Citrus sinensis and Vitis vinifera Protect Cardiomyocytes from Doxorubicin-Induced Oxidative Stress: Evaluation of Onconutraceutical Potential of Vegetable Smoothies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Sample Preparation
2.3. Qualitative Analysis
2.3.1. Instrumentation
2.3.2. Chromatographic Conditions: RP-UHPLC-PDA
2.3.3. LCMS-IT-TOF Parameters
2.4. Quantitative Analysis
2.5. Biological Evaluation
2.5.1. Cell Culture
2.5.2. Cell Treatment
2.5.3. Cell Viability
2.5.4. Measurement of Intracellular ROS Release
2.5.5. HO-1 and NQO1 Detection by Cytofluorimetry
2.5.6. Analysis of Apoptosis
2.6. Data Analysis
3. Results
3.1. Qualitative and Quantitative Profiles of Smoothie Extracts
3.2. Effect of Orange and Grape Smoothies and Their Respective Mixtures, on H9c2 and MCF-7 Cells Viability
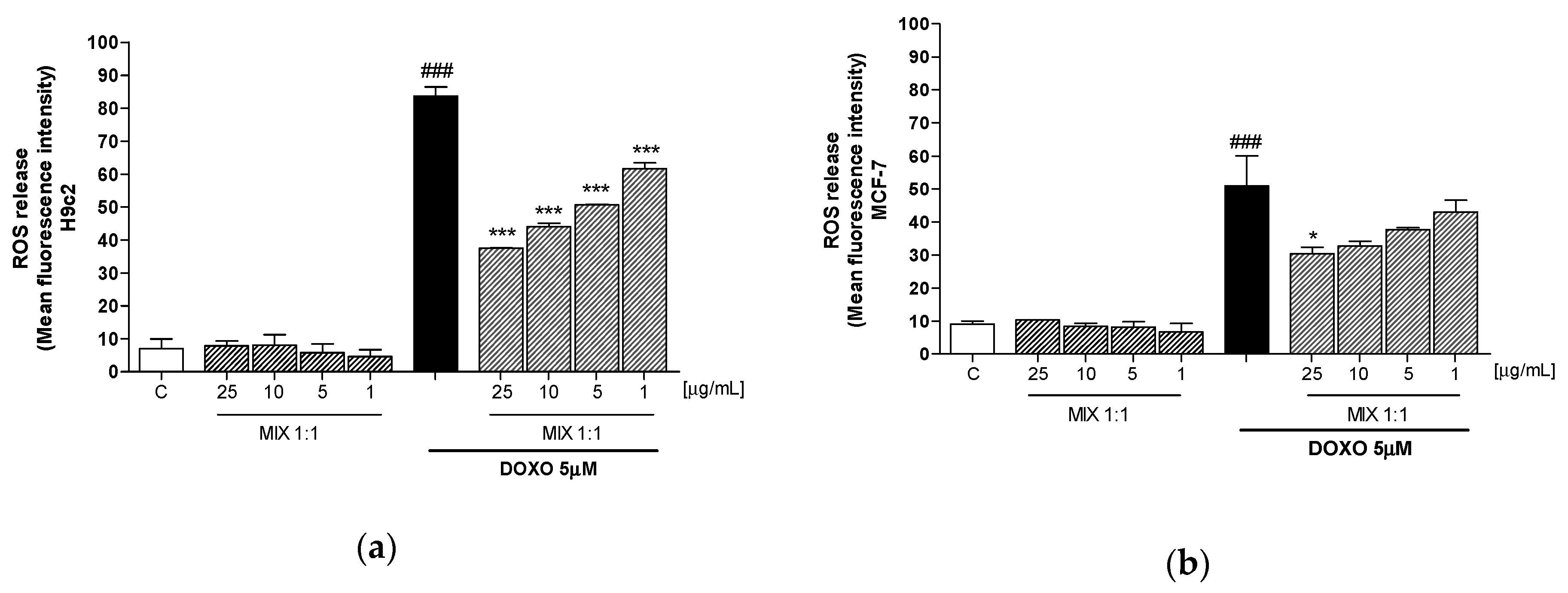
3.3. Effect of Mix 1:1 Reduced on Doxorubicin-Induced ROS Release in H9c2 and MCF-7 Cells
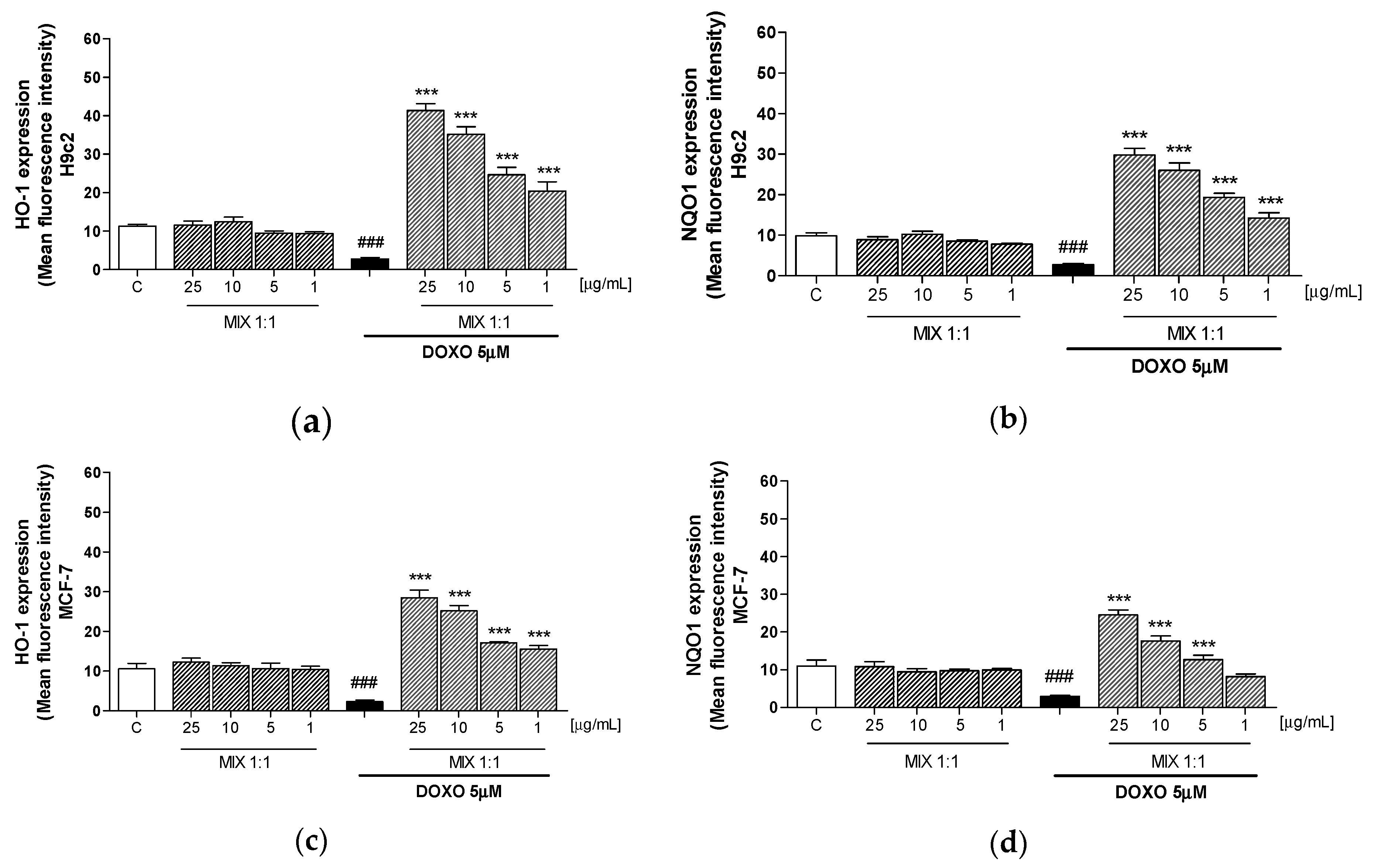
3.4. Effect of MIX 1:1 on Doxorubicin-Induced HO-1 and NQO1 Expression
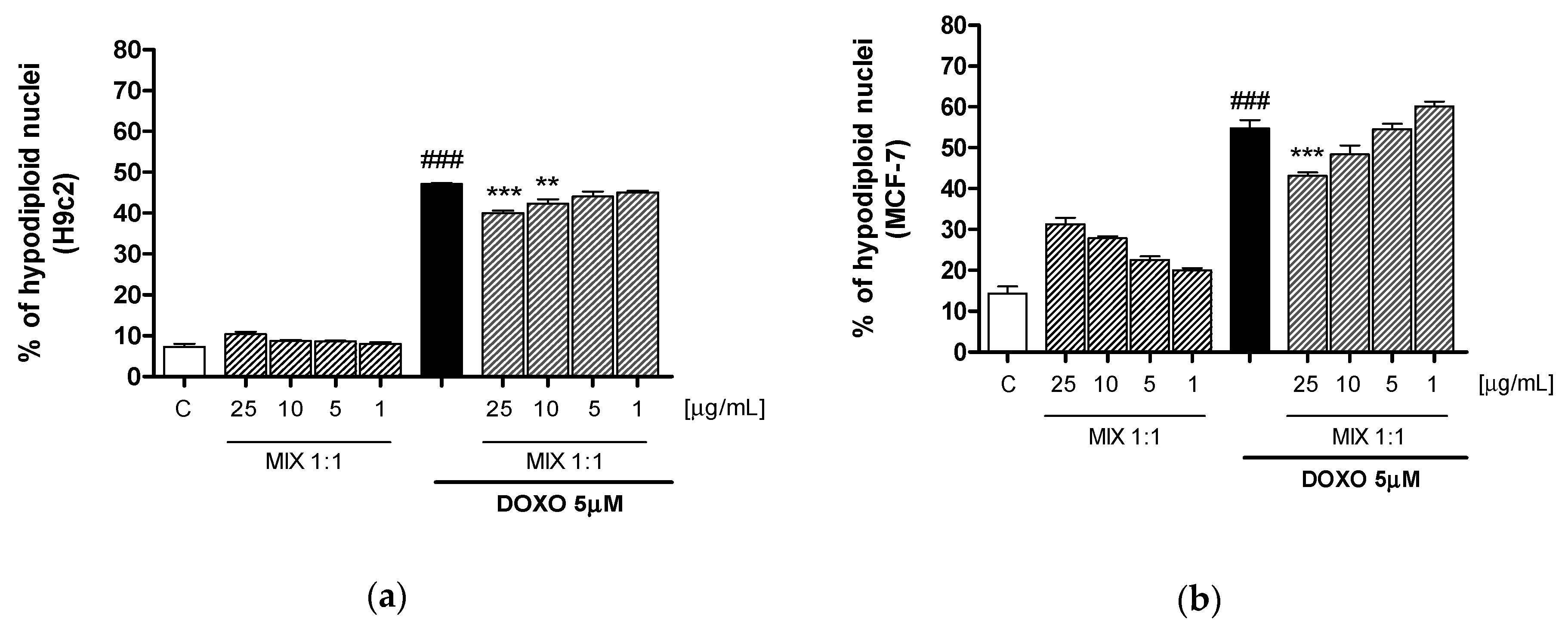
3.5. Effect of MIX 1:1 Reduced Doxorubicin-Induced Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, S.F.; Basra, M.; Canty, J.M. Takotsubo cardiomyopathy following initial chemotherapy presenting with syncope and cardiogenic shock—A case report and literature review. J. Clinic. Exp. Cardiol. 2011, 2, 124. [Google Scholar] [CrossRef]
- Costa, V.M.; Carvalho, F.; Duarte, J.A.; Bastos, M.d.L.; Remião, F. The heart as a target for xenobiotic toxicity: The cardiac susceptibility to oxidative stress. Chem. Res. Toxicol. 2013, 26, 1285–1311. [Google Scholar] [CrossRef] [PubMed]
- Volkova, M.; Russell, R. Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment. Curr. Cardiol. Rev. 2011, 7, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennig, B.; Ettinger, A.S.; Jandacek, R.J.; Koo, S.; McClain, C.; Seifried, H.; Silverstone, A.; Watkins, B.; William, A.; Suk, W.A. Using Nutrition for Intervention and Prevention against Environmental Chemical Toxicity and Associated Diseases. Environ. Health Perspect. 2007, 115, 493–495. [Google Scholar] [CrossRef]
- Adesso, S.; Pepe, G.; Sommella, E.; Manfra, M.; Scopa, A.; Sofo, A.; Tenore, G.C.; Russo, M.; Di Gaudio, F.; Autore, G.; et al. Anti-inflammatory and antioxidant activity of polyphenolic extracts from Lactuca sativa (var. Maravilla de Verano) under different farming methods. J. Sci. Food Agric. 2016, 96, 4194–4206. [Google Scholar] [CrossRef]
- Pepe, G.; Sommella, E.; Manfra, M.; De Nisco, M.; Tenore, G.C.; Scopa, A.; Sofo, A.; Marzocco, S.; Adesso, S.; Novellino, T.; et al. Evaluation of anti-inflammatory activity and fast UHPLC-DAD-IT-TOF profiling of polyphenolic compounds extracted from green lettuce (Lactuca sativa L.; Var. Maravilla de Verano). Food Chem. 2015, 167, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Pepe, G.; Sommella, E.; Cianciarulo, D.; Ostacolo, C.; Manfra, M.; Di Sarno, V.; Musella, S.; Russo, M.; Messore, A.; Parrino, B.; et al. Polyphenolic extract from tarocco (Citrus sinensis l. osbeck) clone “lempso” exerts anti-inflammatory and antioxidant effects via NF-KB and Nrf-2 activation in murine macrophages. Nutrients 2018, 10, 1961. [Google Scholar] [CrossRef] [Green Version]
- Smith-Warner, S.A.; Elmer, P.J.; Tharp, T.M.; Fosdick, L.; Randall, B.; Gross, M.; Wood, J.; Potter, J.D. Increasing vegetable and fruit intake: Randomized intervention and monitoring in an at-risk population. Cancer Epidemiol. Biomarkers Prev. 2000, 9, 307–317. [Google Scholar]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic Content and Antioxidant Activities of Selected Potato Varieties and Their Processing By-Products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Rhodes, V.A.; McDaniel, R.W. Nausea, vomiting, and retching: Complex problems in palliative care. Cancer J. Clin. 2001, 51, 232–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serino, A.; Salazar, G. Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients 2019, 11, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzoni, L.; Giampieri, F.; Suarez, J.M.A.; Gasparrini, M.; Mezzetti, B.; Hernandez, T.Y.F.; Battino, M.A. Isolation of strawberry anthocyanin-rich fractions and their mechanisms of action against murine breast cancer cell lines. Food Funct. 2019, 10, 7103–7120. [Google Scholar] [CrossRef] [PubMed]
- Limer, J.L.; Speirs, V. Phyto-oestrogens and breast cancer chemoprevention. Breast Cancer Res. 2004, 6, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenore, G.C.; Manfra, M.; Stiuso, P.; Coppola, L.; Russo, M.; Gomez Monterrey, I.M.; Campiglia, P. Antioxidant profile and in vitro cardiac radical-scavenging versus pro-oxidant effects of commercial red grape juices (Vitis vinifera L. cv. Aglianico N.). J. Agric. Food Chem. 2012, 60, 9680–9687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepe, G.; Pagano, F.; Adesso, S.; Sommella, E.; Ostacolo, C.; Manfra, M.; Chieppa, M.; Sala, M.; Russo, M.; Marzocco, S.; et al. Bioavailable Citrus sinensis Extract: Polyphenolic Composition and Biological Activity. Molecules 2017, 22, 623. [Google Scholar] [CrossRef] [Green Version]
- Sommella, E.; Pagano, F.; Pepe, G.; Ostacolo, C.; Manfra, M.; Chieppa, M.; Di Sanzo, R.; Carabetta, S.; Campiglia, P.; Russo, M. Flavonoid Composition of Tarocco (Citrus sinensis L. Osbeck) Clone “Lempso” and Fast Antioxidant Activity Screening by DPPH-UHPLC-PDA-IT-TOF. Phytochem Anal. 2017, 28, 521–528. [Google Scholar] [CrossRef]
- Sommella, E.; Pepe, G.; Pagano, F.; Conte, G.; Carimi, F.; Tenore, G.C.; Novellino, E.; Manfra, M.; Russo, M.; Campiglia, P. Rapid Screening of Antioxidant Anthocyanins in Autochthonous Nero d’Avola Grape Clones by Pre-column DPPH Reaction Coupled to UHPLC-UV/Vis-IT-TOF: A Strategy to Combine Chemical data and Genetic Diversity. Food Anal. Methods 2016, 10, 2780–2790. [Google Scholar] [CrossRef]
- Marzocco, S.; Adesso, S.; Alilou, M.; Stuppner, H.; Schwaiger, S. Anti-Inflammatory and Anti-Oxidant Potential of the Root Extract and Constituents of Doronicum austriacum. Molecules 2017, 22, 1003. [Google Scholar] [CrossRef]
- Adesso, S.; Ruocco, M.; Rapa, S.F.; Dal Piaz, F.; Di Iorio, R.B.; Popolo, A.; Autore, G.; Nishijima, F.; Pinto, A.; Marzocco, S. Effect of Indoxyl Sulfate on the Repair and Intactness of Intestinal Epithelial Cells: Role of Reactive Oxygen Species’ Release. Int. J. Mol. Sci. 2019, 20, 2280. [Google Scholar] [CrossRef] [Green Version]
- Adesso, S.; Magnus, T.; Cuzzocrea, S.; Campolo, M.; Rissiek, B.; Paciello, O.; Autore, G.; Pinto, A.; Marzocco, S. Indoxyl Sulfate Affects Glial Function Increasing Oxidative Stress and Neuroinflammation in Chronic Kidney Disease: Interaction between Astrocytes and Microglia. Front. Pharmacol. 2017, 12, 370. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Popolo, A.; Bianco, G.; Pinto, A.; Autore, G. Pro-apoptotic effect of methylguanidine on hydrogen peroxide-treated rat glioma cell line. Neurochem. Int. 2010, 57, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Raffoul, J.J. Potential anticancer properties of grape antioxidants. J Oncol. 2012, 2012, 803294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirmi, S.; Maugeri, A.; Ferlazzo, N.; Gangemi, S.; Calapai, G.; Schumacher, U.; Navarra, M. Anticancer Potential of Citrus Juices and Their Extracts: A Systematic Review of Both Preclinical and Clinical Studies. Front Pharmacol. 2017, 8, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salviati, E.; Ciaglia, E.; Sommella, E.; Montella, F.; Bertamino, A.; Ostacolo, C.; Parrino, B.; Rubino, R.; Vecchione, C.; Puca, A.; et al. Immunomodulatory activity of Humulus Lupulus bitter acids fraction: Enhancement of natural killer cells function by NKp44 activating receptor stimulation. J. Funct. Foods 2019, 61, 103469. [Google Scholar] [CrossRef]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [Green Version]
- Gorini, S.; De Angelis, A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxid. Med. Cell. Longev. 2018, 2018, 7582730. [Google Scholar] [CrossRef] [Green Version]
- Kaiserová, H.; Simunek, T.; van der Vijgh, W.J.; Bast, A.; Kvasnicková, E. Flavonoids as protectors against doxorubicin cardiotoxicity: Role of iron, chelation, antioxidant activity and inhibition of carbonyl reductase. Biochim. Biophys. Acta 2007, 1772, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Yang, B.; Qiao, Y.; Zhou, Q.; He, H. Kaempferol protects mitochondria and alleviates damages against endotheliotoxicity induced by doxorubicin. Biomed. Pharmacother. 2020, 126, 110040. [Google Scholar] [CrossRef]
- Crespo, I.; García-Mediavilla, M.V.; Almar, M.; González, P.; Tuñón, M.J.; Sánchez-Campos, S.; González-Gallego, J. Differential effects of dietary flavonoids on reactive oxygen and nitrogen species generation and changes in antioxidant enzyme expression induced by proinflammatory cytokines in Chang Liver cells. Food Chem. Toxicol. 2008, 46, 1555–1569. [Google Scholar] [CrossRef]
- Abushouk, A.I.; Ismail, A.; Salem, A.M.A.; Afifi, A.M.; Abdel-Daim, M.M. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 2017, 90, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ren, D.; Fan, P.; Shen, T.; Lou, H. Protective effects of naringenin-7-O-glucoside on doxorubicin-induced apoptosis in H9c2 cells. Eur. J. Pharmacol. 2008, 581, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, A.M.; Hernández Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, C.; Guo, Z.; Li, D.; Li, H.; He, J.; Wen, D.; Luo, B. Preventive effect of hesperidin modulates inflammatory responses and antioxidant status following acute myocardial infarction through the expression of PPAR-γ and Bcl-2 in model mice. Mol. Med. Rep. 2018, 17, 1261–1268. [Google Scholar] [CrossRef]
- Trivedi, P.P.; Kushwaha, S.; Tripathi, D.N.; Jena, G.B. Cardioprotective effects of hesperetin against doxorubicin-induced oxidative stress and DNA damage in Rat. Cardiovasc. Toxicol. 2011, 11, 215–225. [Google Scholar] [CrossRef]
- Chularojmontri, L.; Gerdprasert, O.; Wattanapitayakul, S.K. Pummelo protects doxorubicin-induced cardiac cell death by reducing oxidative otress, modifying glutathione transferase expression, and preventing cellular senescence. Evid. Based Complementary Altern. Med. 2013, 2013, 1–9. [Google Scholar]
- Mokni, M.; Hamlaoui-Guesmi, S.; Amri, M.; Marzouki, L.; Limam, F.; Aouani, E. Grape Seed and Skin Extract Protects Against acute chemotherapy toxicity induced by Doxorubicin in Rat Heart. Cardiovasc. Toxicol. 2012, 12, 158–165. [Google Scholar] [CrossRef]
- Du, Y.; Guo, H.; Lou, H. Grape seed polyphenols protect cardiac cells from apoptosis via induction of endogenous antioxidant enzymes. J. Agric. Food Chem. 2007, 55, 1695–1701. [Google Scholar] [CrossRef]
- Choi, H.C.; Chang, H.J.; Cho, J.Y.; Chun, H.S. Cytoprotective effect of anthocyanins against doxorubicin-induced toxicity in H9c2 cardiomyocytes in relation to their antioxidant activities. Food Chem. Toxicol. 2007, 45, 1873–1881. [Google Scholar] [CrossRef]
- Petroni, K.; Trinei, M.; Fornari, M.; Calvenzani, V.; Marinelli, A.; Micheli, L.A.; Pilu, R.; Matros, A.; Mock, H.P.; Tonelli, C.; et al. Dietary cyanidin 3-glucoside from purple corn ameliorates doxorubicin-induced cardiotoxicity in mice. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 462–469. [Google Scholar] [CrossRef]
- Choi, E.H.; Park, J.H.; Kim, M.K.; Chun, H.S. Alleviation of doxorubicin-induced toxicities by anthocyanin-rich bilberry (Vaccinium myrtillus L.) extract in rats and mice. BioFactors 2010, 36, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.M.; de Sylos, C.M. Effect of the manufacturing process on the citrus concentrations of flavanones. Alim. Nutr. Araraquara 2008, 19, 379–384. [Google Scholar]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure-radical Scavenging Activity Relationships of Phenolic Compounds from Traditional Chinese Medicinal Plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, G.; Bennett, A.C.; González-Burgos, E.; Gómez-Serranillos, M.P.; López, V.; Smith, C. Polyphenol-associated oxidative stress and inflammation in a model of LPS-induced inflammation in glial cells: Do we know enough for responsible compounding? Inflammopharmacology 2019, 27, 189–197. [Google Scholar]
- Suzuki, S.; Okada, M.; Shibuya, K.; Seino, M.; Sato, A.; Takeda, H.; Seino, S.; Yoshioka, T.; Kitanaka, C. JNK suppression of chemotherapeutic agents-induced ROS confers chemoresistance on pancreatic cancer stem cells. Oncotarget 2015, 6, 458–470. [Google Scholar] [CrossRef] [Green Version]
- Kirkby, K.A.; Adin, C.A. Products of heme oxygenase and their potential therapeutic applications. Am. J. Physiol. Renal Physiol. 2006, 290, F563–F571. [Google Scholar] [CrossRef] [Green Version]
- Siegel, D.; Gustafson, D.L.; Dehn, D.L.; Han, J.Y.; Boonchoong, P.; Berliner, L.J.; Ross, D. NAD(P)H: Quinone oxidoreductase 1: Role as a superoxide scavenger. Mol. Pharmacol. 2004, 65, 1238–1247. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cao, Z.; Zhu, H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol. Res. 2006, 53, 6–15. [Google Scholar] [CrossRef]
- Yao, P.; Nussler, A.; Liu, L.; Hao, L.; Song, F.; Schirmeier, A.; Nussler, N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007, 47, 253–261. [Google Scholar] [CrossRef]
- Wang, S.; Konorev, E.A.; Kotamraju, S.; Joseph, J.; Kalivendi, S.; Kalyanaraman, B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms: Intermediacy of H2O2− and p53-dependent pathways. J. Biol. Chem. 2004, 279, 25535–25543. [Google Scholar] [CrossRef] [Green Version]
- Adina, A.B.; Goenadi, F.A.; Handoko, F.F.; Nawangsari, D.A.; Hermawan, A.; Jenie, R.I.; Meiyan, E. Combination of Ethanolic Extract of Citrus aurantifolia Peels with Doxorubicin Modulate Cell Cycle and Increase Apoptosis Induction on MCF-7 Cells. Iran J. Pharm. Res. 2014, 13, 919–926. [Google Scholar] [PubMed]
- Leone, A.; Longo, C.; Gerardi, C.; Trosko, E.J. Pro-apoptotic effect of grape seed extract on MCF-7 involves transient increase of gap junction intercellular communication and Cx43 up-regulation: A mechanism of chemoprevention. Int. J. Mol. Sci. 2019, 20, 3244. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepe, G.; Salviati, E.; Rapa, S.F.; Ostacolo, C.; Cascioferro, S.; Manfra, M.; Autore, G.; Marzocco, S.; Campiglia, P. Citrus sinensis and Vitis vinifera Protect Cardiomyocytes from Doxorubicin-Induced Oxidative Stress: Evaluation of Onconutraceutical Potential of Vegetable Smoothies. Antioxidants 2020, 9, 378. https://doi.org/10.3390/antiox9050378
Pepe G, Salviati E, Rapa SF, Ostacolo C, Cascioferro S, Manfra M, Autore G, Marzocco S, Campiglia P. Citrus sinensis and Vitis vinifera Protect Cardiomyocytes from Doxorubicin-Induced Oxidative Stress: Evaluation of Onconutraceutical Potential of Vegetable Smoothies. Antioxidants. 2020; 9(5):378. https://doi.org/10.3390/antiox9050378
Chicago/Turabian StylePepe, Giacomo, Emanuela Salviati, Shara Francesca Rapa, Carmine Ostacolo, Stella Cascioferro, Michele Manfra, Giuseppina Autore, Stefania Marzocco, and Pietro Campiglia. 2020. "Citrus sinensis and Vitis vinifera Protect Cardiomyocytes from Doxorubicin-Induced Oxidative Stress: Evaluation of Onconutraceutical Potential of Vegetable Smoothies" Antioxidants 9, no. 5: 378. https://doi.org/10.3390/antiox9050378
APA StylePepe, G., Salviati, E., Rapa, S. F., Ostacolo, C., Cascioferro, S., Manfra, M., Autore, G., Marzocco, S., & Campiglia, P. (2020). Citrus sinensis and Vitis vinifera Protect Cardiomyocytes from Doxorubicin-Induced Oxidative Stress: Evaluation of Onconutraceutical Potential of Vegetable Smoothies. Antioxidants, 9(5), 378. https://doi.org/10.3390/antiox9050378









