Antirhea borbonica Aqueous Extract Protects Albumin and Erythrocytes from Glycoxidative Damages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Preparation of Plant Extracts
2.2. Identification of Polyphenols in Medicinal Plant Extract
2.3. Quantification and Identification of Polyphenols in Medicinal Plant Extracts
2.4. Determination of Antioxidant Capacity of Plant Extracts
2.5. BSA and AGE Preparation and Biochemical Characterization
2.6. Fructosamine and Fluorescent AGE Determination
2.7. Free Primary Amino and Sulphydryl Group Determination
2.8. Quenching of Intrinsic and Thioflavin T Fluorescence Determination
2.9. Erythrocyte Preparations
2.10. Zebrafish Maintenance and Treatment
2.11. Mass Spectroscopy for Hemoglobin Glycation Level Determination
2.12. Erythrocyte Sensitivity to Hemolysis Determination
2.13. Flow Cytometry Assays
2.14. Shear Stress Gradient Ektacytometry
2.15. Osmotic Gradient Ektacytometry
2.16. Statistical Analysis
3. Results
3.1. Phenolic Composition of Antirhea borbonica Plant Extracts
3.2. Antirhea borbonica Plant Extracts Prevent Albumin from Glycation and Oxidation
3.3. Antirhea borbonica Plant Extracts Impact on Albumin β-Aggregation
3.4. Effects of Antirhea borbonica Plant Extracts on MGO-Induced Hemoglobin Glycation
3.5. Antirhea borbonica Plant Extracts Prevent Erythrocytes from MGO-Induced Injuries
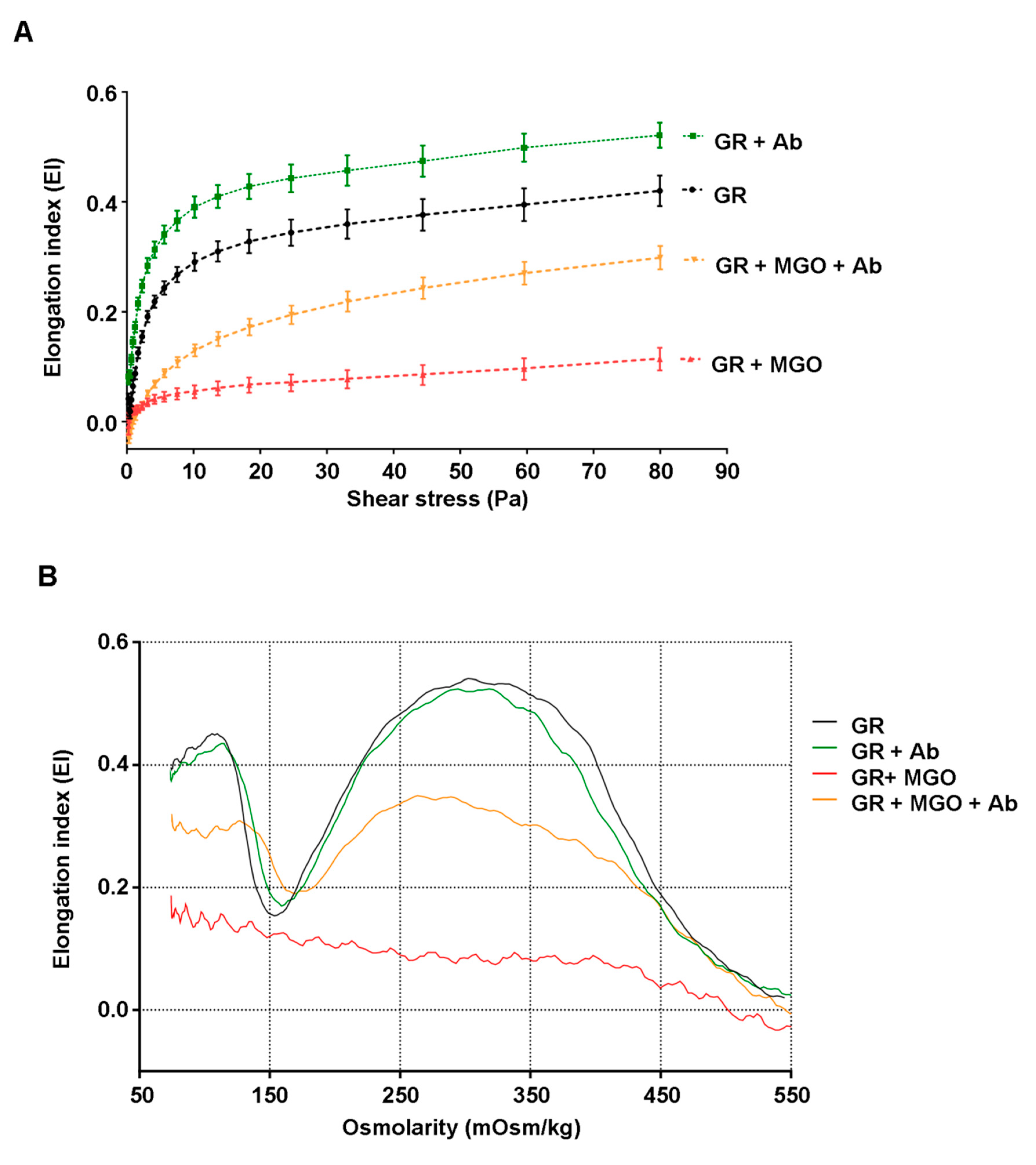
3.6. Antirhea borbonica Plant Extracts Preserve the Deformability Capacity of Erythrocyte Membrane from Glycation Impairments
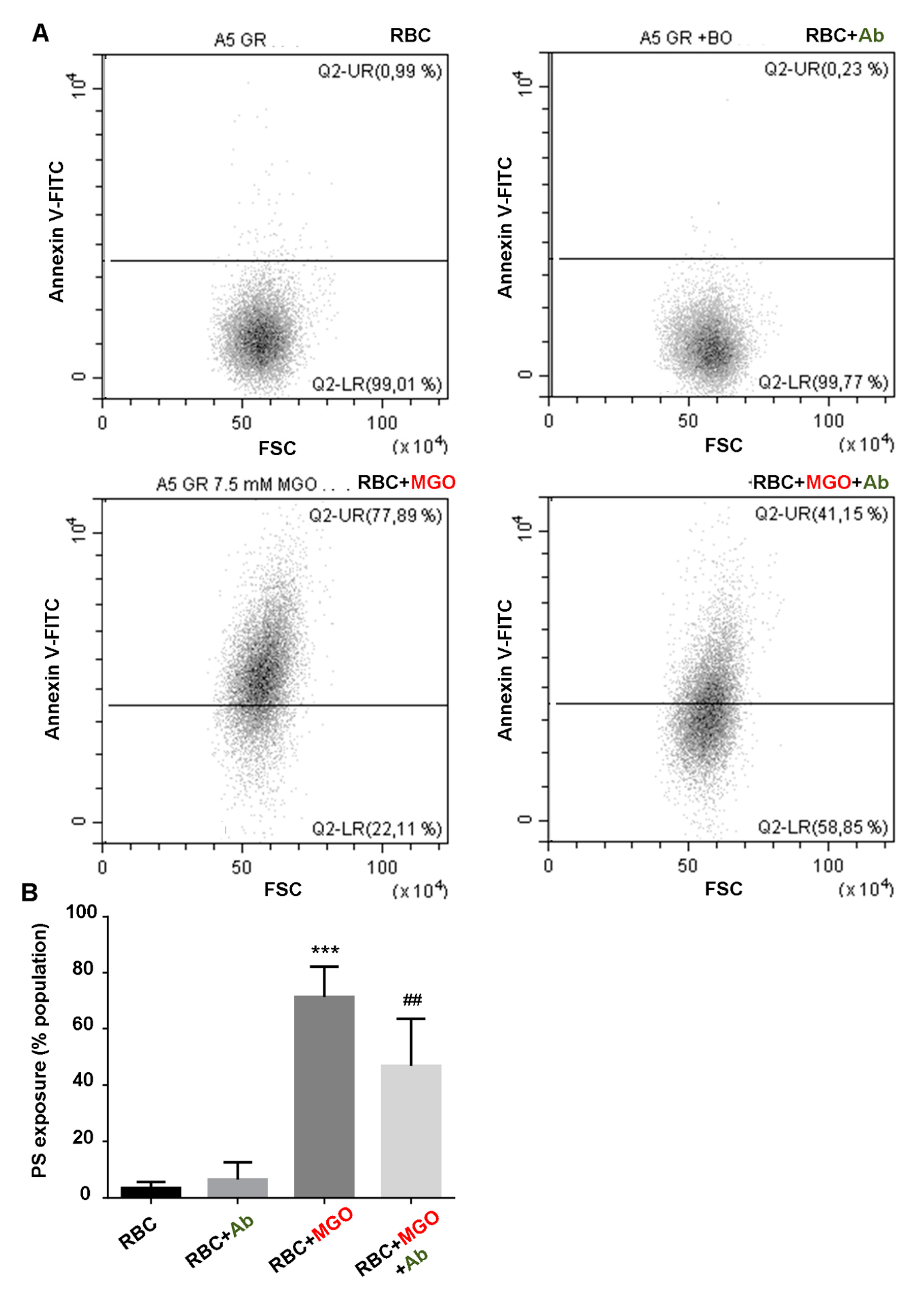
3.7. Antirhea borbonica Plant Extracts Protects Erythrocytes from MGO-Induced Oxidative Stress and PS Exposure
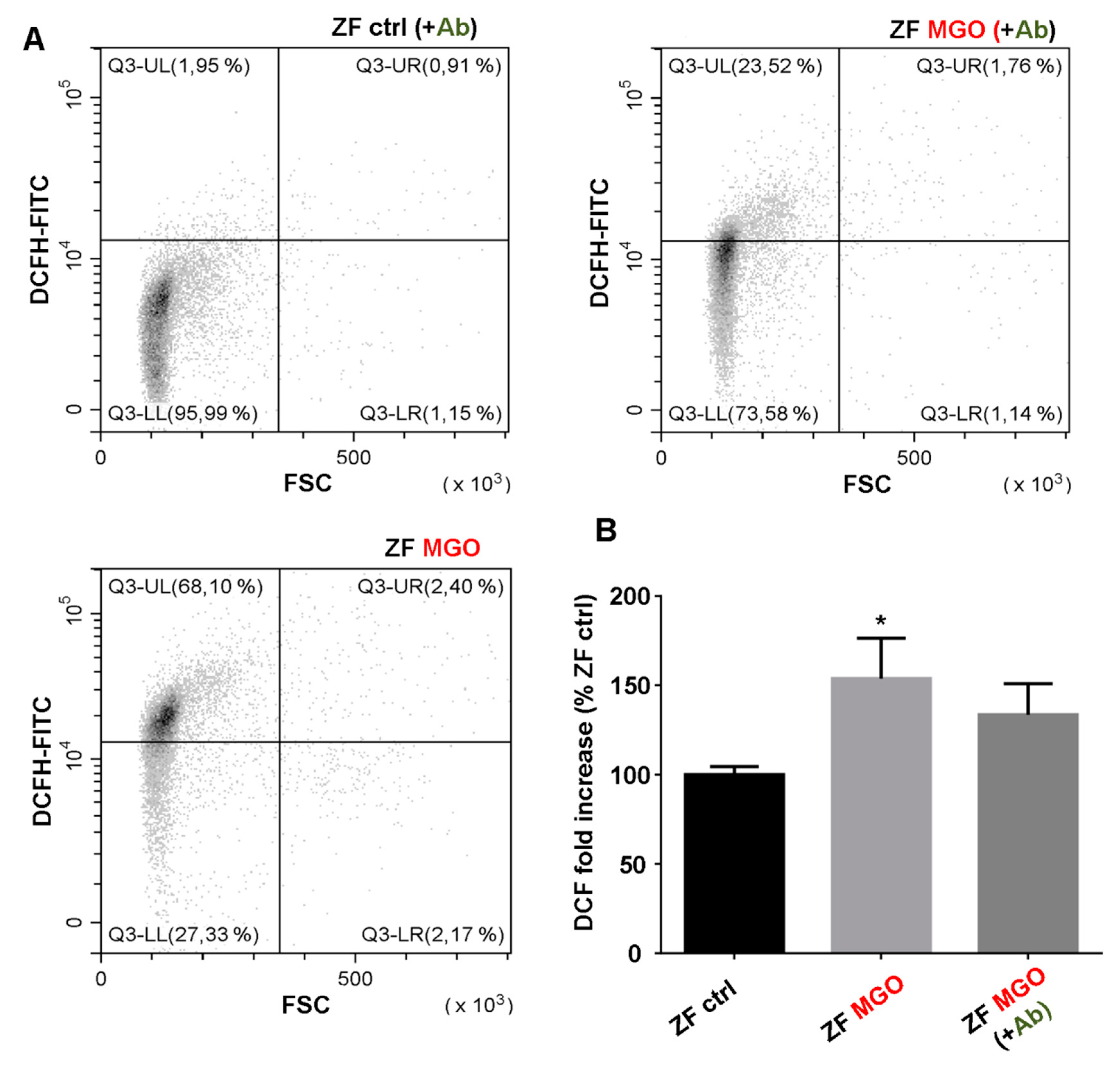
3.8. In Vivo Effects of Antirhea borbonica in Zebrafish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orasanu, G.; Plutzky, J. The pathologic continuum of diabetic vascular disease. J. Am. Coll. Cardiol. 2009, 53, S35–S42. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [Green Version]
- Vlassopoulos, A.; Lean, M.E.; Combet, E. Role of oxidative stress in physiological albumin glycation: A neglected interaction. Free Radic. Biol. Med. 2013, 60, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344 Pt 1, 109–116. [Google Scholar] [CrossRef]
- Goudarzi, M.; Kalantari, H.; Rezaei, M. Glyoxal toxicity in isolated rat liver mitochondria. Hum. Exp. Toxicol. 2018, 37, 532–539. [Google Scholar] [CrossRef]
- Rondeau, P.; Bourdon, E. The glycation of albumin: Structural and functional impacts. Biochimie 2011, 93, 645–658. [Google Scholar] [CrossRef]
- Bouma, B.; Kroon-Batenburg, L.M.; Wu, Y.P.; Brunjes, B.; Posthuma, G.; Kranenburg, O.; de Groot, P.G.; Voest, E.E.; Gebbink, M.F. Glycation induces formation of amyloid cross-beta structure in albumin. J. Biol. Chem. 2003, 278, 41810–41819. [Google Scholar] [CrossRef] [Green Version]
- Sattarahmady, N.; Moosavi-Movahedi, A.A.; Ahmad, F.; Hakimelahi, G.H.; Habibi-Rezaei, M.; Saboury, A.A.; Sheibani, N. Formation of the molten globule-like state during prolonged glycation of human serum albumin. Biochim. Biophys. Acta 2007, 1770, 933–942. [Google Scholar] [CrossRef]
- Baraka-Vidot, J.; Guerin-Dubourg, A.; Bourdon, E.; Rondeau, P. Impaired drugs-binding capacities of in vitro and in vivo glycated albumin. Biochimie 2012, 94, 1960–1967. [Google Scholar] [CrossRef]
- Gajahi Soudahome, A.; Catan, A.; Giraud, P.; Assouan Kouao, S.; Guerin-Dubourg, A.; Debussche, X.; Le Moullec, N.; Bourdon, E.; Bravo, S.B.; Paradela-Dobarro, B.; et al. Glycation of human serum albumin impairs binding to the glucagon-like peptide-1 analogue liraglutide. J. Biol. Chem. 2018, 293, 4778–4791. [Google Scholar] [CrossRef] [Green Version]
- Neelofar, K.; Ahmad, J. An overview of in vitro and in vivo glycation of albumin: A potential disease marker in diabetes mellitus. Glycoconj. J. 2017, 34, 575–584. [Google Scholar] [CrossRef]
- Wautier, J.L.; Wautier, M.P.; Schmidt, A.M.; Anderson, G.M.; Hori, O.; Zoukourian, C.; Capron, L.; Chappey, O.; Yan, S.D.; Brett, J.; et al. Advanced glycation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: A link between surface-associated AGEs and diabetic complications. Proc. Natl. Acad. Sci. USA 1994, 91, 7742–7746. [Google Scholar] [CrossRef] [Green Version]
- Babu, N.; Singh, M. Influence of hyperglycemia on aggregation, deformability and shape parameters of erythrocytes. Clin. Hemorheol. Microcirc. 2004, 31, 273–280. [Google Scholar]
- Buttari, B.; Profumo, E.; Rigano, R. Crosstalk between red blood cells and the immune system and its impact on atherosclerosis. Biomed. Res. Int. 2015, 2015, 616834. [Google Scholar] [CrossRef] [Green Version]
- Catan, A.; Turpin, C.; Diotel, N.; Patche, J.; Guerin-Dubourg, A.; Debussche, X.; Bourdon, E.; Ah-You, N.; Le Moullec, N.; Besnard, M.; et al. Aging and glycation promote erythrocyte phagocytosis by human endothelial cells: Potential impact in atherothrombosis under diabetic conditions. Atherosclerosis 2019, 291, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Mokken, F.C.; Kedaria, M.; Henny, C.P.; Hardeman, M.R.; Gelb, A.W. The clinical importance of erythrocyte deformability, a hemorrheological parameter. Ann. Hematol. 1992, 64, 113–122. [Google Scholar] [CrossRef]
- Noad, R.L.; Rooney, C.; McCall, D.; Young, I.S.; McCance, D.; McKinley, M.C.; Woodside, J.V.; McKeown, P.P. Beneficial effect of a polyphenol-rich diet on cardiovascular risk: A randomised control trial. Heart 2016, 102, 1371–1379. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Giraud-Techer, S.; Amédé, J.; Girard-Valenciennes, E.; Thomas, H.; Brillant, S.; Grondin, I.; Marodon, C.; Smadja, J. Plantes médicinales de La Réunion inscrites à la Pharmacopée française. Ethnopharmacologia 2016, 56, 7–33. [Google Scholar]
- Lavergne, R. Tisaneurs et Plantes Médicinales Indigenes a la Réunion; Orphie Edition: Saint-Denis, France, 2016; pp. 440–445. [Google Scholar]
- Gurib-Fakim, A. Novel Plant Bioresources: Applications in Food, Medicine and Cosmetics; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Smadja, J.; Marodon, C. Le Grand Livre des Plantes Médicinales de l’ile de La Réunion: Inscrites à la Pharmacopée Française; Orphie Edition: Saint-Denis, France, 2016. [Google Scholar]
- Le Sage, F.; Meilhac, O.; Gonthier, M.P. Anti-inflammatory and antioxidant effects of polyphenols extracted from Antirhea borbonica medicinal plant on adipocytes exposed to Porphyromonas gingivalis and Escherichia coli lipopolysaccharides. Pharmacol. Res. 2017, 119, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Marimoutou, M.; Le Sage, F.; Smadja, J.; Lefebvre d’Hellencourt, C.; Gonthier, M.P.; Robert-Da Silva, C. Antioxidant polyphenol-rich extracts from the medicinal plants Antirhea borbonica, Doratoxylon apetalum and Gouania mauritiana protect 3T3-L1 preadipocytes against H2O2, TNFalpha and LPS inflammatory mediators by regulating the expression of superoxide dismutase and NF-kappaB genes. J. Inflamm. 2015, 12, 10. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Yang, H.; Protiva, P.; Cui, B.; Ma, C.; Baggett, S.; Hequet, V.; Mori, S.; Weinstein, I.B.; Kennelly, E.J. New Bioactive Polyphenols from Theobroma grandiflorum (“Cupuaçu”). J. Nat. Prod. 2003, 66, 1501–1504. [Google Scholar] [CrossRef]
- Baraka-Vidot, J.; Planesse, C.; Meilhac, O.; Militello, V.; van den Elsen, J.; Bourdon, E.; Rondeau, P. Glycation alters ligand binding, enzymatic and pharmacological properties of human albumin. Biochemistry 2015, 54, 3051–3062. [Google Scholar] [CrossRef]
- Baraka-Vidot, J.; Denemont, I.; Ali Mcolo, Z.; Bourdon, E.; Rondeau, P. Ammonium Sulfate Precipitation but not Delipidation is a Good Method for Human Albumin Preparation for Biological Studies. Int. J. Diabetes Clin. Diagn. 2015, 2, 109. [Google Scholar] [CrossRef]
- Paradela-Dobarro, B.; Rodino-Janeiro, B.; Alonso, J.; Raposeiras-Roubin, S.; Gonzalez-Peteiro, M.; Gonzalez-Juanatey, J.; Alvarez, E. Key structural and functional differences between early and advanced glycation products. J. Mol. Endocrinol. 2016, 56, 23–37. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Hardeman, M.R.; Uyuklu, M.; Ulker, P.; Cengiz, M.; Nemeth, N.; Shin, S.; Alexy, T.; Meiselman, H.J. Parameterization of red blood cell elongation index--shear stress curves obtained by ektacytometry. Scand. J. Clin. Lab. Investig. 2009, 69, 777–788. [Google Scholar] [CrossRef]
- Nemeth, N.; Kiss, F.; Miszti-Blasius, K. Interpretation of osmotic gradient ektacytometry (osmoscan) data: A comparative study for methodological standards. Scand. J. Clin. Lab. Investig. 2015, 75, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Arias, C.F.; Arias, C.F. How do red blood cells know when to die? R. Soc. Open Sci. 2017, 4, 160850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorsemans, A.C.; Soule, S.; Weger, M.; Bourdon, E.; Lefebvre d’Hellencourt, C.; Meilhac, O.; Diotel, N. Impaired constitutive and regenerative neurogenesis in adult hyperglycemic zebrafish. J. Comp. Neurol. 2017, 525, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Intine, R.V.; Olsen, A.S.; Sarras, M.P., Jr. A zebrafish model of diabetes mellitus and metabolic memory. J. Vis. Exp. 2013, 72, e50232. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.M.; Byun, K.; Cho, K.; Kim, J.Y.; Yoo, J.S.; Kim, D.; Paek, S.H.; Kim, S.U.; Simpson, R.J.; Lee, B. Human microglial cells synthesize albumin in brain. PLoS ONE 2008, 3, e2829. [Google Scholar] [CrossRef] [Green Version]
- Machado, A.P.; Pinto, R.S.; Moyses, Z.P.; Nakandakare, E.R.; Quintao, E.C.; Passarelli, M. Aminoguanidine and metformin prevent the reduced rate of HDL-mediated cell cholesterol efflux induced by formation of advanced glycation end products. Int. J. Biochem. Cell Biol. 2006, 38, 392–403. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Danino, O.; Gottlieb, H.E.; Grossman, S.; Bergman, M. Antioxidant activity of 1,3-dicaffeoylquinic acid isolated from Inula viscosa. Food Res. Int. 2009, 42, 1273–1280. [Google Scholar] [CrossRef]
- McCarty, M.F. A chlorogenic acid-induced increase in GLP-1 production may mediate the impact of heavy coffee consumption on diabetes risk. Med. Hypotheses 2005, 64, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.H.; Wishart, K. Effects of chlorogenic acid and bovine serum albumin on the oxidative stability of low density lipoproteins in vitro. J. Agric. Food. Chem. 2010, 58, 5828–5833. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Boitier, A.; Stewart, A.J.; Crozier, A. Flavonoid metabolites in human plasma and urine after the consumption of red onions: Analysis by liquid chromatography with photodiode array and full scan tandem mass spectrometric detection. J. Chromatogr. A 2004, 1058, 163–168. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: Identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef] [Green Version]
- Rondeau, P.; Navarra, G.; Cacciabaudo, F.; Leone, M.; Bourdon, E.; Militello, V. Thermal aggregation of glycated bovine serum albumin. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 789–798. [Google Scholar] [CrossRef]
- Kang, J.; Liu, Y.; Xie, M.-X.; Li, S.; Jiang, M.; Wang, Y.-D. Interactions of human serum albumin with chlorogenic acid and ferulic acid. Biochim. Biophys. Acta Gen. Subj. 2004, 1674, 205–214. [Google Scholar] [CrossRef]
- Tang, D.; Li, H.-J.; Li, P.; Wen, X.-D.; Qian, Z.-M. Interaction of Bioactive Components Caffeoylquinic Acid Derivatives in Chinese Medicines with Bovine Serum Albumin. Chem. Pharm. Bull. 2008, 56, 360–365. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, D.; Wang, G.; Lu, Y. Study of interaction between human serum albumin and three antioxidants: Ascorbic acid, a-tocopherol and proanthocyanidins. Eur. J. Med. Chem. 2013, 70, 22–36. [Google Scholar] [CrossRef]
- Lo, C.-Y.; Hsiao, W.-T.; Chen, X.-Y. Efficiency of Trapping Methylglyoxal by Phenols and Phenolic Acids. J. Food Sci. 2011, 76, H90–H96. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Martini, S.; Conte, A. Protocatechuic and 3,4-Dihydroxyphenylacetic Acids Inhibit Protein Glycation by Binding Lysine through a Metal-Catalyzed Oxidative Mechanism. J. Agric. Food Chem. 2019, 67, 7821–7831. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, E.; Loreau, N.; Blache, D. Glucose and free radicals impair the antioxidant properties of serum albumin. FASEB J. 1999, 13, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Tupe, R.S.; Sankhe, N.M.; Shaikh, S.A.; Phatak, D.V.; Parikh, J.U.; Khaire, A.A.; Kemse, N.G. Aqueous extract of some indigenous medicinal plants inhibits glycation at multiple stages and protects erythrocytes from oxidative damage-an in vitro study. J. Food Sci. Technol. 2015, 52, 1911–1923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiruthiga, P.V.; Shafreen, R.B.; Pandian, S.K.; Devi, K.P. Silymarin protection against major reactive oxygen species released by environmental toxins: Exogenous H2O2 exposure in erythrocytes. Basic Clin. Pharmacol. Toxicol. 2007, 100, 414–419. [Google Scholar] [CrossRef]
- Kiruthiga, P.V.; Shafreen, R.B.; Pandian, S.K.; Arun, S.; Govindu, S.; Devi, K.P. Protective effect of silymarin on erythrocyte haemolysate against benzo(a)pyrene and exogenous reactive oxygen species (H2O2) induced oxidative stress. Chemosphere 2007, 68, 1511–1518. [Google Scholar] [CrossRef]
- Konyalioglu, S.; Karamenderes, C. The protective effects of Achillea L. species native in Turkey against H(2)O(2)-induced oxidative damage in human erythrocytes and leucocytes. J. Ethnopharmacol. 2005, 102, 221–227. [Google Scholar] [CrossRef]
- Asgary, S.; Naderi, G.; Askari, N. Protective effect of flavonoids against red blood cell hemolysis by free radicals. Exp. Clin. Cardiol. 2005, 10, 88–90. [Google Scholar]
- Lee, P.; Wu, X. Review: Modifications of human serum albumin and their binding effect. Curr. Pharm. Des. 2015, 21, 1862–1865. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.S.; Nowak, R.B.; Zhou, S.; Giannetto, M.; Gokhin, D.S.; Papoin, J.; Ghiran, I.C.; Blanc, L.; Wan, J.; Fowler, V.M. Myosin IIA interacts with the spectrin-actin membrane skeleton to control red blood cell membrane curvature and deformability. Proc. Natl. Acad. Sci. USA 2018, 115, E4377–E4385. [Google Scholar] [CrossRef] [Green Version]
- Paiva-Martins, F.; Fernandes, J.; Rocha, S.; Nascimento, H.; Vitorino, R.; Amado, F.; Borges, F.; Belo, L.; Santos-Silva, A. Effects of olive oil polyphenols on erythrocyte oxidative damage. Mol. Nutr. Food Res. 2009, 53, 609–616. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Fernandes, J.; Santos, V.; Silva, L.; Borges, F.; Rocha, S.; Belo, L.; Santos-Silva, A. Powerful protective role of 3,4-dihydroxyphenylethanol-elenolic acid dialdehyde against erythrocyte oxidative-induced hemolysis. J. Agric. Food Chem. 2010, 58, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Martins, F.; Goncalves, P.; Borges, J.E.; Przybylska, D.; Ibba, F.; Fernandes, J.; Santos-Silva, A. Effects of the olive oil phenol metabolite 3,4-DHPEA-EDAH2 on human erythrocyte oxidative damage. Food Funct. 2015, 6, 2350–2356. [Google Scholar] [CrossRef] [PubMed]
- Heckler, K.; Kroll, J. Zebrafish as a Model for the Study of Microvascular Complications of Diabetes and Their Mechanisms. Int. J. Mol. Sci. 2017, 18, 2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgens, K.; Stoll, S.J.; Pohl, J.; Fleming, T.H.; Sticht, C.; Nawroth, P.P.; Hammes, H.P.; Kroll, J. High tissue glucose alters intersomitic blood vessels in zebrafish via methylglyoxal targeting the VEGF receptor signaling cascade. Diabetes 2015, 64, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Rastegar, S.; Parimisetty, A.; Cassam Sulliman, N.; Narra, S.S.; Weber, S.; Rastegar, M.; Viranaicken, W.; Couret, D.; Planesse, C.; Strahle, U.; et al. Expression of adiponectin receptors in the brain of adult zebrafish and mouse: Links with neurogenic niches and brain repair. J. Comp. Neurol. 2019, 527, 2317–2333. [Google Scholar] [CrossRef] [PubMed]
- Patche, J.; Girard, D.; Catan, A.; Boyer, F.; Dobi, A.; Planesse, C.; Diotel, N.; Guerin-Dubourg, A.; Baret, P.; Bravo, S.B.; et al. Diabetes-induced hepatic oxidative stress: A new pathogenic role for glycated albumin. Free Radic. Biol. Med. 2017, 102, 133–148. [Google Scholar] [CrossRef] [PubMed]


| Peak N | Compound Name | Retention Time (min) | [M – H] | Formula |
|---|---|---|---|---|
| 1 | Gallic acid (traces) | 1.2 | 169.0142 | C7H5O5 |
| 2 | Chlorogenic acid | 2.7 | 353.0878 | C16H16O9 |
| 3 | Chlorogenic acid (isomer) | 3.5 | 353.0878 | C16H16O9 |
| 4 | Quercetin hexoside | 5.0 | 463.0882 | C21H19O12 |
| 5 | Kaempferol hexoside | 5.0 | 593.1512 | C21H19O11 |
| 6 | Kaempferol hexoside (isomer) | 5.2 | 593.1512 | C21H19O11 |
| 7 | Kaempferol hexoside (isomer) | 5.4 | 593.1512 | C21H19O11 |
| 8 | Dicaffeoylquinic acid | 6.1 | 515.1195 | C25H23O12 |
| 9 | Dicaffeoylquinic acid (isomer) | 6.4 | 515.1195 | C25H23O12 |
| 10 | Chlorogenic acid (isomer) | 6.6 | 353.0878 | C16H16O9 |
| 11 | Dicaffeoylquinic acid (isomer) | 6.6 | 515.1195 | C25H23O12 |
| 12 | Quercetin | 7.4 | 301.0354 | C15H9O7 |
| Total Polyphenol Content (mg GAE/g Plant) | Total Flavonoid Content (mg EE/g Plant) | Radical Scavenging Capacity (mM Trolox eq.) | |
|---|---|---|---|
| Antirhea borbonica plant extract | 7.69 ± 0.59 | 2.70 ± 0.04 | 16.30 ± 2.73 |
| Antirhea borbonica (10 mM GAE) | Gallic Acid (10 mM) | Ascorbic Acid (10 mM) | Caffeic Acid (8.5 mM) | |
|---|---|---|---|---|
| Radical scavenging capacity | 82.59 ± 2.71 | 92.00 ± 2.54 | 93.20 ± 3.30 | 41.22 ± 1.22 |
| (% DPPH reduced) | 82.59 ± 2.71 | 92.00 ± 2.54 | 93.20 ± 3.30 | 41.22 ± 1.22 |
| Ketoamine/BSA (mol/mol) | Fluorescent AGE (% / BSA) | Free Amine/BSA (mol/mol) | Thiols/BSA (mol/mol) | β-Amyloid formation (%/BSA) | Intrinsic Fluo Quenching (%/BSA) | |
|---|---|---|---|---|---|---|
| BSA | 0.07 ± 0.29 | 0 ± 96.38 | 58.86 ± 2.43 | 0.968 ± 0.089 | 0 ± 29.04 | 0 ± 1.54 |
| BSA + Ab | 0.59 ± 0.03 | 97.72 ± 5.42 | 60.81 ± 6.20 | 0.178 ± 0.023 ### | 1725.9 ± 374.7 ### | 49.63 ± 2.38 ### |
| BSA + MGO | 6.30 ± 0.44 *** | 707.69 ± 193.15 *** | 46.94 ± 1.06 ** | 0.384 ± 0.024 ** | 722.5 ± 64.9 *** | 88.40 ± 0.75 *** |
| BSA + MGO + Ab | 3.97 ± 0.07 # | 331.80 ± 43.18 # | 49.55 ± 0.93 # | 0.426 ± 0.070 | 848.8 ± 198.5 *** | 87.92 ± 0.29 *** |
| α-Hemoglobin | β-Hemoglobin | |||
|---|---|---|---|---|
| ∆Mass | Relative % Glycation | ∆Mass | Relative % Glycation | |
| RBC | 194.0 ± 4.0 | 17.8 ± 0.9 | 188.3 ± 4.0 | 25.2 ± 1.1 |
| RBC + Ab | 187.7 ± 3.8 | 20.5 ± 1.2 | 184.0 ± 3.0 | 27.4 ± 0.7 |
| RBC + MGO | 194.4 ± 3.6 | 25.3 ± 1.8 * | 192.0 ± 2.2 | 32.7 ± 2.9 * |
| RBC+MGO + Ab | 196.3 ± 2.7 | 25.1 ± 1.0 # | 193.6 ± 1.9 | 33.7 ± 1.1 # |
| RBC | RBC + Ab | RBC + MGO | RBC + MGO + Ab | |
|---|---|---|---|---|
| Elongation parameters | ||||
| EImax | 0.428 ± 0.038 | 0.492 ± 0.026 | 0.113 ± 0.022 *** | 0.239 ± 0.103 ## |
| SS1/2 | 4.447 ± 0.978 | 2.300 ± 0.090 | 12.259 ± 4.284 ** | 14.197 ± 2.765 |
| Osmoscan parameters | ||||
| EIos-max | 0.494 ± 0.066 | 0.495 ± 0.057 | 0.206 ± 0.035 ** | 0.302 ± 0.032 # |
| Eios-min | 0.200 ± 0.064 | 0.200 ± 0.040 | 0.226 ± 0.055 | 0.192 ± 0.002 |
| rEI | 2.669 ± 0.660 | 2.573 ± 0.719 | 0.970 ± 0.052 * | 1.572 ± 0.237 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delveaux, J.; Turpin, C.; Veeren, B.; Diotel, N.; Bravo, S.B.; Begue, F.; Álvarez, E.; Meilhac, O.; Bourdon, E.; Rondeau, P. Antirhea borbonica Aqueous Extract Protects Albumin and Erythrocytes from Glycoxidative Damages. Antioxidants 2020, 9, 415. https://doi.org/10.3390/antiox9050415
Delveaux J, Turpin C, Veeren B, Diotel N, Bravo SB, Begue F, Álvarez E, Meilhac O, Bourdon E, Rondeau P. Antirhea borbonica Aqueous Extract Protects Albumin and Erythrocytes from Glycoxidative Damages. Antioxidants. 2020; 9(5):415. https://doi.org/10.3390/antiox9050415
Chicago/Turabian StyleDelveaux, Jade, Chloé Turpin, Bryan Veeren, Nicolas Diotel, Susana B. Bravo, Floran Begue, Ezequiel Álvarez, Olivier Meilhac, Emmanuel Bourdon, and Philippe Rondeau. 2020. "Antirhea borbonica Aqueous Extract Protects Albumin and Erythrocytes from Glycoxidative Damages" Antioxidants 9, no. 5: 415. https://doi.org/10.3390/antiox9050415
APA StyleDelveaux, J., Turpin, C., Veeren, B., Diotel, N., Bravo, S. B., Begue, F., Álvarez, E., Meilhac, O., Bourdon, E., & Rondeau, P. (2020). Antirhea borbonica Aqueous Extract Protects Albumin and Erythrocytes from Glycoxidative Damages. Antioxidants, 9(5), 415. https://doi.org/10.3390/antiox9050415







