Piceatannol-Loaded Emulsomes Exhibit Enhanced Cytostatic and Apoptotic Activities in Colon Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.2.1. Preparation of PIC-E
2.2.2. Measurement of Particle Size
2.2.3. Drug Entrapment Determination
2.2.4. Optimization of PIC-E
2.3. Characterization of Optimized Formulation
2.3.1. Transmission Electron Microscopy
2.3.2. Drug Release (In Vitro)
2.3.3. Determination of IC50 Values
2.3.4. Cell Cycle Analysis
2.3.5. Annexin V Assay
2.3.6. Mitochondrial Membrane Potential
2.3.7. Cleaved Caspase-3 Content
2.3.8. Real-Time Polymerase Chain Reaction
2.3.9. Assessment of Nitric Oxide (NO) Production
2.3.10. Reactive Oxygen Species (ROS) Determination
2.3.11. Statistical Analysis
3. Results
3.1. Statistical Analysis for Model Selection
3.1.1. Variables’ Influence on Particle Size
3.1.2. Variables’ Influence on Particle Size and PIC-E
3.1.3. Variables’ Influence on Particle Size
3.2. Optimization of PIC-E
3.3. Characterization and Evaluation of Optimized Formulation
3.3.1. Transmission Electron Microscopy
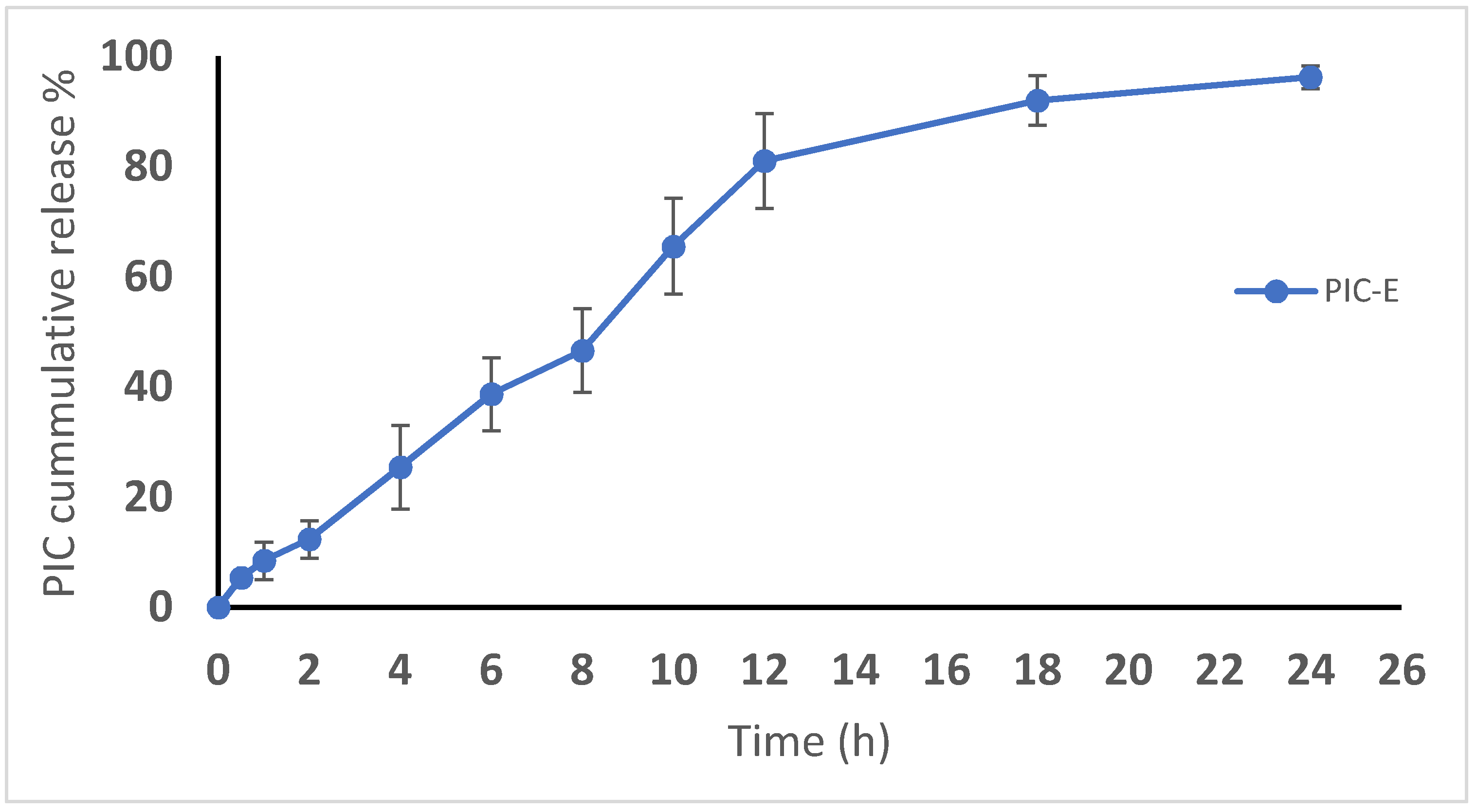
3.3.2. Drug Release (In Vitro)
3.3.3. Determination of IC50 Values
3.3.4. Cell Cycle Analysis
3.3.5. Annexin V Staining
3.3.6. Mitochondrial Membrane Potential
3.3.7. Cleaved Caspase-3 Content
3.3.8. mRNA Expression of Bax and Bcl-2
3.3.9. Nitric Oxide Determination
3.3.10. ROS Determination
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiello, P.; Sharghi, M.; Mansourkhani, S.M.; Ardekan, A.P.; Jouybari, L.; Daraei, N.; Peiro, K.; Mohamadian, S.; Rezaei, M.; Heidari, M.; et al. Medicinal Plants in the Prevention and Treatment of Colon Cancer. Oxid. Med. Cell. Longev. 2019, 2019, 2075614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Yang, Z.; Xie, Q.; Zhang, Z.; Zhang, H.; Ma, J. Natural products for treating colorectal cancer: A mechanistic review. Biomed. Pharm. 2019, 117, 109142. [Google Scholar] [CrossRef] [PubMed]
- Varamenti, E.I.; Kyparos, A.; Veskoukis, A.S.; Bakou, M.; Kalaboka, S.; Jamurtas, A.Z.; Koutedakis, Y.; Kouretas, D. Oxidative stress, inflammation and angiogenesis markers in elite female water polo athletes throughout a season. Food Chem. Toxicol. 2013, 61, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Seyed, M.A.; Jantan, I.; Bukhari, S.N.A.; Vijayaraghavan, K. A Comprehensive Review on the Chemotherapeutic Potential of Piceatannol for Cancer Treatment, with Mechanistic Insights. J. Agric. Food Chem. 2016, 64, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Farrand, L.; Byun, S.; Kim, J.Y.; Im-Aram, A.; Lee, J.; Lim, S.; Lee, K.W.; Suh, J.-Y.; Lee, H.J.; Tsang, B.K. Piceatannol Enhances Cisplatin Sensitivity in Ovarian Cancer via Modulation of p53, X-linked Inhibitor of Apoptosis Protein (XIAP), and Mitochondrial Fission. J. Biol. Chem. 2013, 288, 23740–23750. [Google Scholar] [CrossRef] [Green Version]
- Wesołowska, O.; Wiśniewski, J.; Bielawska-Pohl, A.; Paprocka, M.; Duarte, N.; Ferreira, M.-J.U.; Duś, D.; Michalak, K. Stilbenes as Multidrug Resistance Modulators and Apoptosis Inducers in Human Adenocarcinoma Cells. Anticancer Res. 2010, 30, 4587–4593. [Google Scholar]
- Zhang, H.; Jia, R.; Wang, C.; Hu, T.; Wang, F. Piceatannol promotes apoptosis via up-regulation of microRNA-129 expression in colorectal cancer cell lines. Biochem. Biophys. Res. Commun. 2014, 452, 775–781. [Google Scholar] [CrossRef]
- AA Aljabali, A.; A Bakshi, H.; L Hakkim, F.; Haggag, A.Y.; M Al-Batanyeh, K.; S Al Zoubi, M.; Al-Trad, B.; M Nasef, M.; Satija, S.; Mehta, M.; et al. Albumin Nano-Encapsulation of Piceatannol Enhances Its Anticancer Potential in Colon Cancer Via Downregulation of Nuclear p65 and HIF-1α. Cancers 2020, 12, 113. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.D. Lipid-Based Nanoparticles in Cancer Diagnosis and Therapy. J. Drug Deliv. 2013, 2013, 165981. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-based nanoparticles: Application and recent advances in cancer treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ucisik, M.H.; Sleytr, U.B.; Schuster, B. Emulsomes Meet S-layer Proteins: An Emerging Targeted Drug Delivery System. Curr. Pharm. Biotechnol. 2015, 16, 392–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paliwal, R.; Paliwal, S.R.; Mishra, N.; Mehta, A.; Vyas, S.P. Engineered chylomicron mimicking carrier emulsome for lymph targeted oral delivery of methotrexate. Int. J. Pharm. 2009, 380, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Ucisik, M.H.; Küpcü, S.; Schuster, B.; Sleytr, U.B. Characterization of CurcuEmulsomes: Nanoformulation for enhanced solubility anddelivery of curcumin. J. Nanobiotechnol. 2013, 11, 37. [Google Scholar] [CrossRef] [Green Version]
- Varshosaz, J.; Raghami, F.; Rostami, M.; Jahanian, A. PEGylated trimethylchitosan emulsomes conjugated to octreotide for targeted delivery of sorafenib to hepatocellular carcinoma cells of HepG2. J. Liposome Res. 2019, 29, 383–398. [Google Scholar] [CrossRef]
- Messiad, H.; Amira-Guebailia, H.; Houache, O. Reversed phase High Performance Liquid Chromatography used for the physicochemical and thermodynamic characterization of piceatannol/β-cyclodextrin complex. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 926, 21–27. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, Q.; Ding, T.; Sun, J. SiO2 nanoparticle-induced impairment of mitochondrial energy metabolism in hepatocytes directly and through a Kupffer cell-mediated pathway in vitro. Int. J. Nanomed. 2014, 9, 2891–2903. [Google Scholar]
- Hussain, S. Measurement of Nanoparticle-Induced Mitochondrial Membrane Potential Alterations BT—Nanotoxicity: Methods and Protocols; Zhang, Q., Ed.; Springer: New York, NY, USA, 2019; pp. 123–131. ISBN 978-1-4939-8916-4. [Google Scholar]
- Luna-Vital, D.A.; González de Mejía, E.; Loarca-Piña, G. Selective mechanism of action of dietary peptides from common bean on HCT116 human colorectal cancer cells through loss of mitochondrial membrane potential and DNA damage. J. Funct. Foods 2016, 23, 24–39. [Google Scholar] [CrossRef]
- Baharara, J.; Ramezani, T.; Divsalar, A.; Mousavi, M.; Seyedarabi, A. Induction of Apoptosis by Green Synthesized Gold Nanoparticles Through Activation of Caspase-3 and 9 in Human Cervical Cancer Cells. Avicenna J. Med. Biotechnol. 2016, 8, 75–83. [Google Scholar]
- Ucisik, M.H.; Küpcü, S.; Breitwieser, A.; Gelbmann, N.; Schuster, B.; Sleytr, U.B. S-layer fusion protein as a tool functionalizing emulsomes and CurcuEmulsomes for antibody binding and targeting. Colloids Surf. B Biointerfaces 2015, 128, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Raza, K.; Prakash, O.; Setia, A.; Bhatia, A.; Singh, B. Improved therapeutic performance of dithranol against psoriasis employing systematically optimized nanoemulsomes. J. Microencapsul. 2012, 30, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.P.; Subhedar, R.; Jain, S. Development and characterization of emulsomes for sustained and targeted delivery of an antiviral agent to liver. J. Pharm. Pharmacol. 2006, 58, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Ahmed, O.A.A.; Fahmy, U.A.; Alkhalidi, H.M. Nanovesicular systems loaded with a recently approved second generation type-5 phospodiesterase inhibitor (avanafil): I. Plackett-Burman screening and characterization. J. Drug Deliv. Sci. Technol. 2018, 43, 154–159. [Google Scholar] [CrossRef]
- Fahmy, U.A.; Aljaeid, B.M. Tadalafil transdermal delivery with alpha-lipoic acid self nanoemulsion for treatment of erectile dysfunction by diabetes mellitus. Int. J. Pharmacol. 2018, 14, 945–951. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Elfaky, M.A.; Fahmy, U.A.; Aljaeid, B.M.; Alshareef, O.A.; El-Say, K.M. Development of a fluvastatin-loaded self-nanoemulsifying system to maximize therapeutic efficacy in human colorectal carcinoma cells. J. Drug Deliv. Sci. Technol. 2018, 46, 7–13. [Google Scholar] [CrossRef]
- Fahmy, U.A.; El-Sisi, A.E.; El-Ghamry, H.A.; Zidan, A.S. Effect of concomitant administration of amoxicillin on the pharmacokinetics and bioavailability of metformin. Lat. Am. J. Pharm. 2013, 32. [Google Scholar]
- Al-Gethmy, H.A.; Fahmy, U.A.; Alhakamy, N.A.; Ahmed, O.A.A.; El-Say, K.M. Optimization of the factors affecting the absorption of vardenafil from oral disintegrating tablets: A clinical pharmacokinetic investigation. Pharmaceutics 2019, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Aldawsari, H. Intranasal optimized solid lipid nanoparticles loaded in situ gel for enhancing trans-mucosal delivery of simvastatin. J. Drug Deliv. Sci. Technol. 2018, 48, 499–508. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, L.; Mollica, M.; Re, A.T.; Wu, S.; Zuo, L. Nitric oxide in cancer metastasis. Cancer Lett. 2014, 353, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Xu, D.; Li, Z.; Gao, Y.; Chen, H. Synthesis and potent cytotoxic activity of a novel diosgenin derivative and its phytosomes against lung cancer cells. Beilstein J. Nanotechnol. 2019, 10, 1933–1942. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Mattingly, C.A.; Tseng, M.T.; Cho, M.J.; Liu, Y.; Adams, V.R.; Mumper, R.J. Doxorubicin and paclitaxel-loaded lipid-based nanoparticles overcome multidrug resistance by inhibiting P-glycoprotein and depleting ATP. Cancer Res. 2009, 69, 3918–3926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hershman, D.L.; Shao, T.; Kushi, L.H.; Buono, D.; Tsai, W.Y.; Fehrenbacher, L.; Kwan, M.; Gomez, S.L.; Neugut, A.I. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res. Treat. 2011, 126, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Hodnick, W.F.; MllosavljeviĆ, E.B.; Nelson, J.H.; Pardini, R.S. Electrochemistry of flavonoids. Relationships between redox potentials, inhibition of mitochondrial respiration, and production of oxygen radicals by flavonoids. Biochem. Pharmacol. 1988, 37, 2607–2611. [Google Scholar] [CrossRef]
- Kuo, P.-L.; Hsu, Y.-L. The grape and wine constituent piceatannol inhibits proliferation of human bladder cancer cells via blocking cell cycle progression and inducing Fas/membrane bound Fas ligand-mediated apoptotic pathway. Mol. Nutr. Food Res. 2008, 52, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. Rev. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Bolat, Z.B.; Islek, Z.; Demir, B.N.; Yilmaz, E.N.; Sahin, F.; Ucisik, M.H. Curcumin- and Piperine-Loaded Emulsomes as Combinational Treatment Approach Enhance the Anticancer Activity of Curcumin on HCT116 Colorectal Cancer Model. Front. Bioeng. Biotechnol. 2020, 8, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkempinck, S.H.E.; Kyomugasho, C.; Salvia-Trujillo, L.; Denis, S.; Bourgeois, M.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Emulsion stabilizing properties of citrus pectin and its interactions with conventional emulsifiers in oil-in-water emulsions. Food Hydrocoll. 2018, 85, 144–157. [Google Scholar] [CrossRef]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (δψm) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef]
- Passos, C.L.A.; Ferreira, C.; Soares, D.C.; Saraiva, E.M. Leishmanicidal effect of synthetic trans-resveratrol analogs. PLoS ONE 2015, 10, e0141778. [Google Scholar] [CrossRef] [Green Version]
- Madreiter-Sokolowski, C.T.; Gottschalk, B.; Parichatikanond, W.; Eroglu, E.; Klec, C.; Waldeck-Weiermair, M.; Malli, R.; Graier, W.F. Resveratrol Specifically Kills Cancer Cells by a Devastating Increase in the Ca2+ Coupling between the Greatly Tethered Endoplasmic Reticulum and Mitochondria. Cell. Physiol. Biochem. 2016, 39, 1404–1420. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M.; Wickramasinghe, N.S.; Ivanova, M.M.; Dougherty, S.M. Resveratrol stimulates nitric oxide production by increasing estrogen receptor α-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008, 22, 2185–2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, J.R.; Kim, S.M.; Hwang, K.A.; Kang, J.H.; Choi, K.C. Resveratrol induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Int. J. Mol. Med. 2018, 42, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.-E. Oxidative stress and its possible relation to lower urinary tract functional pathology. BJU Int. 2018, 121, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Enríquez, S.; Pacheco-Velázquez, S.C.; Marín-Hernández, Á.; Gallardo-Pérez, J.C.; Robledo-Cadena, D.X.; Hernández-Reséndiz, I.; García-García, J.D.; Belmont-Díaz, J.; López-Marure, R.; Hernández-Esquivel, L.; et al. Resveratrol inhibits cancer cell proliferation by impairing oxidative phosphorylation and inducing oxidative stress. Toxicol. Appl. Pharmacol. 2019, 370, 65–77. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Kawakami, S.; Yanae, K.; Sano, S.; Uchida, H.; Inagaki, H.; Ito, T. Effect of long-term piceatannol treatment on eNOS levels in cultured endothelial cells. Biochem. Biophys. Res. Commun. 2013, 430, 1164–1168. [Google Scholar] [CrossRef]
- Islam, S.; Hassan, F.; Mu, M.M.; Ito, H.; Koide, N.; Mori, I.; Yoshida, T.; Yokochi, T. Piceatannol Prevents Lipopolysaccharide (LPS)-Induced Nitric Oxide (NO) Production and Nuclear Factor (NF)-κB Activation by Inhibiting IκB Kinase (IKK). Microbiol. Immunol. 2004, 48, 729–736. [Google Scholar] [CrossRef]
- Youn, J.; Lee, J.-S.; Na, H.-K.; Kundu, J.K.; Surh, Y.-J. Resveratrol and Piceatannol Inhibit iNOS Expression and NF-κ B Activation in Dextran Sulfate Sodium-Induced Mouse Colitis. Nutr. Cancer 2009, 61, 847–854. [Google Scholar] [CrossRef]











| Independent Variables | Levels | ||
| (−1) | (0) | (+1) | |
| X1: PIC concentration (%w/w) | 0.10 | 0.30 | 0.50 |
| X2: Lipoid® S 100 concentration (%w/w) | 1.00 | 2.50 | 4.00 |
| X3: pH of hydration medium | 5.00 | 6.50 | 8.00 |
| Responses | Desirability constraints | ||
| Y1: Particle size (nm) | Minimize | ||
| Y2: Entrapment efficiency (%) | Maximize | ||
| Run # | Independent Variables | Particle Size (nm) ± SD | Entrapment Efficiency (%) ± SD | ||
|---|---|---|---|---|---|
| PIC Concentration (%w/w) | Lipoid® S 100 Concentration (%w/w) | Hydration Medium pH | |||
| F1 | 0.30 | 4.00 | 8.00 | 191.10 ± 3.45 | 88.81 ± 1.45 |
| F2 | 0.10 | 2.50 | 8.00 | 158.81 ± 2.98 | 82.98 ± 1.99 |
| F3 | 0.30 | 2.50 | 6.50 | 169.43 ± 3.99 | 86.81 ± 2.31 |
| F4 | 0.10 | 4.00 | 6.50 | 196.75 ± 4.53 | 88.83 ± 2.16 |
| F5 | 0.30 | 4.00 | 5.00 | 192.32 ± 6.54 | 86.82 ± 1.56 |
| F6 | 0.50 | 4.00 | 6.50 | 212.32 ± 7.32 | 96.51 ± 1.99 |
| F7 | 0.30 | 2.50 | 6.50 | 169.72 ± 3.51 | 87.10 ± 2.54 |
| F8 | 0.50 | 2.50 | 5.00 | 189.76 ± 5.14 | 93.61 ± 2.32 |
| F9 | 0.30 | 2.50 | 6.50 | 172.80 ± 5.89 | 86.19 ± 2.27 |
| F10 | 0.10 | 1.00 | 6.50 | 99.31 ± 2.12 | 81.60 ± 1.49 |
| F11 | 0.50 | 2.50 | 8.00 | 182.81 ± 8.32 | 90.12 ± 1.89 |
| F12 | 0.10 | 2.50 | 5.00 | 169.83 ± 4.46 | 81.21 ± 2.17 |
| F13 | 0.30 | 1.00 | 8.00 | 107.34 ± 2.79 | 82.30 ± 2.39 |
| F14 | 0.30 | 2.50 | 6.50 | 175.71 ± 7.45 | 85.82 ± 1.39 |
| F15 | 0.30 | 1.00 | 5.00 | 113.42 ± 3.11 | 85.41 ± 1.83 |
| F16 | 0.50 | 1.00 | 6.50 | 124.50 ± 3.62 | 91.70 ± 1.92 |
| F17 | 0.30 | 2.50 | 6.50 | 171.81 ± 4.19 | 86.91 ± 2.71 |
| Gene | Primer Sequence from 5′–3′ |
|---|---|
| β-actin | F: TCCGTCGCCGGTCCACACCC R: TCACCAACTGGGACGATATG |
| Bax | F: CCTGAGCTGACCTTGGAGCA R: GGTGGTTGCCCTTTTCTACT |
| Bcl-2 | F: TGATAACCGGGAGATCGTGA R: AAAGCACATCCAATAAAAAGC |
| Responses | Sequential p-Value | Lack of Fit p-Value | R2 | Adjusted R2 | Predicted R2 | Adequate Precision | PRESS * | Significant Terms |
|---|---|---|---|---|---|---|---|---|
| Y1: Vesicle size (nm) | 0.0001 | 0.1069 | 0.9941 | 0.9865 | 0.9269 | 36.3396 | 1305.69 | X1, X2, X12, X22 |
| Y2: Entrapment Efficiency (%) | 0.0007 | 0.1821 | 0.9864 | 0.9689 | 0.8476 | 26.4061 | 42.26 | X1, X2, X1X3, X2X3, X12, X22, X32 |
| Variables | X1: PIC Concentration (w/w) | X2: Lipoid® S 100 Concentration (w/w) | X3: Hydration Medium pH |
| Optimum values | 0.50 | 1.00 | 5.20 |
| Predicted value | Observed value | Residual | |
| Vesicle size (nm) | 129.21 | 125.45 | −3.76 |
| Entrapment efficiency (%) | 93.71 | 93.14 | −0.57 |
| Samples- | IC50 Value (µM) | ||
|---|---|---|---|
| HCT 116 | HCT 29 | EA.hy926 | |
| Plain- E | 118.3 ± 5.4 | 131.0 ± 2.6 | 159.8 ± 3.6 |
| PIC-raw | 18.9 ± 1.9 * | 18.4 ± 1.7 * | 50.7 ± 4.1 * |
| PIC-E | 7.02 ± 0.2 * # | 6.3 ± 0.2 * # | 38.6 ± 3.2 * # |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhakamy, N.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Asfour, H.Z.; Aldawsari, H.M.; Algandaby, M.M.; Eid, B.G.; Abdel-Naim, A.B.; Awan, Z.A.; Alghaith, A.F.; et al. Piceatannol-Loaded Emulsomes Exhibit Enhanced Cytostatic and Apoptotic Activities in Colon Cancer Cells. Antioxidants 2020, 9, 419. https://doi.org/10.3390/antiox9050419
Alhakamy NA, Badr-Eldin SM, Ahmed OAA, Asfour HZ, Aldawsari HM, Algandaby MM, Eid BG, Abdel-Naim AB, Awan ZA, Alghaith AF, et al. Piceatannol-Loaded Emulsomes Exhibit Enhanced Cytostatic and Apoptotic Activities in Colon Cancer Cells. Antioxidants. 2020; 9(5):419. https://doi.org/10.3390/antiox9050419
Chicago/Turabian StyleAlhakamy, Nabil A., Shaimaa M. Badr-Eldin, Osama A. A. Ahmed, Hani Z. Asfour, Hibah M. Aldawsari, Mardi M. Algandaby, Basma G. Eid, Ashraf B. Abdel-Naim, Zuhier A. Awan, Adel F. Alghaith, and et al. 2020. "Piceatannol-Loaded Emulsomes Exhibit Enhanced Cytostatic and Apoptotic Activities in Colon Cancer Cells" Antioxidants 9, no. 5: 419. https://doi.org/10.3390/antiox9050419
APA StyleAlhakamy, N. A., Badr-Eldin, S. M., Ahmed, O. A. A., Asfour, H. Z., Aldawsari, H. M., Algandaby, M. M., Eid, B. G., Abdel-Naim, A. B., Awan, Z. A., Alghaith, A. F., Alaofi, A. L., Mohamed, A. I., Okbazghi, S. Z., Al-Rabia, M. W., & Fahmy, U. A. (2020). Piceatannol-Loaded Emulsomes Exhibit Enhanced Cytostatic and Apoptotic Activities in Colon Cancer Cells. Antioxidants, 9(5), 419. https://doi.org/10.3390/antiox9050419





