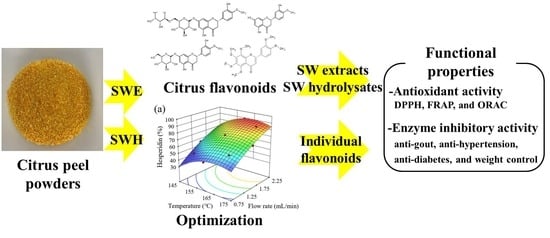
Semi-Continuous Subcritical Water Extraction of Flavonoids from Citrus unshiu Peel: Their Antioxidant and Enzyme Inhibitory Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Chemicals
2.3. Subcritical Water Extraction (SWE)
2.4. Methanol Extraction
2.5. Acid and Base Hydrolysis
2.6. HPLC Analysis
2.7. Response Surface Design
2.8. Antioxidant Activity Measurement
2.9. Enzyme Inhibitory Activity Measurement
2.10. Statistical Analysis
3. Result and Discussion
3.1. Composition of Flavonoids in C. unshiu Peel
3.2. Optimization of SWE Process
3.3. Effects of Extraction Parameters on Individual Flavonoid Yields
3.4. Comparison of Flavonoid Compositions in the SW Extract and Acid and Base Hydrolysis
3.5. Antioxidant and Enzyme Inhibitory Activities in the SW Extracts
3.6. Correlations between Flavonoid Yields and Functional Properties of the SW Extracts
3.7. Antioxidant Activities of Individual Citrus Flavonoids
3.8. Enzyme Inhibitory Activities of Individual Citrus Flavonoids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- An, H.J.; Park, K.J.; Kim, S.S. Flavonoids composition and antioxidant activity of by-products of five orange cultivars during maturation. Korean J. Food Preserv. 2016, 23, 1012–1017. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, H.J.; Kim, K.J.; Kim, M.J.; Lee, J.A.; Lee, A.R.; Roh, S.S. Antioxidant activity of citrus peel and effect on its glucose metabolism in L6 rat skeletal muscle cells. Kor. J. Herbol. 2018, 33, 101–108. [Google Scholar]
- Kim, M.Y.; Bo, H.H.; Ji, S.Y.; Hong, S.H.; Choi, S.H.; Kim, S.O.; Park, C.; Choi, Y.H. Relationship between reactive oxygen species and adenosine monophosphate-activated protein kinase signaling in apoptosis induction of human breast adenocarcinoma MDA-MB-231 cells by ethanol extract of Citrus unshiu peel. J. Life Sci. 2019, 29, 410–420. [Google Scholar]
- Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Morrow, M.R.; Sawyez, C.G.; Edwards, J.Y.; Drangova, M.; Huff, M.W. Intervention with citrus flavonoids reverses obesity and improves metabolic syndrome and atherosclerosis in obese Ldlr−/− mice. J. Lipid Res. 2018, 59, 1714–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.Y.; Choi, E.O.; Bo, H.H.; Kwon, D.H.; Ahn, K.I.; Kim, H.J.; Ji, S.Y.; Hong, S.H.; Jeong, J.W.; Kim, G.Y.; et al. Reactive oxygen species-dependent apoptosis induction by water extract of Citrus unshiu peel in MDA-MB-231 human breast carcinoma cells. Nutr. Res. Pract. 2018, 12, 129–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revathy, J.; Srinivasan, S.; Abdullah, S.H.S.; Muruganathan, U. Antihyperglycemic effect of hesperetin, a citrus flavonoid, extenuates hyperglycemia and exploring the potential role in antioxidant and antihyperlipidemic in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 97, 98–106. [Google Scholar]
- Lachos-Perez, D.; Baseggio, A.M.; Mayanga-Torres, P.C.; Junior, M.R.M.; Rostagno, M.A.; Martínez, J.; Forster-Carneiro, T. Subcritical water extraction of flavanones from defatted orange peel. J. Supercrit. Fluids 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Nipornram, S.; Tochampa, W.; Rattanatraiwong, P.; Singanusong, R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018, 1, 338–345. [Google Scholar] [CrossRef]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical water extraction of biological materials. Sep. Purif. Rev. 2016, 46, 21–34. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, T. Pressurized hot water extraction of bioactives. Trac-Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Shitu, A.; Izhar, S.; Tahir, T.M. Sub-critical water as a green solvent for production of valuable materials from agricultural waste biomass: A review of recent work. Global J. Environ. Sci. Manag. 2015, 1, 255–264. [Google Scholar]
- Cheigh, C.I.; Chung, E.Y.; Chung, M.S. Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. J. Food Eng. 2012, 110, 472–477. [Google Scholar] [CrossRef]
- Chen, P.X.; Tang, Y.; Marcone, M.F.; Pauls, P.K.; Zhang, B.; Liu, R.; Tsao, R. Characterization of free, conjugated and bound phenolics and lipophilic antioxidants in regular- and non-darkening cranberry beans (Phaseolus vulgaris L.). Food Chem. 2015, 185, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Amaretti, A.; Raimondi, S.; Leonardi, A.; Quartieri, A.; Rossi, M. Hydrolysis of the rutinose-conjugates flavonoids rutin and hesperidin by the gut microbiota and bifidobacteria. Nutrients 2015, 7, 2788–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Chang, X.; Brennan, M.A.; Brennan, C.S.; Guo, X. Comparison of phytochemical profiles, cellular antioxidant and anti-proliferative activities in five varieties of wampee (Clausena lansium) fruits. Int. J. Food Sci. Technol. 2019, 54, 2487–2493. [Google Scholar] [CrossRef]
- Yoon, K.N.; Jang, H.S. Anti-xanthine oxidase, anti-cholinesterase, and anti-inflammatory activities of fruiting bodies of Phellinus gilvus. Korean J. Clin. Lab. Sci. 2018, 50, 225–235. [Google Scholar] [CrossRef]
- Hussain, F.; Jahan, N.; Rahman, K.; Sultana, B.; Jamil, A. Identification of hypotensive biofunctional compounds of Coriandrum sativum and evaluation of their angiotensin-converting enzyme (ACE) inhibition potential. Oxidative Med. Cell. Longev. 2018, 2018, 4643736. [Google Scholar] [CrossRef] [Green Version]
- Proenca, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tome, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E.; et al. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure–activity relationship study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Lee, E.W.; Eom, S.H.; Kim, T.H. Pancreatic lipase inhibitory stilbenoids from the roots of Vitis vinifera. Int. J. Food Sci. Nutr. 2014, 65, 97–100. [Google Scholar] [CrossRef]
- Ravber, M.; Knez, Z.; Škerget, M. Optimization of hydrolysis of rutin in subcritical water using response surface methodology. J. Supercrit. Fluids 2015, 104, 145–152. [Google Scholar] [CrossRef]
- Brunner, G. Hydrothermal and Supercritical Water Process; Elsevier: Amsterdam, The Netherlands, 2014; p. 73. [Google Scholar]
- Céliz, G.; Rodriguez, G.; Soria, F.; Daz, M. Synthesis ofhesperetin7-O-glucosidefrom flavonoids extracted from Citrus waste using both free and immobilized α-L-rhamnosidases. Biocatal. Agric. Biotechnol. 2015, 4, 335–341. [Google Scholar] [CrossRef]
- Ruen-ngam, D.; Quitain, A.T.; Tanaka, M.; Sasaki, M.; Goto, M. Reaction kinetics of hydrothermal hydrolysis of hesperidin into more valuable compounds under supercritical carbon dioxide conditions. J. Supercrit. Fluids 2012, 66, 215–220. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F. Effect of processing techniques at industrial scale on orange juice antioxidant and beneficial health compounds. J. Agric. Food Chem. 2002, 50, 5107–5114. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Park, Y.; Lee, Y.; Noh, S.K.; Sung, E.G.; Choi, I. Effect of oral administration of water-soluble extract from citrus peel (Citrus unshiu) on suppressing alcohol-induced fatty liver in rats. Food Chem. 2012, 130, 598–604. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, G.; Zhao, C.; Han, Y.; DiMarco-Crook, C.; Lu, C.; Bao, Y.; Li, C.; Xiao, H.; Zheng, J.; et al. Characterization of polymethoxyflavone demethylation during drying processes of citrus peels. Food Funct. 2019, 10, 5707–5717. [Google Scholar] [CrossRef] [PubMed]
- Mottahedin, P.; Asl, A.H.; Khajenoori, M. Extraction of curcumin and essential oil from Curcuma longa L. by subcritical water via response surface methodology. J. Food Process. Preserv. 2017, 41, e13095. [Google Scholar] [CrossRef]
- Kim, D.S.; Lim, S.B. Subcritical water extraction of rutin from the aerial parts of common buckwheat. J. Supercrit. Fluids 2019, 152, 104561. [Google Scholar] [CrossRef]
- Peng, H.; Li, W.; Li, H.; Deng, Z.; Zhang, B. Extractable and non-extractable bound phenolic compositions and their antioxidant properties in seed coat and cotyledon of black soybean (Glycinemax (L.) merr). J. Funct. Food 2018, 32, 296–312. [Google Scholar] [CrossRef]
- Andujar, S.A.; Filippa, M.A.; Ferretti, F.H.; Blanco, S.E. Isomerization of 4’-methoxy-flavanone in alkaline medium. Determination of the enolate formation constant. Theochem.-J. Mol. Struct. 2003, 636, 157–166. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.; Gansukh, E.; Baskar, V.; Kai, G. Subcritical water extraction of withanosides and withanolides from ashwagandha (Withania somnifera L) and their biological activities. Food Chem. Toxicol. 2019, 132, 110659. [Google Scholar] [CrossRef] [PubMed]
- Gironés-Vilaplana, A.; Moreno, D.A.; García-Viguera, C. Phytochemistry and biological activity of Spanish Citrus fruits. Food Funct. 2014, 5, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Mhiri, N.; Veys-Renaux, D.; Rocca, E.; Ioannou, I.; Boudhrioua, N.M.; Ghoula, M. Corrosion inhibition of carbon steel in acidic medium by orange peel extract and its main antioxidant compounds. Corros. Sci. 2016, 102, 55–62. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreca, D.; Bisignano, C.; Ginestra, G.; Bisignano, G.; Bellocco, E.; Leuzzi, U.; Gattuso, G. C- and O-glycosyl flavonoids in tangelo (Citrus reticulata × Citrus paradisi) juice and their influence on antioxidant properties. Food Chem. 2013, 141, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Liu, Y.; Xiao, A.; Leng, J.; Liao, L.; Ma, L.; Liu, L. The interaction of dietary flavonoids with xanthine oxidase in vitro: Molecular property-binding affinity relationship aspects. RSC Adv. 2019, 9, 10781–10788. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J.; Gong, D. Dietary flavonoids as xanthine oxidase inhibitors: Structure-affinity and structure-activity relationships. J. Agric. Food Chem. 2015, 63, 7784–7794. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Kurita, I.; Maeda-Yamamoto, M.; Tachibana, H.; Kamei, M. Antihypertensive effect of benifuuki tea containing O-methylated EGCG. J. Agric. Food Chem. 2010, 58, 1903–1908. [Google Scholar] [CrossRef]


| No | X1 | X2 | Hesperidin and Its Hydrolysis Products (μg/g Dry Sample) | Narirutin and Its Hydrolysis Products (μg/g Dry Sample) | Polymethoxyflavones (μg/g Dry Sample) | Total Flavonoids (μg/g Dry Sample) | Total Soluble Solids (%) | Total Flavonoid Concentration in the Extract (mg/g Dry Extract) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hesperidin | Hesperetin-7-O-glucoside | Hesperetin | Narirutin | Prunin | Naringenin | Sinensetin | Nobiletin | Tangeretin | ||||||
| 1 | 150 | 1 | 24,700.4 | N.D. | N.D. | 5442.7 | N.D. | N.D. | 13.7 | 68.8 | 29.0 | 30,253.5 | 45.2 ± 0.3 d | 66.6 |
| 2 | 150 | 2 | 37,995.1 | N.D. | N.D. | 8040.6 | N.D. | N.D. | 18.4 | 85.4 | 45.5 | 46,184.3 | 56.0 ± 1.7 b,c | 82.4 |
| 3 | 170 | 1 | 30,498.0 | 1640.8 | 1591.0 | 5631.8 | 237.8 | 222.3 | 13.2 | 76.4 | 37.4 | 39,947.9 | 58.2 ± 2.4 a,b | 68.5 |
| 4 | 170 | 2 | 42,975.4 | 756.8 | 937.1 | 8027.2 | 200.4 | 145.7 | 15.7 | 90.6 | 49.6 | 53,197.9 | 57.9 ± 1.5 a,b | 91.8 |
| 5 | 145 | 1.5 | 27,935.1 | N.D. | N.D. | 6687.7 | N.D. | N.D. | 15.6 | 76.5 | 37.5 | 34,751.6 | 38.5 ± 1.1 e | 90.1 |
| 6 | 175 | 1.5 | 34,749.8 | 2048.0 | 1802.7 | 6595.3 | 326.0 | 268.1 | 14.2 | 82.2 | 39.1 | 45,924.3 | 58.1 ± 0.7 a,b | 79.0 |
| 7 | 160 | 0.75 | 24,345.7 | 698.2 | 433.6 | 4848.1 | 162.5 | 102.3 | 11.1 | 62.4 | 28.2 | 30,691.3 | 45.3 ± 1.2 d | 67.6 |
| 8 | 160 | 2.25 | 44,617.3 | 370.6 | 642.5 | 8760.0 | N.D. | 135.2 | 17.7 | 91.2 | 54.5 | 54,688.7 | 60.6 ± 2.2 a | 90.1 |
| 9 | 160 | 1.5 | 36,858.1 | 757.8 | 870.2 | 6856.2 | 141.4 | 126.1 | 15.9 | 82.5 | 41.6 | 45,749.5 | 55.1 ± 0.6 c | 82.9 |
| 10 | 160 | 1.5 | 36,786.7 | 701.2 | 856.7 | 6832.4 | 135.9 | 127.7 | 16.6 | 83.9 | 40.8 | 45,580.8 | --- | 75.1 |
| 11 | 160 | 1.5 | 37,486.7 | 739.4 | 884.1 | 6762.1 | 142.1 | 135.1 | 15.9 | 83.2 | 42.1 | 46,289.9 | --- | 76.3 |
| Flavonoid Content (μg/g Dry Sample) | |||
|---|---|---|---|
| Subcritical Water Extract * | Acid Hydrolysate | Base Hydrolysate | |
| Hesperidin | 34,749.8 | 12,081.2 ± 1188.3 | 16,124.1 ± 491.9 |
| Hesperetin-7-O-glucoside | 2048.0 | 2462.4 ± 96.0 | N.D. |
| Hesperetin | 1802.7 | 2204.0 ± 39.9 | N.D. |
| Narirutin | 6595.3 | 1240.7 ± 54.2 | 1620.8 ± 8.3 |
| Prunin | 326.0 | 703.9 ± 23.6 | N.D. |
| Naringenin | 268.1 | 863.0 ± 34.5 | N.D. |
| Sinensetin | 14.2 | 18.3 ± 0.6 | 15.4 ± 0.3 |
| Nobiletin | 82.2 | 91.1 ± 0.7 | 74.3 ± 1.7 |
| Tangeretin | 39.1 | 57.6 ± 2.5 | 41.8 ± 1.1 |
| Total | 45,924.3 | 19,722.2 ± 1236.3 | 17,876.4 ± 483.9 |
| X1 | X2 | Antioxidant Activity | Enzyme Inhibition Activity | |||||
|---|---|---|---|---|---|---|---|---|
| DPPH Radical Scavenging Activity (mg AAE/g Dry Sample) | FRAP (mmol FSE 100/g Dry Sample)) | ORAC (mg TE/g Dry Sample) | Xanthine Oxidase (%) | ACE (%) | α-Glucosidase (%) | Pancreatic Lipase (%) | ||
| 145 | 1.5 | 8.3 ± 0.1 f | 24.7 ± 1.1 f | 169.5 ± 5.2 d | 11.6 ± 1.3 f | 12.8 ± 1.5 f | 12.8 ± 1.1 f | 52.0 ± 1.9 g |
| 150 | 1 | 8.2 ± 0.3 f | 24.9 ± 0.9 f | 122.8 ± 5.8 e | 16.6 ± 1.4 e | 14.2 ± 0.6 e, f | 16.5 ± 0.8 e | 59.8 ± 1.9 e, f |
| 150 | 2 | 14.8 ± 0.5 c | 28.9 ± 0.7 e | 338.8 ± 16.6 b | 20.8 ± 1.8 d | 22.5 ± 1.3 d | 21.4 ± 1.4 d | 66.8 ± 2.0 d |
| 160 | 0.75 | 9.5 ± 0.6 e | 25.5 ± 0.9 f | 281.3 ± 17.5 c | 15.3 ± 1.0 e | 16.2 ± 0.7 e | 20.7 ± 1.2 d | 58.0 ± 1.6 f |
| 160 | 1.5 | 13.3 ± 0.7 d | 45.3 ± 1.3 c | 316.2 ± 26.3 b, c | 21.4 ± 0.8 d | 21.6 ± 0.5 d | 23.1 ± 1.1 d | 64.1 ± 2.7 d, e |
| 160 | 2.25 | 18.6 ± 0.3 a | 48.8 ± 1.3 b | 397.4 ± 21.8 a | 30.9 ± 1.7 c | 35.6 ± 1.5 b | 41.0 ± 1.7 b, c | 73.3 ± 1.8 c |
| 170 | 1 | 14.0 ± 0.0 c, d | 39.3 ± 1.1 d | 327.6 ± 5.0 b | 32.3 ± 1.8 c | 32.6 ± 1.7 c | 39.2 ± 2.1 c | 78.0 ± 1.4 b |
| 170 | 2 | 17.2 ± 0.4 b | 44.4 ± 1.4 c | 423.4 ± 34.9 a | 38.1 ± 1.5 b | 35.5 ± 1.2 b | 42.1 ± 1.2 b | 81.3 ± 3.1 b |
| 175 | 1.5 | 18.6 ± 0.4 a | 52.6 ± 1.8 a | 424.9 ± 32.5 a | 52.6 ± 2.5 a | 48.9 ± 2.6 a | 55.5 ± 2.4 a | 86.8 ± 3.4 a |
| Antioxidant Activity | Enzyme Inhibition Activity (IC50) | ||||||
|---|---|---|---|---|---|---|---|
| DPPH Radical Scavenging Activity (mg AAE/g) | FRAP (mmol FSE 100/g) | ORAC (mg TE/100 mg) | Xanthine Oxidase (mg/L) | ACE (mg/L) | α-Glucosidase (mg/L) | Pancreatic Lipase (mg/L) | |
| Hesperidin | 45.3 ± 0.9 c | 399.7 ± 21.1 c | 247.3 ± 13.2 f | >2000 | 1375.8 ± 62.1 b | >2000 | 418.4 ± 21.7 a |
| Hesperetin-7-O-glucoside | 51.6 ± 1.4 b | 439.8 ± 18.4 b | 323.9 ± 13.7 d | >2000 | 106.5 ± 3.8 f | 1395.5 ± 69.9 b | 84.4 ± 4.2 d |
| Hesperetin | 115.8 ± 3.9 a | 549.1 ± 21.1 a | 710.9 ± 12.0 a | 275.4 ± 17.7 c | 358.2 ± 7.8 d | 131.8 ± 7.3 e | 104.6 ± 5.2 d |
| Narirutin | 17.6 ± 1.2 e | 102.6 ± 4.1 e | 283.4 ± 15.7 e | 1104.7 ± 40.7 a | 1,937.1 ± 42.5 a | 1004.0 ± 42.6 c | >2000 |
| Prunin | 26.5 ± 0.2 d | 99.5 ± 3.6 e | 363.9 ± 18.1 c | 862.5 ± 36.6 b | 221.2 ± 6.2 e | 622.7 ± 28.0 d | >2000 |
| Naringenin | 52.6 ± 3.3 b | 150.3 ± 3.7 d | 475.1 ± 13.3 b | 129.3 ± 6.4 d | 459.7 ± 15.0 c | 72.2 ± 3.8 e | >2000 |
| Sinensetin | N.D. | N.D. | 7.6 ± 0.4 g | >2000 | 364.0 ± 13.4 d | >2000 | 301.4 ± 22.2 b |
| Nobiletin | N.D. | N.D. | 6.0 ± 0.4 g | >2000 | 322.5 ± 4.7 d | >2000 | 426.4 ± 27.6 a |
| Tangeretin | N.D. | N.D. | 5.8 ± 0.4 g | >2000 | 352.0 ± 8.9 d | 1763.1 ± 66.6 a | 148.7 ± 6.5 c |
| Positive control | 116.7 ± 3.7 d (Allopurinol) | 6.9 ± 0.2 g (Captopril) | 1366.9 ± 73.2 b (Acarbose) | 54.4 ± 3.6 e (Orlistat) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-S.; Lim, S.-B. Semi-Continuous Subcritical Water Extraction of Flavonoids from Citrus unshiu Peel: Their Antioxidant and Enzyme Inhibitory Activities. Antioxidants 2020, 9, 360. https://doi.org/10.3390/antiox9050360
Kim D-S, Lim S-B. Semi-Continuous Subcritical Water Extraction of Flavonoids from Citrus unshiu Peel: Their Antioxidant and Enzyme Inhibitory Activities. Antioxidants. 2020; 9(5):360. https://doi.org/10.3390/antiox9050360
Chicago/Turabian StyleKim, Dong-Shin, and Sang-Bin Lim. 2020. "Semi-Continuous Subcritical Water Extraction of Flavonoids from Citrus unshiu Peel: Their Antioxidant and Enzyme Inhibitory Activities" Antioxidants 9, no. 5: 360. https://doi.org/10.3390/antiox9050360
APA StyleKim, D.-S., & Lim, S.-B. (2020). Semi-Continuous Subcritical Water Extraction of Flavonoids from Citrus unshiu Peel: Their Antioxidant and Enzyme Inhibitory Activities. Antioxidants, 9(5), 360. https://doi.org/10.3390/antiox9050360