Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress
Abstract
:1. Introduction
2. Background Information of Cytokines
3. Dynamics and Sources of Cytokines in Response to Exercise
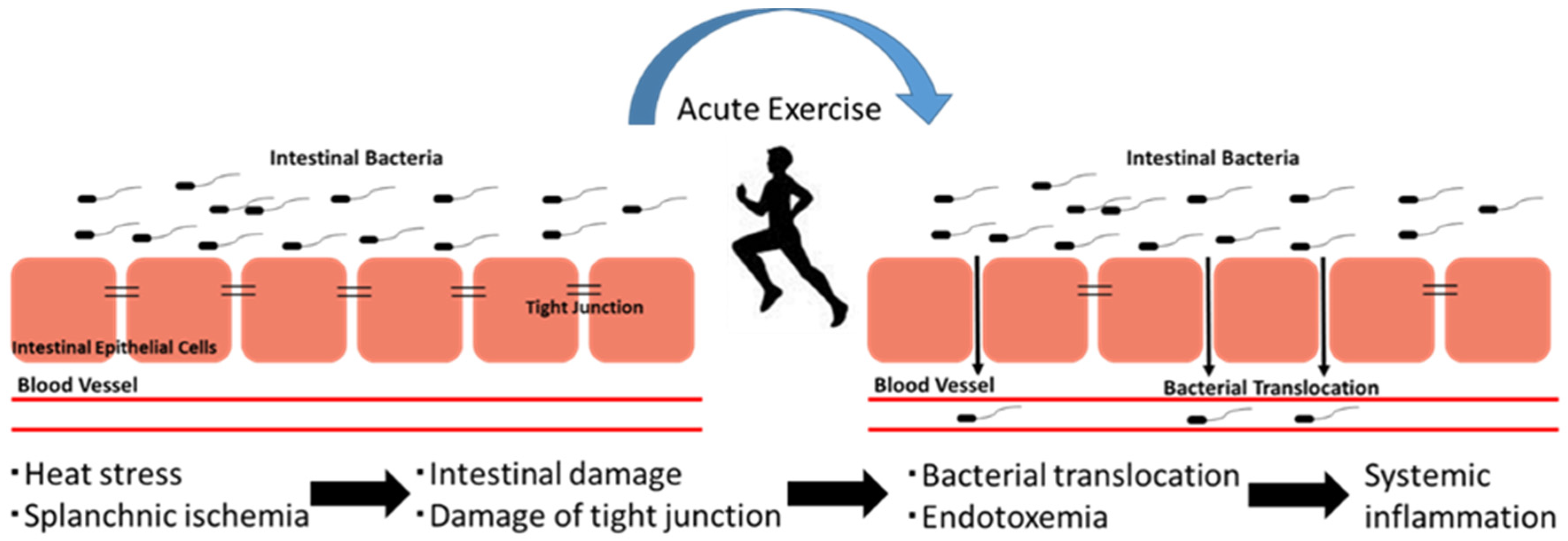
4. The Exercise-Induced Endotoxemia and Systemic Inflammation
5. The Exercise-Induced Inflammation and Organ Damage
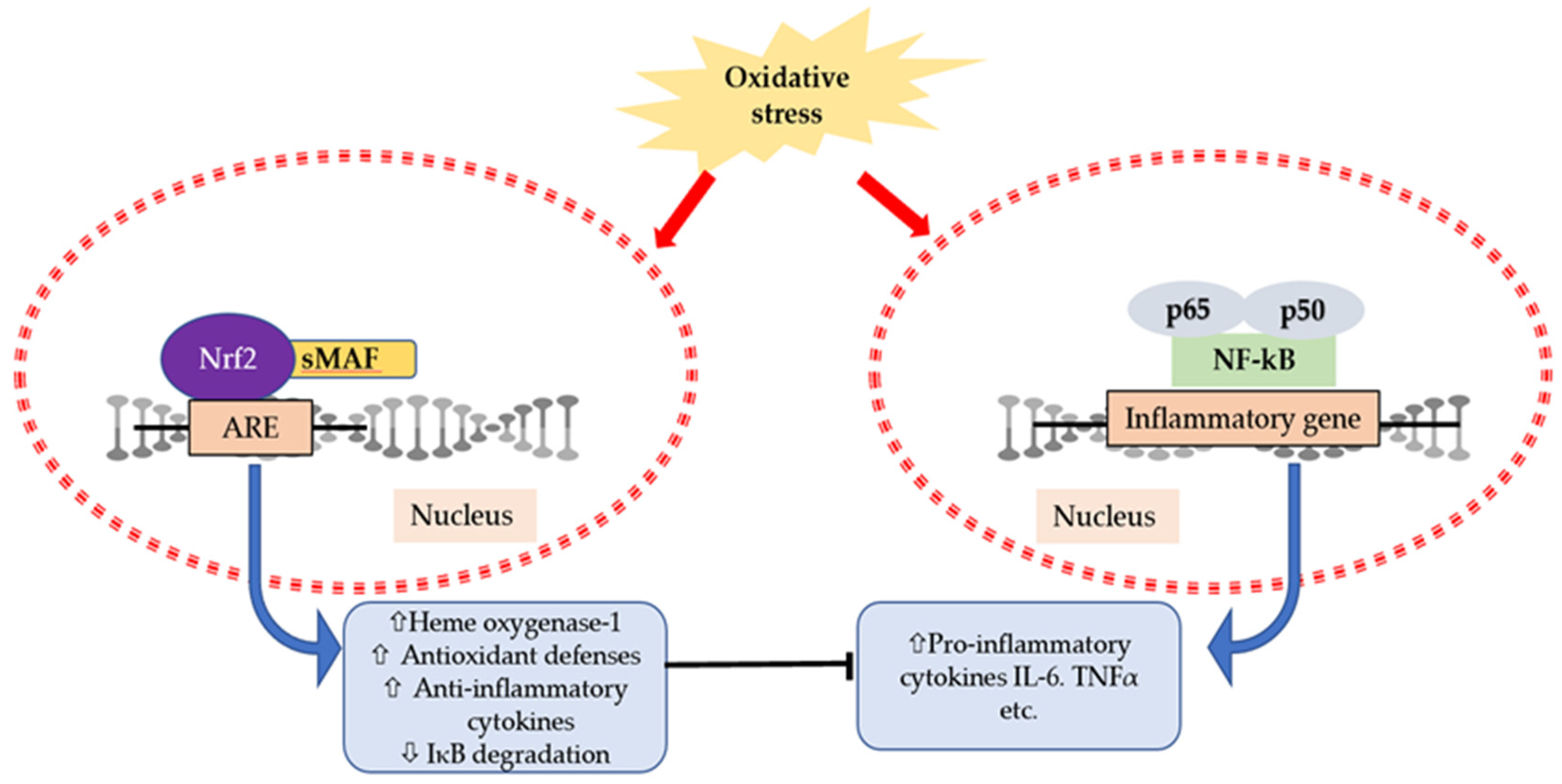
6. Interaction of Cytokines and Oxidative Stress
7. Potential Strategy/Countermeasures to Reduce Exercise-Induced Inflammation and Oxidative Stress
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hung, Y.-L.; Suzuki, K. The pattern recognition receptors and lipopolysaccharides (LPS)-induced systemic inflammation. Int. J. Res. Stud. Med. Health Sci. 2017, 2, 1–7. [Google Scholar]
- Suzuki, K. Involvement of neutrophils in exercise-induced muscle damage. Gen. Intern. Med. Clin. Innov. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Ma, S.; Suzuki, K. Toll-like receptor 4: Target of lipotoxicity and exercise-induced anti-inflammatory effect? Ann. Nutr. Food Sci. 2018, 2, 1027. [Google Scholar]
- Suzuki, K. Characterization of exercise-induced cytokine release, the impacts on the body, the mechanisms and modulations. Int. J. Sports Exerc. Med. 2019, 5, 122. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radak, Z.; Torma, F.; Berkes, I.; Goto, S.; Mimura, T.; Posa, A.; Balogh, L.; Boldogh, I.; Suzuki, K.; Higuchi, M.; et al. Exercise effects on physiological function during aging. Free Radic. Biol. Med. 2019, 132, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Aw, N.H.; Canetti, E.; Suzuki, K.; Goh, J. Monocyte subsets in atherosclerosis and modification with exercise in humans. Antioxidants 2018, 7, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization “Global strategy on diet, physical activity and health”. Available online: https://www.who.int/dietphysicalactivity/pa/en/ (accessed on 14 April 2020).
- Cerqueira, E.; Marinho, D.A.; Neiva, H.P.; Lourenco, O. Inflammatory effects of high and moderate intensity exercise—A systematic review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Cytokine response to exercise and its modulation. Antioxidants 2018, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Peake, J.; Suzuki, K. Neutrophil activation, antioxidant supplements and exercise-induced oxidative stress. Exerc. Immunol. Rev. 2004, 10, 129–141. [Google Scholar] [PubMed]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Totsuka, M.; Sato, K.; Sugawara, K. Systemic inflammatory response to exhaustive exercise: Cytokine kinetics. Exerc. Immunol. Rev. 2002, 8, 6–48. [Google Scholar] [PubMed]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Kometani, T. IL-17, neutrophil activation and muscle damage following endurance exercise. Exerc. Immunol. Rev. 2012, 18, 116–127. [Google Scholar] [PubMed]
- Suzuki, K.; Nakaji, S.; Kurakake, S.; Totsuka, M.; Sato, K.; Kuriyama, T.; Fujimoto, H.; Shibusawa, K.; Machida, K.; Sugawara, K. Exhaustive exercise and type-1/type-2 cytokine balance in special focus on interleukin-12 p40/p70. Exerc. Immunol. Rev. 2003, 9, 48–57. [Google Scholar]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Miura, S.; Yoshioka, H.; Mori, Y.; Kometani, T. Changes of thioredoxin, oxidative stress markers, inflammation and muscle/renal damage following intensive endurance exercise. Exerc. Immunol. Rev. 2015, 21, 130–142. [Google Scholar]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Kometani, T. Urinary excretion of cytokines versus their plasma levels after endurance exercise. Exerc. Immunol. Rev. 2013, 19, 29–48. [Google Scholar]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med. Sci. Sports Exerc. 2003, 35, 348–355. [Google Scholar] [CrossRef]
- Suzuki, K.; Shiraishi, K.; Yoshitani, K.; Sugama, K.; Kometani, T. The effect of a sports drink based on highly branched cyclic dextrin on cytokine responses to exhaustive endurance exercise. J. Sports Med. Phys. Fit. 2014, 54, 622–630. [Google Scholar]
- Ma, S.; Tominaga, T.; Kanda, K.; Sugama, K.; Omae, C.; Hashimoto, S.; Aoyama, K.; Yoshikai, Y.; Suzuki, K. Effects of an 8-week protein supplementation regimen with hyperimmunized cow milk on exercise-induced organ damage and inflammation in male runners: A randomized, placebo controlled, cross-over study. Biomedicines 2020, 8, 51. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Naganuma, S.; Totsuka, M.; Suzuki, K.J.; Mochizuki, M.; Shiraishi, M.; Nakaji, S.; Sugawara, K. Effects of exhaustive endurance exercise and its one-week daily repetition on neutrophil count and functional status in untrained men. Int. J. Sports Med. 1996, 17, 205–212. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamada, M.; Kurakake, S.; Okamura, N.; Yamaya, K.; Liu, Q.; Kudoh, S.; Kowatari, K.; Nakaji, S.; Sugawara, K. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur. J. Appl. Physiol. 2000, 81, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.M.; Lim, C.L.; Suzuki, K. Effects of endurance-, strength-, and concurrent training on cytokines and inflammation. In Concurrent Aerobic and Strength Training: Scientific Basics and Practical Applications; Schumann, M., Ronnestad, B., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 125–138. ISBN 978-3-319-75547-2. [Google Scholar]
- Suzuki, K.; Peake, J.; Nosaka, K.; Okutsu, M.; Abbiss, C.R.; Surriano, R.; Bishop, D.; Quod, M.J.; Lee, H.; Martin, D.T.; et al. Changes in markers of muscle damage, inflammation and HSP70 after an Ironman triathlon race. Eur. J. Appl. Physiol. 2006, 98, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.; Della Gatta, P.; Suzuki, K.; Nieman, D. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar] [PubMed]
- Suzuki, K.; Totsuka, M.; Nakaji, S.; Yamada, M.; Kudoh, S.; Liu, Q.; Sugawara, K.; Yamaya, K.; Sato, K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J. Appl. Physiol. 1999, 87, 1360–1367. [Google Scholar] [CrossRef]
- Kim, H.K.; Konishi, M.; Takahashi, M.; Tabata, H.; Endo, N.; Numao, S.; Lee, S.K.; Suzuki, K.; Kim, Y.H.; Sakamoto, S. Effects of acute endurance exercise performed in the morning and evening on inflammatory cytokine and metabolic hormone responses. PLoS ONE 2015, 10, e0137567. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Sato, H.; Kikuchi, T.; Abe, T.; Nakaji, S.; Sugawara, K.; Totsuka, M.; Sato, K.; Yamaya, K. Capacity of circulating neutrophils to produce reactive oxygen species after exhaustive exercise. J. Appl. Physiol. 1996, 81, 1213–1222. [Google Scholar] [CrossRef]
- Nieman, D.C.; Davis, J.M.; Henson, D.A.; Walberg-Rankin, J.; Shute, M.; Dumke, C.L.; Utter, A.C.; Vinci, D.M.; Carson, J.A.; Brown, A.; et al. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J. Appl. Physiol. 2003, 94, 1917–1925. [Google Scholar] [CrossRef]
- Nieman, D.C.; Davis, J.M.; Henson, D.A.; Gross, S.J.; Dumke, C.L.; Utter, A.C.; Vinci, D.M.; Carson, J.A.; Brown, A.; McAnulty, S.R.; et al. Muscle cytokine mRNA changes after 2.5 h of cycling: Influence of carbohydrate. Med. Sci. Sports Exerc. 2005, 37, 1283–1290. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Davis, J.M.; Dumke, C.L.; Gross, S.J.; Jenkins, D.P.; Murphy, E.A.; Carmichael, M.D.; Quindry, J.C.; McAnulty, S.R.; et al. Quercetin ingestion does not alter cytokine changes in athletes competing in the Western States Endurance Run. J. Interf. Cytokine Res. 2007, 27, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K. Exhaustive exercise-induced neutrophil-associated tissue damage and possibility of its prevention. J. Nanomed. Biother. Discov. 2017, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Peake, J.M.; Suzuki, K.; Wilson, G.; Hordern, M.; Nosaka, K.; Mackinnon, L.; Coombs, J. Exercise-induced muscle damage, plasma cytokines, and markers of neutrophil activation. Med. Sci. Sports Exerc. 2005, 37, 737–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashida, H.; Shimura, M.; Sugama, K.; Kanda, K.; Suzuki, K. Exercise-induced inflammation during different phases of the menstrual cycle. Physiother. Rehabil. 2016, 1, 4. [Google Scholar] [CrossRef]
- Peake, J.; Peiffer, J.J.; Abbiss, C.R.; Nosaka, K.; Okutsu, M.; Laursen, P.B.; Suzuki, K. Body temperature and its effect on leukocyte mobilization, cytokines and markers of neutrophil activation during and after exercise. Eur. J. Appl. Physiol. 2008, 102, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.; Peiffer, J.J.; Abbiss, C.R.; Nosaka, K.; Laursen, P.B.; Suzuki, K. Carbohydrate gel ingestion and immunoendocrine responses to cycling in temperate and hot conditions. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 229–246. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Suzuki, K.; Kudo, S.; Totsuka, M.; Nakaji, S.; Sugawara, K. Raised plasma G-CSF and IL-6 after exercise may play a role in neutrophil mobilization into the circulation. J. Appl. Physiol. 2002, 92, 1789–1794. [Google Scholar] [CrossRef]
- Peake, J.; Wilson, G.; Hordern, M.; Suzuki, K.; Yamaya, K.; Nosaka, K.; Mackinnon, L.; Coombes, J.S. Changes in neutrophil surface receptor expression, degranulation, and respiratory burst activity after moderate- and high-intensity exercise. J. Appl. Physiol. 2004, 97, 612–618. [Google Scholar] [CrossRef]
- Mezil, Y.A.; Allison, D.; Kish, K.; Ditor, D.; Ward, W.E.; Tsiani, E.; Klentrou, P. Response of bone turnover markers and cytokines to high-intensity low-impact exercise. Med. Sci. Sports Exerc. 2015, 47, 1495–1502. [Google Scholar] [CrossRef]
- Lira, F.S.; dos Santos, T.; Caldeira, R.S.; Inoue, D.S.; Panissa, V.L.G.; Cabral-Santos, C.; Campos, E.Z.; Rodrigues, B.; Monteiro, P.A. Short-term high- and moderate-intensity training modifies inflammatory and metabolic factors in response to acute exercise. Front. Physiol. 2017, 8, 856. [Google Scholar] [CrossRef] [Green Version]
- Brenner, I.K.; Natale, V.M.; Vasiliou, P.; Moldoveanu, A.I.; Shek, P.N.; Shephard, R.J. Impact of three different types of exercise on components of the inflammatory response. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 452–460. [Google Scholar] [CrossRef]
- Kanda, K.; Sugama, K.; Hayashida, H.; Sakuma, J.; Kawakami, Y.; Miura, S.; Yoshioka, H.; Mori, Y.; Suzuki, K. Eccentric exercise-induced delayed-onset muscle soreness and changes in markers of muscle damage and inflammation. Exerc. Immunol. Rev. 2013, 19, 74–87. [Google Scholar]
- Kanda, K.; Sugama, K.; Sakuma, J.; Kawakami, Y.; Suzuki, K. Evaluation of serum leaking enzymes and investigation into new biomarkers for exercise-induced muscle damage. Exerc. Immunol. Rev. 2014, 20, 39–54. [Google Scholar] [PubMed]
- Peake, J.M.; Roberts, L.A.; Figueiredo, V.C.; Egner, I.; Krog, S.; Aas, S.N.; Suzuki, K.; Markworth, J.F.; Coombes, J.S.; Cameron-Smith, D.; et al. The effects of cold water immersion and active recovery on inflammation and cell stress responses in human skeletal muscle after resistance exercise. J. Physiol. 2017, 595, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Galvão, D.A.; Nosaka, K.; Taaffe, D.R.; Spry, N.; Kristjanson, L.J.; McGuigan, M.R.; Suzuki, K.; Yamaya, K.; Newton, R.U. Resistance training and reduction of treatment side effects in prostate cancer patients. Med. Sci. Sports Exerc. 2006, 38, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Galvão, D.A.; Nosaka, K.; Taaffe, D.R.; Peake, J.; Spry, N.; Suzuki, K.; Yamaya, K.; McGuigan, M.R.; Kristjanson, L.J.; Newton, R.U. Endocrine and immune responses to resistance training in prostate cancer patients. Prostate Cancer Prostatic Dis. 2008, 11, 160–165. [Google Scholar] [CrossRef]
- Ross, M.L.; Halson, S.L.; Suzuki, K.; Garnham, A.; Hawley, J.A.; Cameron-Smith, D.; Peake, J.M. Cytokine responses to carbohydrate ingestion during recovery from exercise-induced muscle injury. J Interf. Cytokine Res. 2010, 30, 329–337. [Google Scholar] [CrossRef]
- Scott, J.P.R.; Sale, C.; Greeves, J.P.; Casey, A.; Dutton, J.; Fraser, W.D. Effect of exercise intensity on the cytokine response to an acute bout of running. Med. Sci. Sports Exerc. 2011, 43, 2297–2306. [Google Scholar] [CrossRef]
- Starkie, R.L.; Arkinstall, M.J.; Koukoulas, I.; Hawley, J.A.; Febbraio, M.A. Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J. Physiol. 2001, 533, 585–591. [Google Scholar] [CrossRef]
- Keller, C.; Keller, P.; Marshal, S.; Pedersen, B.K. IL-6 gene expression in human adipose tissue in response to exercise--effect of carbohydrate ingestion. J. Physiol. 2003, 550, 927–931. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Steensberg, A.; Keller, C.; Starkie, R.L.; Nielsen, H.B.; Krustrup, P.; Ott, P.; Secher, N.H.; Pedersen, B.K. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J. Physiol. 2003, 549, 607–612. [Google Scholar] [CrossRef]
- Hashimoto, H.; Ishijima, T.; Hayashida, H.; Suzuki, K.; Higuchi, M. Menstrual cycle phase and carbohydrate ingestion alter immune response following endurance exercise and high intensity time trial performance test under hot conditions. J. Int. Soc. Sports Nutr. 2014, 11, 39. [Google Scholar] [CrossRef] [Green Version]
- Gudiksen, A.; Schwartz, C.L.; Bertholdt, L.; Joensen, E.; Knudsen, J.G.; Pilegaard, H. Lack of skeletal muscle IL-6 affects pyruvate dehydrogenase activity at rest and during prolonged exercise. PLoS ONE 2016, 11, e0156460. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Zwetsloot, K.A.; Lomiwes, D.D.; Meaney, M.P.; Hurst, R.D. Muscle glycogen depletion following 75-km of cycling is not linked to increased muscle IL-6, IL-8, and MCP-1 mRNA expression and protein content. Front. Physiol. 2016, 7, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, S.G.; Verma, G.; Pata, R.W.; Martin, T.G.; Perazella, M.A.; Parikh, C.R. Kidney injury and repair biomarkers in marathon runners. Am. J. Dis. 2017, 70, 252–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawanishi, N.; Mizokami, T.; Niihara, H.; Yada, K.; Suzuki, K. Macrophage depletion by clodronate liposome attenuates muscle injury and inflammation following exhaustive exercise. Biochem. Biophys. Rep. 2016, 5, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Kawanishi, N.; Mizokami, T.; Niihara, H.; Yada, K.; Suzuki, K. Neutrophil depletion attenuates muscle injury after exhaustive exercise. Med. Sci. Sports Exerc. 2016, 48, 1917–1924. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Sekiai, S.; Hatakeyama, H.; Koide, M.; Chaweewannakorn, C.; Yaoita, F.; Tan-No, K.; Sasaki, K.; Watanabe, M.; Sugawara, S.; et al. Neutrophils provide a favorable IL-1-mediated immunometabolic niche that primes GLUT4 translocation and performance in skeletal muscles. Cell Rep. 2018, 23, 2354–2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steensberg, A.; Van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef]
- Hiscock, N.; Chan, M.H.S.; Bisucci, T.; Darby, I.A.; Febbraio, M.A. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: Evidence of fiber type specificity. FASEB J. 2004, 18, 992–994. [Google Scholar] [CrossRef]
- Tominaga, T.; Ma, S.; Saitou, K.; Suzuki, K. Glucose ingestion inhibits endurance exercise-induced IL-6 producing macrophage infiltration in mice muscle. Nutrients 2019, 11, 1496. [Google Scholar] [CrossRef] [Green Version]
- Banzet, S.; Koulmann, N.; Simler, N.; Birot, O.; Sanchez, H.; Chapot, R.; Peinnequin, A.; Bigard, X. Fibre-type specificity of interleukin-6 gene transcription during muscle contraction in rat: Association with calcineurin activity. J. Physiol. 2005, 566, 839–847. [Google Scholar] [CrossRef]
- Ma, S.; Huang, Q.; Tominaga, T.; Liu, C.; Suzuki, K. An 8-week ketogenic diet alternated interleukin-6, ketolytic and lipolytic gene expression, and enhanced exercise capacity in mice. Nutrients 2018, 10, 1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, D.; Dubots, P.; Boggio, V.; Guilland, J.C.; Cometti, G. Effects of electromyostimulation and strength training on muscle soreness, muscle damage and sympathetic activation. J. Sports Sci. 1995, 13, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, T.; Machida, K.; Suzuki, K. Importance of correlations between phagocytic activity and superoxide production of neutrophils under conditions of voluntary exercise and stress. J. Clin. Lab. Anal. 1996, 10, 458–464. [Google Scholar] [CrossRef]
- Peake, J.M.; Suzuki, K.; Coombes, J.S. The influence of antioxidant supplementation on markers of inflammation and the relationship to oxidative stress after exercise. J. Nutr. Biochem. 2007, 18, 357–371. [Google Scholar] [CrossRef]
- Suzuki, K. Inflammatory response to exercise and its prevention. Curr. Top. Biochem. Res. 2018, 19, 37–42. [Google Scholar]
- Suzuki, K.; Ohno, S.; Suzuki, Y.; Ohno, Y.; Okuyama, R.; Aruga, A.; Yamamoto, M.; Ishihara, K.O.; Nozaki, T.; Miura, S.; et al. Effect of green tea extract on reactive oxygen species produced by neutrophils from cancer patients. Anticancer Res. 2012, 32, 2369–2375. [Google Scholar]
- Ohno, S.; Ohno, Y.; Suzuki, Y.; Miura, S.; Yoshioka, H.; Mori, Y.; Suzuki, K. Ingestion of Tabebuia avellanedae (Taheebo) inhibits production of reactive oxygen species from human peripheral blood neutrophils. Int. J. Food Sci. Nutr. Diet. 2015, S6, 1–4. [Google Scholar]
- Hung, Y.L.; Miyazaki, H.; Fang, S.H.; Li, C.; Suzuki, K. The structural characteristics of green tea polyphenols on lipopolysaccharide-stimulated RAW cells. J. Nutr. Biol. 2018, 2, 151–157. [Google Scholar] [CrossRef]
- Kawanishi, N.; Kato, K.; Takahashi, M.; Mizokami, T.; Otsuka, Y.; Imaizumi, A.; Shiva, D.; Yano, H.; Suzuki, K. Curcumin attenuates oxidative stress following downhill running-induced muscle damage. Biochem. Biophys. Res. Commun. 2013, 441, 573–578. [Google Scholar] [CrossRef]
- Takahashi, M.; Suzuki, K.; Kim, H.K.; Otsuka, Y.; Imaizumi, A.; Miyashita, M.; Sakamoto, S. Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int. J. Sports Med. 2013, 34, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y.; Suzuki, K.; Hung, Y.L.; Yang, M.S.; Yu, C.P.; Lin, S.P.; Hou, Y.C.; Fang, S.H. Aloe metabolites prevent LPS-induced sepsis and inflammatory response by inhibiting mitogen-activated protein kinase activation. Am. J. Chin. Med. 2017, 45, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.L.; Fang, S.H.; Wang, S.C.; Cheng, W.C.; Liu, P.L.; Su, C.C.; Chen, C.S.; Huang, M.Y.; Hua, K.F.; Shen, K.H.; et al. Corylin protects LPS-induced sepsis and attenuates LPS-induced inflammatory response. Sci. Rep. 2017, 7, 46299. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Yada, K.; Lee, H.; Fukuda, Y.; Iida, A.; Suzuki, K. Taheebo polyphenols attenuate FFA-induced inflammation in murine and human macrophage cell lines as inhibitor of COX-2. Front. Nutr. 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane protects cells against lipopolysaccharide-stimulated inflammation in murine macrophages. Antioxidants 2019, 8, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamura, T.; Suzuki, K.; Takahashi, M.; Tomari, M.; Hara, R.; Gando, Y.; Muraoka, I. Involvement of neutrophil dynamics and function in exercise-induced muscle damage and delayed onset muscle soreness: Effect of hydrogen bath. Antioxidants 2018, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Hirata, N.; Ichimaru, R.; Tominari, T.; Matsumoto, C.; Watanabe, K.; Taniguchi, K.; Hirata, M.; Ma, S.; Suzuki, K.; Grundler, F.M.W.; et al. Beta-cryptoxanthin inhibits lipopolysaccharide-induced osteoclast differentiation and bone resorption via the suppression of inhibitor of NF-κB kinase activity. Nutrients 2019, 11, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yada, K.; Suzuki, K.; Oginome, N.; Ma, S.; Fukuda, Y.; Iida, A.; Radak, Z. Single dose administration of taheebo polyphenol enhances endurance capacity in mice. Sci. Rep. 2018, 8, 14625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Protective effects of sulforaphane on exercise-induced organ damage via inducing antioxidant defense responses. Antioxidants 2020, 9, 136. [Google Scholar] [CrossRef] [Green Version]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Miyashita, M.; Kawanishi, N.; Park, J.H.; Hayashida, H.; Kim, H.S.; Nakamura, Y.; Sakamoto, S.; Suzuki, K. Low-volume exercise training attenuates oxidative stress and neutrophils activation in older adults. Eur. J. Appl. Physiol. 2013, 113, 1117–1126. [Google Scholar] [CrossRef]
- Ogawa, K.; Sanada, K.; Machida, S.; Okutsu, M.; Suzuki, K. Resistance exercise training-induced muscle hypertrophy was associated with reduction of inflammatory markers in elderly women. Mediat. Inflamm. 2010, 2010, 171023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashida, H.; Shimura, M.; Sugama, K.; Kanda, K.; Suzuki, K. Effects of the menstrual cycle and acute aerobic exercise on cytokine levels. J. Sports Med. Doping Stud. 2015, 6, 173. [Google Scholar] [CrossRef] [Green Version]
- van Wijck, K.; Lenaerts, K.; van Loon, L.J.; Peters, W.H.; Buurman, W.A.; Dejong, C.H. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS ONE 2011, 6, e22366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Hashimoto, H.; Oh, T.; Ishijima, T.; Mitsuda, H.; Peake, J.M.; Sakamoto, S.; Muraoka, I.; Higuchi, M. The effects of sports drink osmolality on fluid intake and immunoendocrine responses to cycling in hot conditions. J. Nutr. Sci. Vitaminol. 2013, 59, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Tanisawa, K.; Suzuki, K.; Ma, S.; Kondo, S.; Okugawa, S.; Higuchi, M. Effects of ingestion of different amounts of carbohydrate after endurance exercise on circulating cytokines and markers of neutrophil activation. Antioxidants 2018, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.L.; Pyne, D.B.; Horn, P.; Kalz, A.; Saunders, P.; Peake, J.; Suzuki, K.; Wilson, G.; Mackinnon, L.T. The effects of increased endurance training load on biomarkers of heat tolerance during intense exercise in the heat. Appl. Physiol. Nutr. Metab. 2009, 34, 616–624. [Google Scholar] [CrossRef]
- Lim, C.L.; Suzuki, K. Systemic inflammation mediates the effects of endotoxemia in the mechanisms of heat stroke. Biol. Med. 2017, 9, 1000376. [Google Scholar] [CrossRef]
- Leon, L.R.; Helwig, B.G. Heat stroke: Role of the systemic inflammatory response. J. Appl. Physiol. 2010, 109, 1980–1988. [Google Scholar] [CrossRef]
- Kawanishi, N.; Mizokami, T.; Yada, K.; Suzuki, K. Exercise training suppresses scavenger receptor CD36 expression in kupffer cells of nonalcoholic steatohepatitis model mice. Physiol. Rep. 2018, 6, e13902. [Google Scholar] [CrossRef]
- Kawanishi, N.; Niihara, H.; Mizokami, T.; Yada, K.; Suzuki, K. Exercise training attenuates neutrophil infiltration and elastase expression in adipose tissue of high-fat-diet-induced obese mice. Physiol. Rep. 2015, 3, e12534. [Google Scholar] [CrossRef]
- Kawanishi, N.; Niihara, H.; Mizokami, T.; Yano, H.; Suzuki, K. Exercise training attenuates adipose tissue fibrosis in diet-induced obese mice. Biochem. Biophys. Res. Commun. 2013, 440, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Mizokami, T.; Yano, H.; Suzuki, K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med. Sci. Sports Exerc. 2013, 45, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Yano, H.; Mizokami, T.; Takahashi, M.; Oyanagi, E.; Suzuki, K. Exercise training attenuates hepatic inflammation, fibrosis and macrophage infiltration during diet induced-obesity in mice. Brain Behav. Immun. 2012, 26, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Kato, Y.; Yokozeki, K.; Sawada, S.; Sakurai, R.; Fujiwara, Y.; Shinkai, S.; Goda, N.; Suzuki, K. Effects of aging on serum levels of lipid molecular species as determined by lipidomics analysis in Japanese men and women. Lipids Health Dis. 2018, 17, 135. [Google Scholar] [CrossRef] [Green Version]
- Sell, H.; Habich, C.; Eckel, J. Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 2012, 8, 709–716. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic review: Exercise-induced gastrointestinal syndrome—implications for health and intestinal disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef] [Green Version]
- Pires, W.; Veneroso, C.E.; Wanner, S.P.; Pacheco, D.A.S.; Vaz, G.C.; Amorim, F.T.; Tonoli, C.; Soares, D.D.; Coimbra, C.C. Association between exercise-induced hyperthermia and intestinal permeability: A systematic review. Sports Med. 2017, 47, 1389–1403. [Google Scholar] [CrossRef]
- van Wijck, K.; Lenaerts, K.; Grootjans, J.; Wijnands, K.A.; Poeze, M.; van Loon, L.J.; Dejong, C.H.; Buurman, W.A. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: Strategies for evaluation and prevention. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G155–G168. [Google Scholar] [CrossRef] [Green Version]
- Jaeschke, H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1083–G1088. [Google Scholar] [CrossRef] [Green Version]
- Sharfuddin, A.A.; Molitoris, B.A. Pathophysiology of ischemic acute kidney injury. Nat. Rev. Nephrol. 2011, 7, 189–200. [Google Scholar] [CrossRef]
- Luissint, A.-C.; Parkos, C.A.; Nusrat, A. Inflammation and the intestinal barrier: Leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.L.; Chen, Y.S.; Huang, X.D.; Zhang, L.X. Exhaustive swimming exercise related kidney injury in rats-protective effects of acetylbritannilactone. Int. J. Sports Med. 2012, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Victoria, M.; Drago-Serrano, M.E.; Reyna-Garfias, H.; Viloria, M.; Lara-Padilla, E.; Resendiz-Albor, A.A.; Sánchez-Torres, L.E.; Cruz-Hernández, T.R.; Campos-Rodriguez, R. Effects on secretory IgA levels in small intestine of mice that underwent moderate exercise training followed by a bout of strenuous swimming exercise. Brain Behav. Immun. 2012, 26, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Zhou, X.; Yu, L.; Yao, Y.; Zhang, Y.; Huang, Y.; Chen, M.; Yi, L.; Mi, M. Exhaustive exercise induces gastrointestinal syndrome through reduced ILC3 and IL-22 in mouse model. Med. Sci. Sports Exerc. 2020. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, M.H.; Davis, M.J.; Secher, N.H.; van Lieshout, J.J.; Arce-Esquivel, A.A.; Simmons, G.H.; Bender, S.B.; Padilla, J.; Bache, R.J.; Merkus, D.; et al. Peripheral circulation. Compr. Physiol. 2012, 2, 321–447. [Google Scholar] [CrossRef]
- Snipe, R.M.J.; Khoo, A.; Kitic, C.M.; Gibson, P.R.; Costa, R.J.S. The impact of exertional-heat stress on gastrointestinal integrity, gastrointestinal symptoms, systemic endotoxin and cytokine profile. Eur. J. Appl. Physiol. 2018, 118, 389–400. [Google Scholar] [CrossRef]
- Sureda, A.; Mestre-Alfaro, A.; Banquells, M.; Riera, J.; Drobnic, F.; Camps, J.; Joven, J.; Tur, J.A.; Pons, A. Exercise in a hot environment influences plasma anti-inflammatory and antioxidant status in well-trained athletes. J. Therm. Biol. 2015, 47, 91–98. [Google Scholar] [CrossRef]
- Steinberg, J.G.; Ba, A.; Bregeon, F.; Delliaux, S.; Jammes, Y. Cytokine and oxidative responses to maximal cycling exercise in sedentary subjects. Med. Sci. Sports Exerc. 2007, 39, 964–968. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Nemes, R.; Koltai, E.; Taylor, A.W.; Suzuki, K.; Gyori, F.; Radak, Z. Reactive oxygen and nitrogen species regulate key metabolic, anabolic, and catabolic pathways in skeletal muscle. Antioxidants 2018, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Albensi, B.C. What is nuclear factor kappa B (NF-κB) doing in and to the mitochondrion? Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Wang, H.; Yan, W.; Xu, L.; Wang, X.; Zhao, X.; Yang, X.; Chen, G.; Ji, Y. Disruption of Nrf2 enhances upregulation of nuclear factor-B activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injury. Mediat. Inflamm. 2008, 2008, 725174. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.; Wang, H.; Qiao, L.; Wang, X. Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediat. Inflamm. 2010, 2010, 238321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellezza, I.; Mierla, A.L.; Minelli, A. Nrf2 and NF-κB and their concerted modulation in cancer pathogenesis and progression. Cancers 2010, 2, 483–497. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Henkel, T. Function and activation of NF-kappaB in the immune system. Ann. Rev Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef]
- Ji, L.L.; Gomez-Cabrera, M.C.; Steinhafel, N.; Vina, J. Acute exercise activates nuclear factor (NF)-κB signaling pathway in rat skeletal muscle. FASEB J. 2004, 18, 1499–1506. [Google Scholar] [CrossRef]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef]
- Cleto, L.S.; Oleto, A.; Sousa, L.; Barreto, T.O.; Cruz, J.d.S.; Penaforte, C.L.; Magalhães, J.C.d.; Franco, J.d.S.; Pinto, K.M.d.C.; Azevedo, A.C.C. Plasma cytokine response, lipid peroxidation and NF-κB activation in skeletal muscle following maximum progressive swimming. Braz. J. Med. Biol. Res. 2011, 44, 546–552. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Suzuki, K. Potential application of ketogenic diet to metabolic status and exercise performance: A review. EC Nutr. 2018, 13, 496–499. [Google Scholar]
- Ma, S.; Suzuki, K. Keto-adaptation and endurance exercise capacity, fatigue recovery, and exercise-induced muscle and organ damage prevention. Sports 2019, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Huang, Q.; Yada, K.; Liu, C.; Suzuki, K. An 8-week ketogenic low carbohydrate, high fat diet enhanced exhaustive exercise capacity in mice. Nutrients 2018, 10, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.; Komine, S.; Warabi, E.; Akiyama, K.; Ishii, A.; Ishige, K.; Mizokami, Y.; Kuga, K.; Horie, M.; Miwa, Y. Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malaguti, M.; Angeloni, C.; Garatachea, N.; Baldini, M.; Leoncini, E.; Collado, P.S.; Teti, G.; Falconi, M.; Gonzalez-Gallego, J.; Hrelia, S. Sulforaphane treatment protects skeletal muscle against damage induced by exhaustive exercise in rats. J. Appl. Physiol. 2009, 107, 1028–1036. [Google Scholar] [CrossRef] [Green Version]
- de Figueiredo, S.M.; Binda, N.S.; Nogueira-Machado, J.A.; Vieira-Filho, S.A.; Caligiorne, R.B. The antioxidant properties of organosulfur compounds (sulforaphane). Recent Pat. Endocr. Metab. Immune Drug Discov. 2015, 9, 24–39. [Google Scholar] [CrossRef]
- Jeffery, E.H.; Araya, M. Physiological effects of broccoli consumption. Phytochem. Rev. 2009, 8, 283–298. [Google Scholar] [CrossRef]
- Sun, C.-C.; Li, S.-J.; Yang, C.-L.; Xue, R.-L.; Xi, Y.-Y.; Wang, L.; Zhao, Q.-L.; Li, D.-J. Sulforaphane attenuates muscle inflammation in dystrophin-deficient Mdx mice via Nrf2-mediated inhibition of NF-κB signaling pathway. J. Biol. Chem. 2015, 290, 17784–17795. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Konkimalla, V.B. Sulforaphane regulates phenotypic and functional switching of both induced and spontaneously differentiating human monocytes. Int. Immunopharmacol. 2016, 35, 85–98. [Google Scholar] [CrossRef]
- Shing, C.M.; Peake, J.; Suzuki, K.; Okutsu, M.; Pereira, R. Effects of bovine colostrum supplementation on immune variables in highly trained cyclists. J. Appl. Physiol. 2007, 102, 1113–1122. [Google Scholar] [CrossRef] [Green Version]
- Shing, C.M.; Peake, J.M.; Suzuki, K.; Jenkins, D.G.; Coombes, J.S. Bovine colostrum modulates cytokine production in human peripheral blood mononuclear cells stimulated with lipopolysaccharide and phytohemagglutinin. J. Interf. Cytokine Res. 2009, 29, 37–44. [Google Scholar] [CrossRef]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Arazi, H.; Suzuki, K. Effects of β-hydroxy-β-methylbutyrate-free acid supplementation on strength, power and hormonal adaptations following resistance training. Nutrients 2017, 9, 1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Takahashi, M.; Li, C.Y.; Lin, S.P.; Tomari, M.; Shing, C.M.; Fang, S.H. The acute effects of green tea and carbohydrate co-ingestion on systemic inflammation and oxidative stress during sprint cycling. Appl. Physiol. Nutr. Metab. 2015, 40, 997–1003. [Google Scholar] [CrossRef]
- March, D.S.; Marchbank, T.; Playford, R.J.; Jones, A.W.; Thatcher, R.; Davison, G. Intestinal fatty acid-binding protein and gut permeability responses to exercise. Eur. J. Appl. Physiol. 2017, 117, 931–941. [Google Scholar] [CrossRef] [Green Version]
- Ogden, H.B.; Child, R.B.; Fallowfield, J.L.; Delves, S.K.; Westwood, C.S.; Layden, J.D. The gastrointestinal exertional heat stroke paradigm: Pathophysiology, assessment, severity, aetiology and nutritional countermeasures. Nutrients 2020, 12, 537. [Google Scholar] [CrossRef] [Green Version]
- Shing, C.M.; Ogawa, K.; Zhang, X.; Nagatomi, R.; Peake, J.M.; Suzuki, K.; Jenkins, D.G.; Coombes, J.S. Reduction in resting plasma granulysin as a marker of increased training load. Exerc. Immunol. Rev. 2007, 13, 89–99. [Google Scholar]
- Tsukamoto, K.; Suzuki, K.; Machida, K.; Saiki, C.; Murayama, R.; Sugita, M. Relationships between lifestyle factors and neutrophil functions in the elderly. J. Clin. Lab. Anal. 2002, 16, 266–272. [Google Scholar] [CrossRef]
- Ogawa, K.; Suzuki, K.; Okutsu, M.; Yamazaki, K.; Shinkai, S. The association of elevated reactive oxygen species levels from neutrophils with low-grade inflammation in the elderly. Immun. Ageing 2008, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Roberts, L.; Suzuki, K. Exercise and Inflammation. Antioxidants 2019, 8, 155. [Google Scholar] [CrossRef] [Green Version]
- Special Issue “Anti-Inflammatory and Antioxidant Effects of Dietary Supplementation and Lifestyle Factors”. Available online: https://www.mdpi.com/journal/antioxidants/special_issues/anti-inflammatory_antioxidant_effects (accessed on 25 March 2020).


| References | Subjects | Exercise Protocols | Exercise Duration | Time Points | Measured Substances | The Changes of Substances |
|---|---|---|---|---|---|---|
| Suzuki, et al. [13] | 10 male athletic students | Maximal exercise test by treadmill running | 10.2 ± 1.7 min | IM, Post 1 h, Post 2 h | TNF-α, IL-1β, IL-2, IL-12, IFN-ɤ, IL-1ra, IL-4, IL-10, IL-6, G-CSF, GM-CSF, M-CSF, IL-8, MCP-1 | IM: G-CSF, GM-CSF, M-CSF, MCP-1↑ Post 1 h: IL-1ra, IL-6, G-CSF, GM-CSF↑ Post 2 h: IL-1β, IL-4, G-CSF↑ |
| Sugama, et al. [14,17] | 14 male triathletes | Duathlon race (5-km running, 40-km cycling, 5-km running) | mean time; approx. 2 h | IM, Post 1.5 h, Post 3 h | TNF-α, IL-1β, IL-1ra, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, MCP-1, IL-17, IL-23, MPO, IL-12p40 | IM: IL-1ra, IL-6, IL-8, IL-10, IL-12p40, MCP-1, MPO↑ IL-17, IL-23↓ Post 1.5 h: IL-1β, IL-1ra, IL-6, IL-8, MCP-1, MPO↑ Post 3 h: IL-1ra,↑ |
| Suzuki, et al. [18] | 10 male runners | Full marathon race | mean time; 2.62 h (rang, 2.55–68 h) | IM | TNF-α, IL-1β, IL-6, IL-8, IL-10, G-CSF, M-CSF, GM-CSF, MCP-1 | IM: IL-6, IL-8, IL-10, G-CSF, M-CSF, MCP-1, MPO↑ |
| Suzuki, et al. [19] | 7 male triathletes | Duathlon race (5-km running, 40-km cycling, 5-km running) | mean time; approx. 2 h | IM | IL-6, IL-8, IL-10, IL-1ra, MCP-1 | IM: IL-6, IL-8, IL-10, MCP-1↑ |
| Suzuki, et al. [22] | 16 male runners | Full marathon race | mean time; 2 h 34 min (rang, 2 h 25 min-2 h 40 min) | IM | IL-1β, IL-1ra, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, IFN-α, IFN-ɤ, G-CSF, GM-CSF, TGF-β1 | IM: IL-1ra, IL-6, IL-8, IL-10, G-CSF↑ IL-4↓ |
| Suzuki, et al. [24] | 9 male triathletes | Ironman triathlon race (3.8-km swim, 180-km cycling, 42.2-km running) | mean time; 9 h 59 min | IM | IL-1ra, IL-6, IL-10, G-CSF, IL-12p40, IL-4, IL-1β | IM: IL-1ra, IL-6, IL-10, IL-12p40, G-CSF↑ Post 1 d: IL-1ra, IL-6, G-CSF↑ |
| Suzuki, et al. [26] | 8 male athletic students | Cycling with 90W power output | 90 mim | Ex 30 min, 60 min, IM, Post 1 h, 3 h, 12 h | IL-1β, IL-6, IL-8, TNF-α, IFN-ɤ | IM: IL-6↑ Post 3 h: IL-6 Post 12 h: IL-6↑ |
| Kim, et al. [27] | 14 males with no regular exercise training | 60% VO2max walking | 60 min | IM, Post 2 h | IL-6, TNF-α, IL-1β | IM: IL-6↑ |
| Nieman, et al. [29] | 12 male and 4 female marathon runners | Treadmill running | 3 h | IM | IL-6, IL-8, IL-10, IL-1ra | IM: IL-6, IL-8, IL-10, IL-1ra↑ |
| Nieman, et al. [30] | 15 trained male cyclists | 75% VO2max cycling | 2.5 h | IM, Post 12 h | IL-6, IL-8, IL-10, IL-1ra | IM: IL-6, IL-8, IL-10, IL-1ra↑ |
| Nieman, et al. [31] | 18 male and 3 female ultramarathon runners as the placebo group | 160-km Western States Endurance Run | 27.5 ± 0.6 h | IM | IL-6, IL-8, IL-10, IL-1ra, G-CSF, MCP-1, MIP-1β, TNF-α, MIF-1 | IM: IL-6, IL-8, IL-10, IL-1ra, G-CSF, MCP-1, MIP-1β, TNF-α, MIF-1↑ |
| Peake, et al. [33] | 10 well-trained male runners | Running at 60% VO2max. | 45 min | IM, Post 1 h, 24 h | IL-6, IL-8 | IM: IL-6↑ Post 1 h:IL-6↑ |
| Hayashida, et al. [34] | 10 healthy sedentary females | Cycling at 75% of their individual anaerobic threshold | 60 min | IM, Post 30 min | IL-6, Calprotectin, MPO | IM: IL-6↑ Post 30 min: Calprotectin, MPO↑ |
| Peake, et al. [35] | 10 well-trained male cyclists | Cycling at 60% VO2max + 16.1-km time trial | 90 min | IM, R1: Post 35-40 min; R2: Post 80-85 min | Calprotectin, G-CSF, MPO, TNF-α, IL-1ra, IL-6, IL-8, IL-10 | IM, R1 and R2: G-CSF, IL-8, Calprotectin, MPO, IL-10↑ |
| Peake, et al. [36] | 10 male cyclists | ① 18.1 +/− 0.4 degrees C, 58% +/− 8% relative humidity, 90 min at approximately 60% VO2max and then completed a 16.1-km time trial②32.2 +/− 0.7 degrees C, 55% +/− 2% relative humidity, 90 min at approximately 60% VO2max and then completed a 16.1-km time trial | Cycling for 90 min and a time trial | IM | IL-6, IL-8, IL-10, G-CSF, Calprotectin, MPO | ① and ②: IL-6, IL-8, IL-10, G-CSF, Calprotectin, MPO↑ |
| Yamada, et al. [37] | 12 male winter-sports athletes | A maximal exercise test on a treadmill (started at 220 m/min for the first 2 min and 220 m/min at a 4% grade for the next 2 min) | Mean running time: 10.3 ± 2.3 min | IM, Post 1 h, 2 h | G-CSF, IL-6 | IM: G-CSF↑Post 1 h: IL-6↑ |
| Mezil, et al. [39] | 23 males | High intensity interval exercise | Total 6 min | Post 5 min, 1 h, 24 h | IL-1α, IL-1β, IL-6, TNF-α | Post 5 min: IL-1α, IL-1β, IL-6, TNF-α↑ |
| Lira, et al. [40] | 10 active males | ① High intensity intermittent training ② Running at 70% maximal aerobic speed | Not described (total 5 km running) | IM, Post 1 h | IL-6, IL-10, TNF-α | Not changed |
| Brenner, et al. [41] | 8 males | ① All out cycling (equivalent to 90% VO2 max) ② Standard circuit-training routine ③ Cycling at 60–65% VO2 max | ① 5 min ② Not described ③ 2 h | IM, Post 3 h, 24 h, 72 h | IL-6, TNF-α, IL-10 | ① Post 3 h: IL-10↓ Post 24 h: IL-10↓ Post 72 h: IL-10↓ ② Not changed ③ IM: IL-6↑, Post 3 h: IL-6, TNF-α↑ Post 24 h: TNF-α↑ Post 72 h: TNF-α↑ |
| Kanda, et al. [42,43] | 9 healthy males | 10 sets of 40 repetitions of exercise at 0.5 Hz by the load corresponding to the half of body weight | Not described | Post 2 h, 4 h, 24 h, 48 h, 72 h, 96 h | TNF-α, IL-1β, IL-1ra, IL-2, IL-4, IL-6, IL-8,IL-10, IL-12, MCP-1, IL-17, IL-23,MPO, IL-12p40, IL-12 p70, IFN-γ, MCP-1, G-CSF, Calprotectin, C5a | Not changed |
| Scott, et al. [48] | 10 active males | ① Running at 55% VO2 max ② Running at 65% VO2 max ③ Running at 75% VO2 max | 60 min | Ex 20 min, Ex 40 min, IM, Post 0.5 h, 1 h, 2 h, Post 3 h, 1 d, 2 d, 3 d | TNF-α, IL-6, IL-1ra | ① Ex 40 min~Post 3 h: IL-6↑ ② Ex 40 min~Post 3 h: IL-6↑ Ex 40 min~Post: 1 h IL-1ra↑ ③ Ex 40 min~Post 3 h: IL-6↑ Ex 20 min~Post: 3 h IL-1ra↑ |
| Nieman, et al. [54] | 20 male cyclists | 75 km cycling time trial | 168 ± 26.0 min | IM | IL-6, IL-8, MCP-1 | IM: IL-6, IL-8, MCP-1↑ |
| van Wijck, et al. [85] | 20 males | Cycling at 70% maximal workload | 60 min | IM | MPO, Calprotectin | IM: MPO, Calprotectin↑ |
| Suzuki, et al. [86] | 6 well-trained male cyclists | Cycling at 60% VO2max | 90 min | IM, Post 30 min | IL-1ra, MCP-1, IL-6, Calprotectin, MPO, IL-8, IL-10, IL-12p40 | IM: IL-6↑; Post 30 min: Calprotectin↑ |
| Tanisawa, et al. [87] | 9 healthy males | Cycling at 70% VO2max | 60 min | IM, Post 30 min, 1 h, 2 h | IL-6, G-CSF, MCP-1, IL-8, C5a, MPO, Calprotectin, Elastase | IM: IL-6, IL-8, MPO, Calprotectin, Elastase↑ Post 30 min: IL-6, G-CSF, MCP-1, IL-8, Calprotectin↑ Post 1 h: IL-6, G-CSF, Calprotecin↑ Post 2 h: IL-6, Calprotectin↑ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants 2020, 9, 401. https://doi.org/10.3390/antiox9050401
Suzuki K, Tominaga T, Ruhee RT, Ma S. Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants. 2020; 9(5):401. https://doi.org/10.3390/antiox9050401
Chicago/Turabian StyleSuzuki, Katsuhiko, Takaki Tominaga, Ruheea Taskin Ruhee, and Sihui Ma. 2020. "Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress" Antioxidants 9, no. 5: 401. https://doi.org/10.3390/antiox9050401
APA StyleSuzuki, K., Tominaga, T., Ruhee, R. T., & Ma, S. (2020). Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants, 9(5), 401. https://doi.org/10.3390/antiox9050401








