from a grapevine-shoot extract in healthy humans is significantly increased by micellar solubilization. Mol. Nutr. Food Res. 2018, 62, 1701057. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—Safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, B.; Zier, K.-I.; Khalid, N.; Azarmi, S.; Löbenberg, R. Evaluation of a microemulsion-based gel formulation for topical drug delivery of diclofenac sodium. J. Pharm. Investig. 2018, 48, 351–362. [Google Scholar] [CrossRef]
- Liu, X.; Feng, X.; Williams III, R.O.; Zhang, F. Characterization of amorphous solid dispersions. J. Pharm. Investig. 2018, 48, 19–41. [Google Scholar] [CrossRef]
- Ma, X.; Williams, R.O., III. Polymeric nanomedicines for poorly soluble drugs in oral delivery systems: An update. J. Pharm. Investig. 2018, 48, 61–75. [Google Scholar]
- Singh, D.; Bedi, N.; Tiwary, A.K. Enhancing solubility of poorly aqueous soluble drugs: Critical appraisal of techniques. J. Pharm. Investig. 2018, 48, 509–526. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanoparticles of poorly soluble drugs: Drug bioavailability and physicochemical stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, L.; Xing, Y.; Zhao, Y.; Wang, Z.; Han, J. Enhanced oral bioavailability of celecoxib nanoparticleline solid dispersion based on wet media milling technique: Formulation, optimization and in vitro/in vivo evaluation. Pharmaceutics 2019, 11, 328. [Google Scholar] [CrossRef]
- Kim, M.-S.; Jin, S.-J.; Kim, J.-S.; Park, H.J.; Song, H.S.; Neubert, R.H.H.; Hwang, S.-J. Preparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical antisolvent (SAS) process. Eur. J. Pharm. Biopharm. 2008, 69, 454–465. [Google Scholar] [CrossRef]
- Ha, E.-S.; Kim, J.-S.; Lee, S.-K.; Sim, W.-Y.; Jeong, J.-S.; Kim, M.-S. Solubility and modeling of telmisartan in binary solvent mixtures of dichloromethane and (methanol, ethanol, n-propanol, or n-butanol) and its application to the preparation of nanoparticles using the supercritical antisolvent technique. J. Mol. Liq. 2019, 295, 111710. [Google Scholar] [CrossRef]
- Ha, E.-S.; Kim, J.-S.; Lee, S.-K.; Sim, W.-Y.; Jeong, J.-S.; Kim, M.-S. Equilibrium solubility and solute-solvent interactions of carvedilol (Form I) in twelve mono solvents and its application for supercritical antisolvent precipitation. J. Mol. Liq. 2019, 294, 111622. [Google Scholar] [CrossRef]
- Ha, E.-S.; Kuk, D.-H.; Kim, J.-S.; Kim, M.S. Solubility of trans-resveratrol in transcutol HP + water mixtures at different temperatures and its application to fabrication of nanosuspensions. J. Mol. Liq. 2019, 281, 344–351. [Google Scholar] [CrossRef]
- Ha, E.-S.; Lee, Y.-R.; Kim, M.-S. Solubility of dronedarone hydrochloride in six pure solvents at the range of 298.15 to 323.15K. J. Mol. Liq. 2016, 216, 360–363. [Google Scholar] [CrossRef]
- Ha, E.-S.; Lee, S.-K.; Jeong, J.-S.; Sim, W.-Y.; Yang, J.-I.; Kim, J.-S.; Kim, M.-S. Solvent effect and solubility modeling of rebamipide in twelve solvents at different temperatures. J. Mol. Liq. 2019, 288, 111041. [Google Scholar] [CrossRef]
- Ha, E.-S.; Choo, G.-H.; Baek, I.-H.; Kim, M.-S. Formulation, characterization, and in vivo evaluation of celecoxib-PVP solid dispersion nanoparticles using supercritical antisolvent process. Molecules 2014, 19, 20325–20339. [Google Scholar] [CrossRef]
- Kim, M.-S.; Ha, E.-S.; Kim, J.-S.; Baek, I.; Yoo, J.-W.; Jung, Y.; Moon, H.R. Development of megestrol acetate solid dispersion nanoparticles for enhanced oral delivery by using a supercritical antisolvent process. Drug Des. Devel. Ther. 2015, 9, 4269–4277. [Google Scholar] [CrossRef]
- Ha, E.-S.; Choo, G.-H.; Baek, I.-H.; Kim, J.-S.; Cho, W.; Jung, Y.S.; Jin, S.-E.; Hwang, S.-J.; Kim, M.-S. Dissolution and bioavailability of lercanidipine-hydroxypropylmethyl cellulose nanoparticles with surfactant. Int. J. Biol. Macromol. 2015, 72, 188–222. [Google Scholar] [CrossRef]
- Ramos, C. Development and validation of a headspace gas chromatographic method for determination of residual solvents in five drug substances. Int. J. Pharm. Sci. 2013, 2, 36–41. [Google Scholar]
- Das, S.; Lin, H.S.; Ho, P.C.; Ng, K.Y. The impact of aqueous solubility and dose on the pharmacokinetic profiles of resveratrol. Pharm Res. 2008, 25, 2593–2600. [Google Scholar] [CrossRef]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Das, S.; Ng, K.Y. Quantification of trans-resveratrol in rat plasma by a simple and sensitive high performance liquid chromatography method and its application in pre-clinical study. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1399–1414. [Google Scholar] [CrossRef]
- Patil, S.C.; Tagalpallewar, A.A.l; Kokare, C.R. Natural anti-proliferative agent loaded self-microemulsifying nanoparticles for potential therapy in oral squamous. J. Pharm. Investig. 2019, 49, 527–541. [Google Scholar] [CrossRef]
- Naik, J.B.; Waghulde, M.R. Development of vildagliptin loaded Eudragit® microspheres by screening design: In vitro evaluation. J. Pharm. Investig. 2018, 48, 627–637. [Google Scholar] [CrossRef]
- De Marco, I.; Rossmann, M.; Prosapio, V.; Reverchon, E.; Braeuer, A. Control of particle size, at micrometric and nanometric range, using supercritical antisolvent precipitation from solvent mixtures: Application to PVP. Chem. Eng. J. 2015, 273, 344–352. [Google Scholar] [CrossRef]
- De Marco, I.; Prosapio, V.; Cice, F.; Reverchon, E. Use of solvent mixtures in supercritical antisolvent process to modify precipitates morphology: Cellulose acetate microparticles. J. Supercrit. Fluids 2013, 83, 153–160. [Google Scholar] [CrossRef]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Euro. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Baek, I.; Kim, J.-S.; Ha, E.-S.; Choo, G.-H.; Cho, W.; Hwamg, S.-J.; Kim, M.-S. Dissolution and oral absorption of pranlukast nanosuspensions stabilized by hydroxypropylmethyl cellulose. Int. J. Biol. Macromol. 2014, 67, 53–57. [Google Scholar] [CrossRef]
- Maier-Salamon, A.; Hagenauer, B.; Wirth, M.; Gabor, F.; Szekeres, T.; Jager, W. Increased transport of resveratrol across monolayers of the human intestinal Caco-2 cells is mediated by inhibition and saturation of metabolites. Pharm. Res. 2006, 23, 2107–2115. [Google Scholar] [CrossRef]
- Planas, J.M.; Alfaras, I.; Colom, H.; Juan, M.E. The bioavailability and distribution of trans-resveratrol are constrained by ABC transporters. Arch. Biochem. Biophys. 2012, 527, 67–73. [Google Scholar] [CrossRef]
- Peñalva, R.; Morales, J.; González-Navarro, C.J.; Larrañeta, E.; Quincoces, G.; Peñuelas, I.; Irache, J.M. Increased oral bioavailability of resveratrol by its encapsulation in casein nanoparticles. Int. J. Mol. Sci. 2018, 19, 2816. [Google Scholar] [CrossRef]
- Chang, C.W.; Wong, C.Y.; Wu, Y.T.; Hsu, M.C. Development of a solid dispersion system for improving the oral bioavailability of resveratrol in rats. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 239–249. [Google Scholar] [CrossRef] [PubMed]

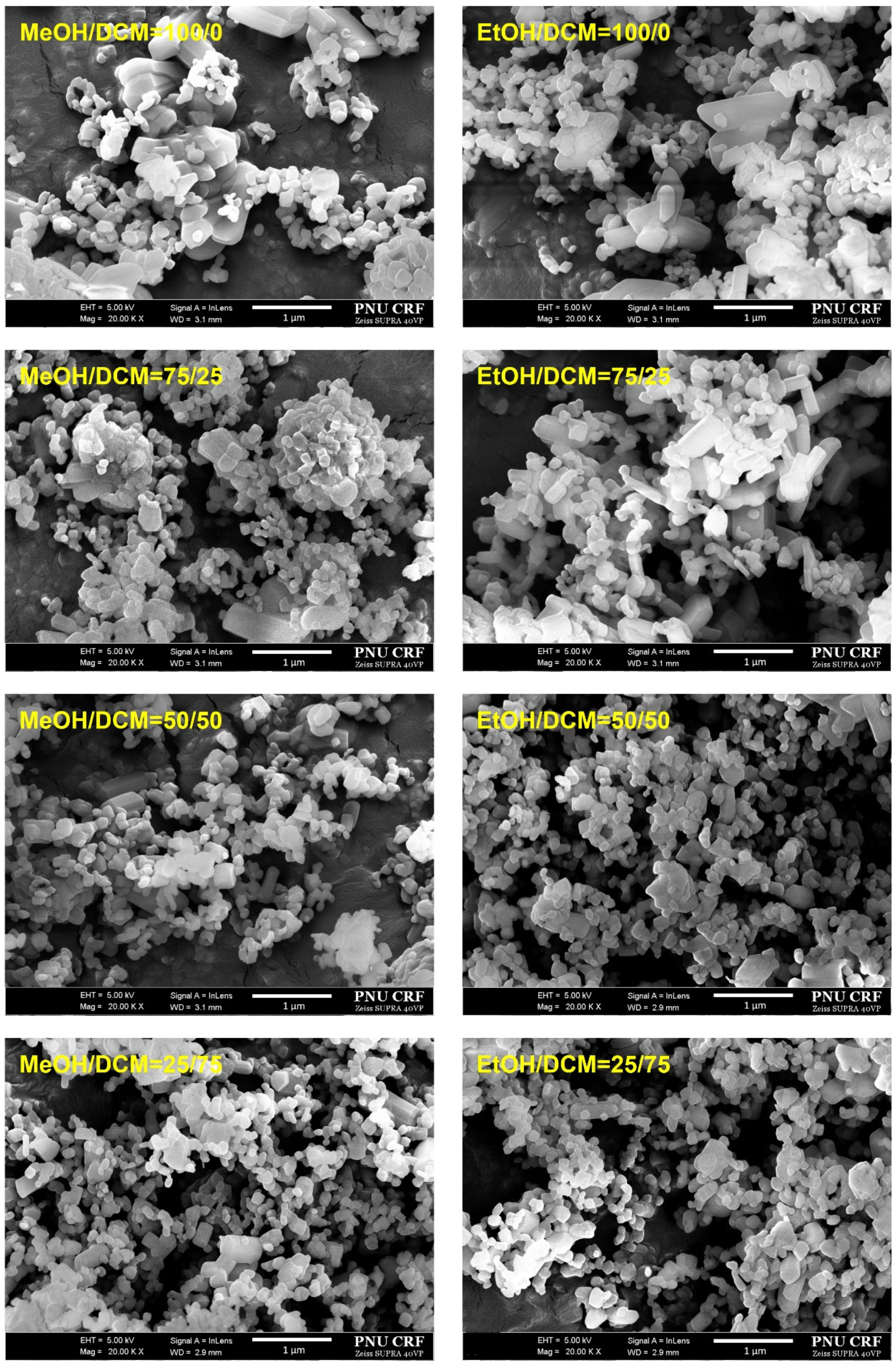

| Solvent Composition Mass% | Z-Average (nm) | PI | Specific Surface Area (m2/g) |
|---|---|---|---|
| MeOH/DCM = 25/75 | 174.5 ± 8.5 | 0.182 ± 0.029 | 56.18 ± 0.88 |
| MeOH/DCM = 50/50 | 208.5 ± 10.5 | 0.195 ± 0.039 | 45.22 ± 0.63 |
| MeOH/DCM = 75/25 | 393.3 ± 13.3 | 0.305 ± 0.079 | 31.14 ± 0.55 |
| MeOH/DCM = 100/0 | 501.7 ± 15.1 | 0.311 ± 0.081 | 27.51 ± 0.83 |
| EtOH/DCM = 25/75 | 151.2 ± 5.9 | 0.174 ± 0.013 | 60.23 ± 0.98 |
| EtOH/DCM = 50/50 | 194.2 ± 9.9 | 0.189 ± 0.023 | 49.31 ± 0.53 |
| EtOH/DCM = 75/25 | 371.8 ± 12.9 | 0.285 ± 0.049 | 35.29 ± 0.65 |
| EtOH/DCM = 100/0 | 481.5 ± 12.5 | 0.291 ± 0.061 | 29.22 ± 0.45 |
| Characterization | Nanoparticles | Microparticles | Microparticles |
|---|---|---|---|
| Preparation method and condition | SAS process: 40 °C and 150 bar, Solvent composition = 25:75 ethanol:dichloromethane (w/w) | Air jet-milling: Injection pressure = 8 bar, Grinding pressure = 8 bar, Feed rate = 0.5 g/min | Fitz milling: Screw speed = 500–1000 rpm and feed rate = 0.5 g/min |
| Morphology (scanning electron microscopy) |  |  |  |
| DSC | 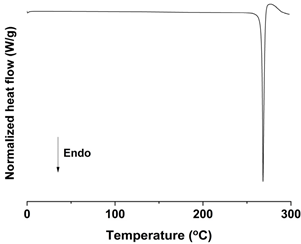 | 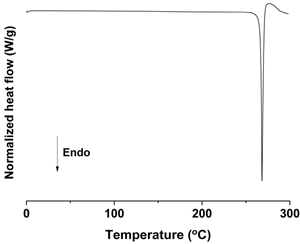 | 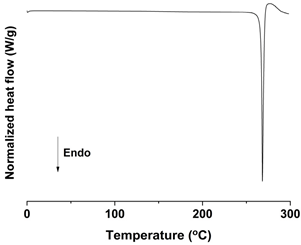 |
| PXRD | 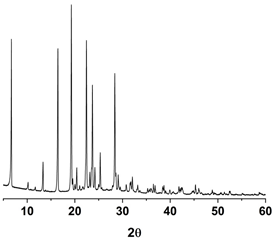 | 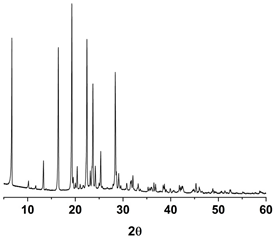 | 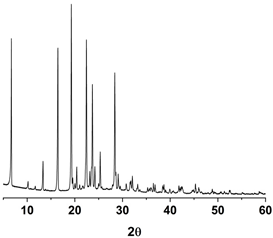 |
| Mean particle size (μm) | 0.17 ± 0.05 | 1.94 ± 0.26 | 18.75 ± 0.53 |
| d10 (μm) | 0.09 | 0.95 | 5.23 |
| d50 (μm) | 0.19 | 2.12 | 19.23 |
| d90 (μm) | 0.33 | 5.23 | 45.39 |
| Span | 1.26 | 2.02 | 2.14 |
| Specific surface area (m2/g) | 60.23 ± 0.98 | 3.43 ± 0.09 | 0.31 ± 0.03 |
| Purity (%) | 99.4 | 99.2 | 99.3 |
| Particle Size | Dissolution Rate, k (mg1/3/min) | Simulated 50% Dissolution Time, t50% (mins) | ||
|---|---|---|---|---|
| FaSSGF | FaSSIF | FaSSGF | FaSSIF | |
| 0.17 μm | 0.8404 | 0.8843 | 0.9 | 0.9 |
| 1.94 μm | 0.0836 | 0.0894 | 9.1 | 8.5 |
| 18.75 μm | 0.0128 | 0.0177 | 59.4 | 42.9 |
| Particle Size, Route | AUC0→8 h (ng·h/mL) | Cmax (ng/mL) | Tmax (h) | F (%) |
|---|---|---|---|---|
| 0.17 μm, oral | 920.4 ± 155.3 a,b | 734.0 ± 284.3 a,b | 0.5 ± 0.1 | 25.2 |
| 1.94 μm, oral | 610.2 ± 75.5 a | 200.8 ± 28.6 | 0.8 ± 0.2 | 16.7 |
| 18.75 μm, oral | 376.5 ± 27.4 | 100.2 ± 14.5 | 1.0 ± 0.3 | 10.3 |
| Intravenous | 1093.6 ± 301.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, E.-S.; Park, H.; Lee, S.-K.; Sim, W.-Y.; Jeong, J.-S.; Baek, I.-h.; Kim, M.-S. Pure Trans-Resveratrol Nanoparticles Prepared by a Supercritical Antisolvent Process Using Alcohol and Dichloromethane Mixtures: Effect of Particle Size on Dissolution and Bioavailability in Rats. Antioxidants 2020, 9, 342. https://doi.org/10.3390/antiox9040342
Ha E-S, Park H, Lee S-K, Sim W-Y, Jeong J-S, Baek I-h, Kim M-S. Pure Trans-Resveratrol Nanoparticles Prepared by a Supercritical Antisolvent Process Using Alcohol and Dichloromethane Mixtures: Effect of Particle Size on Dissolution and Bioavailability in Rats. Antioxidants. 2020; 9(4):342. https://doi.org/10.3390/antiox9040342
Chicago/Turabian StyleHa, Eun-Sol, Heejun Park, Seon-Kwang Lee, Woo-Yong Sim, Ji-Su Jeong, In-hwan Baek, and Min-Soo Kim. 2020. "Pure Trans-Resveratrol Nanoparticles Prepared by a Supercritical Antisolvent Process Using Alcohol and Dichloromethane Mixtures: Effect of Particle Size on Dissolution and Bioavailability in Rats" Antioxidants 9, no. 4: 342. https://doi.org/10.3390/antiox9040342
APA StyleHa, E.-S., Park, H., Lee, S.-K., Sim, W.-Y., Jeong, J.-S., Baek, I.-h., & Kim, M.-S. (2020). Pure Trans-Resveratrol Nanoparticles Prepared by a Supercritical Antisolvent Process Using Alcohol and Dichloromethane Mixtures: Effect of Particle Size on Dissolution and Bioavailability in Rats. Antioxidants, 9(4), 342. https://doi.org/10.3390/antiox9040342