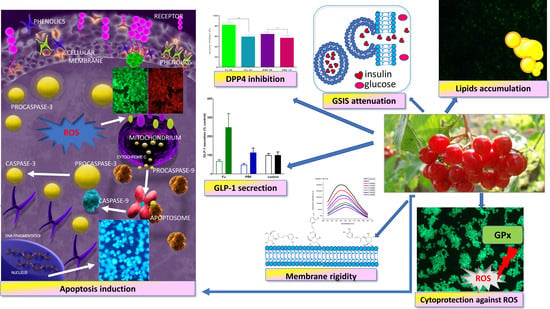
Evaluation of Viburnum opulus L. Fruit Phenolics Cytoprotective Potential on Insulinoma MIN6 Cells Relevant for Diabetes Mellitus and Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of V. opulus Extracts
2.3. Cell Culture and Exposure Conditions
2.4. Cell Viability
2.5. Detection of Intracellular Reactive Oxygen Species Generation
2.6. Measurement of Glutathione Peroxidase Activity and H2O2 Level
2.7. Measurement of ATP Production
2.8. Measurement of Mitochondrial Membrane Potential (MMP)
2.9. Phosphatidylserine Externalization and Membrane Permeabilization
2.10. Detection of Caspases 3/7 and 9 Activities
2.11. Evaluation of Membrane Fluidity and Lateral Phase Separation
2.12. Transmembrane Potential
2.13. Insulin Secretion
2.14. GLP-1 Secretion
2.15. Dipeptidyl Peptidase-4 (DPP4) Inhibition
2.16. Nile Red Staining
2.17. Fatty Acid Uptake
2.18. Human Serum Albumin (HSA) Fluorescence Quenching
2.19. Statistical Analysis
3. Results and Discussion
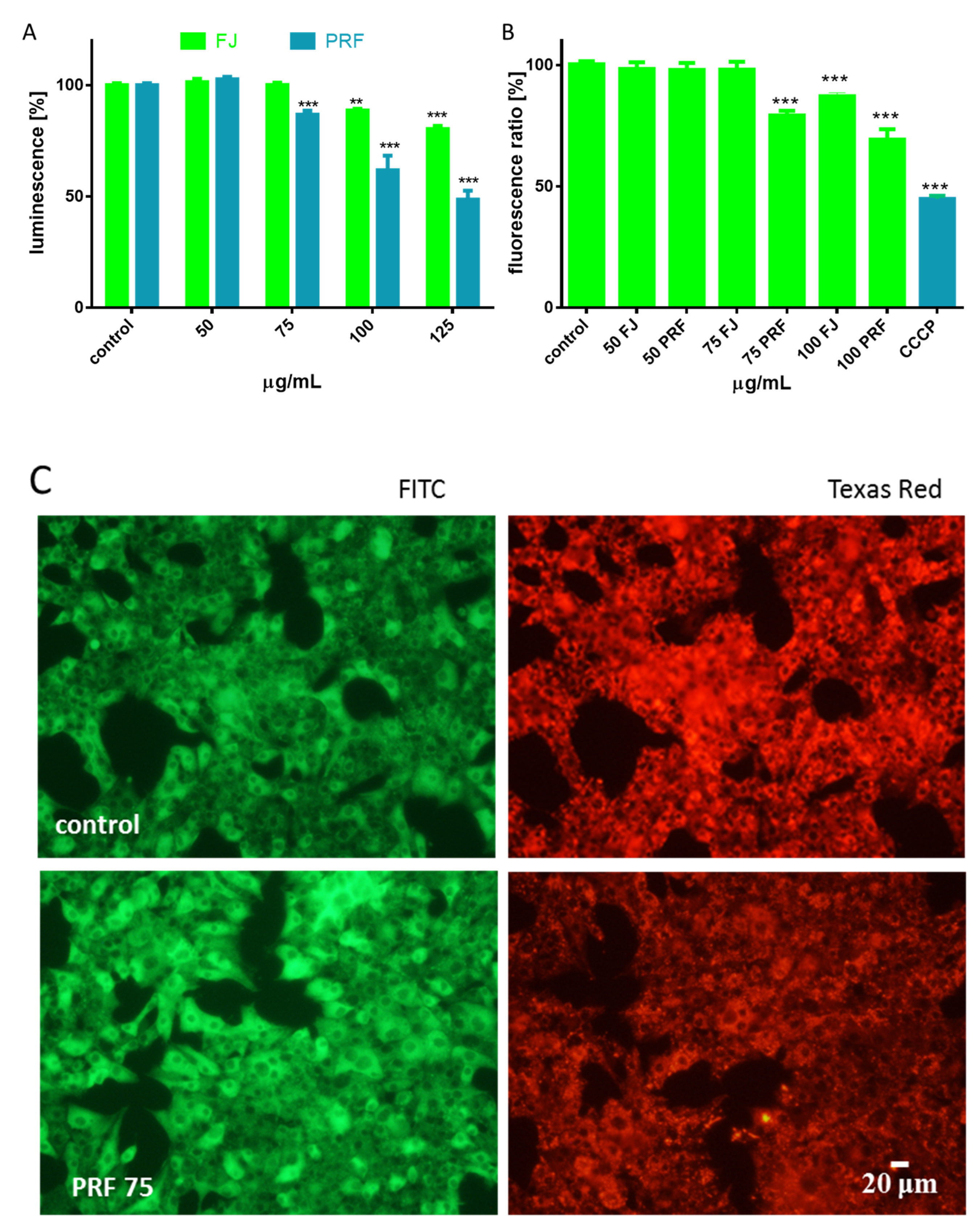
3.1. V. opulus Phenolics Influence on Cellular Viability
3.2. Effect of V. opulus on Intracellular ATP Level, Mitochondrial Membrane Potential, and Apoptosis Induction
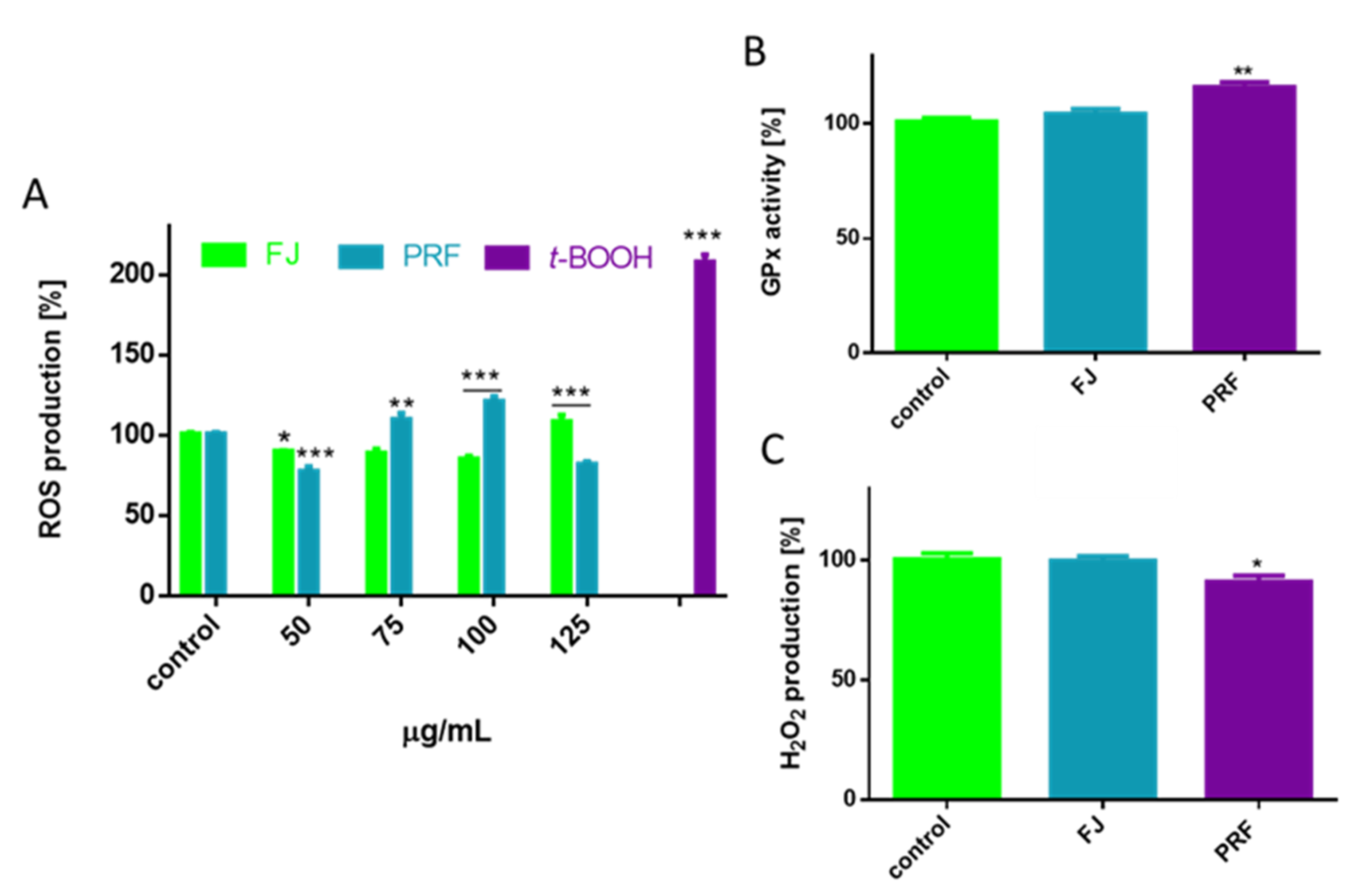
3.3. V. opulus as a Cytoprotective Agent
3.4. V. opulus Phenolics as Modulators of Fatty Acids Uptake
3.5. V. opuluce Influence on Insulin and GLP-1 Secretion, and DPP4 Acivity
3.6. Membrane Effect of V. opulus
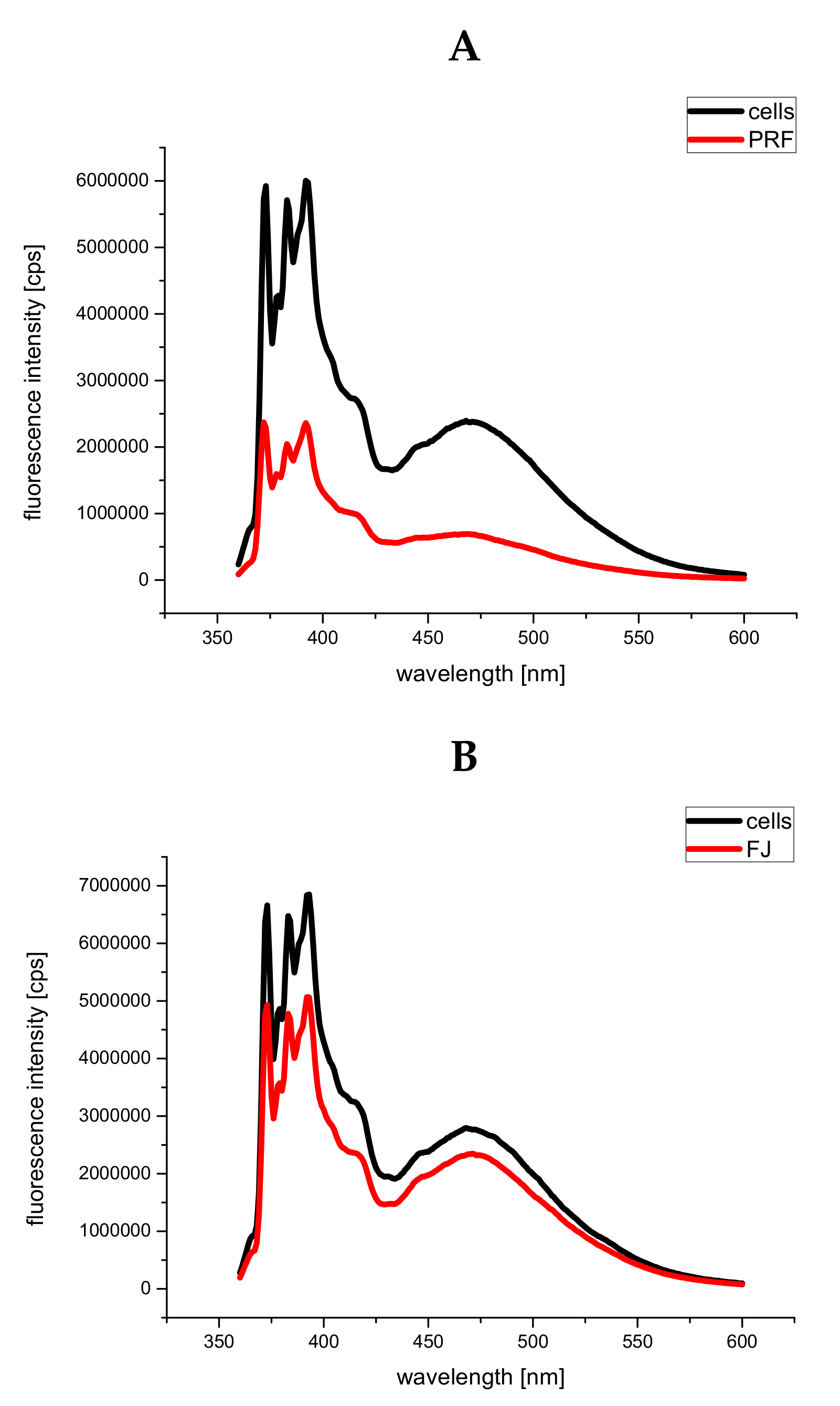
3.7. Human Serum Albumin Fluorescence Quenching by PRF
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Česonienė, L.; Daubaras, R.; Viškelis, P. Evaluation of productivity and biochemical components in fruit of different Viburnum accessions. Biologija 2008, 54, 93–96. [Google Scholar]
- Česoniene, L.; Daubaras, R.; Vencloviene, J.; Viškelis, P. Biochemical and agro-biological diversity of Viburnum opulus genotypes. Cent. Eur. J. Biol. 2010, 5, 864–871. [Google Scholar]
- Bae, K.; Chong, H.; Kim, D.; Choi, Y.; Kim, Y.; Kim, Y. Compounds from Viburnum sargentii Koehne and evaluation of their cytotoxic effects on human cancer cell lines. Molecules 2010, 15, 4599–4609. [Google Scholar] [CrossRef] [PubMed]
- Stępień, A.; Aebisher, D.; Aebisher, D. Anticancer properties of Viburnum. Eur. J. Clin. Exp. Med. 2018, 16, 47–52. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pawlik, N.; Polka, D.; Nowak, A.; Koziołkiewicz, M.; Podsędek, A. Viburnum opulus fruit phenolic compounds as cytoprotective agents able to decrease free fatty acids and glucose uptake by Caco-2 cells. Antioxidants 2019, 8, 262. [Google Scholar]
- Karaçelik, A.A.; Küçük, M.; Iskefiyeli, Z.; Aydemir, S.; De Smet, S.; Miserez, B.; Sandra, P. Antioxidant components of Viburnum opulus L. determined by on-line HPLC–UV–ABTS radical scavenging and LC–UV–ESI-MS methods. Food Chem. 2015, 175, 106–114. [Google Scholar] [CrossRef]
- Polka, D.; Podsędek, A.; Koziołkiewicz, M. Comparison of chemical composition and antioxidant capacity of fruit, flower and bark of Viburnum opulus. Plant Foods Hum. Nutr. 2019, 74, 436–442. [Google Scholar] [CrossRef]
- Han, B.; Wu, J.; Huang, L. Induction of apoptosis in lung cancer cells by Viburnum grandiflorum via mitochondrial pathway. Med. Sci. Monit. 2020, 26, 1–8. [Google Scholar] [CrossRef]
- Ceylan, D.; Aksoy, A.; Ertekin, T.; Yay, A.H.; Nisari, M.; Karatoprak, G.Ş.U.H. The effects of gilaburu (Viburnum opulus) juice on experimentally induced Ehrlich ascites tumor in mice. J. Cancer Res. Ther. 2018, 14, 310–320. [Google Scholar]
- Kraujalyte, V.; Rimantas, P.; Pukalskas, A.; Laima, C. Antioxidant properties and polyphenolic compositions of fruits from different European cranberrybush (Viburnum opulus L.) genotypes. Food Chem. 2013, 141, 3695–3702. [Google Scholar] [CrossRef]
- Perova, I.B.; Zhogova, A.A.; Cherkashin, A.V.; Éller, K.I.; Ramenskaya, G.V. Biologically active substances from european guelder berry fruits. Pharm. Chem. J. 2014, 48, 332–339. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pawlik, N. The influence of Viburnum opulus polyphenolic compounds on metabolic activity and migration of HeLa and MCF cells. Acta Innov. 2019, 33, 33–42. [Google Scholar] [CrossRef]
- Eken, A.; Yücel, O.; İpek, İ.; Ayşe, B.; Endİrlİk, B.Ü. An investigation on protective effect of Viburnum opulus L. fruit extract against ischemia/reperfusion-induced oxidative stress after lung transplantation in rats. Kafkas Univ. Vet. Fak. Derg. 2017, 23, 437–444. [Google Scholar]
- Zhang, Y.; Zhou, W.Y.; Song, X.Y.; Yao, G.D.; Song, S.J. Neuroprotective terpenoids from the leaves of Viburnum odoratissimum. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef]
- Yang, K.; Chan, C.B. Epicatechin potentiation of glucose-stimulated insulin secretion in INS-1 cells is not dependent on its antioxidant activity. Acta Pharmacol. Sin. 2018, 39, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H. Oxidative stress in pancreatic beta cell regeneration. Oxid. Med. Cell. Longev. 2017. [Google Scholar] [CrossRef]
- Razali, N.; Mat Junit, S.; Ariffin, A.; Ramli, N.S.F.; Abdul Aziz, A. Polyphenols from the extract and fraction of T. indica seeds protected HepG2 cells against oxidative stress. BMC Complement. Altern. Med. 2015, 15, 438. [Google Scholar] [CrossRef]
- Avila, J.A.D.; García, J.R.; Aguilar, G.A.G.; De La Rosa, L.A. The antidiabetic mechanisms of polyphenols related to increased glucagon-like peptide-1 (GLP1) and insulin signaling. Molecules 2017, 22, 903. [Google Scholar]
- Kim, B.R.; Kim, H.Y.; Choi, I.; Kim, J.B.; Jin, C.H.; Han, A.R. DPP-IV inhibitory potentials of flavonol glycosides isolated from the seeds of lens culinaris: In vitro and molecular docking analyses. Molecules 2018, 23, 1998. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Majewska, I.; Redzynia, M.; Koziołkiewicz, M. Antidiabetic effect of polyphenolic extracts from selected edible plants as α-amylase, α-glucosidase and PTP1B inhibitors, and β pancreatic cells cytoprotective agents-a comparative study. Curr. Top. Med. Chem. 2015, 15, 2431–2444. [Google Scholar] [CrossRef]
- Cheng, K.; de lghingaro-Augusto, V.; Nolan, C.J.; Turner, N.; Hallahan, N.; Andrikopoulos, S.; Gunton, J.E. High passage MIN6 cells have impaired insulin secretion with impaired glucose and lipid oxidation. PLoS ONE 2012, 7, e40868. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.I.; Araki, K.; Yamato, E.; Ikegami, H.; Asano, T.; Shibasaki, Y.; Oka, Y.; Yamamura, K.I.; Miyazaki, J.I. Establishment of a pancreatic β cell line that retains glucose-inducible insulin secretion: Special reference to expression of glucose transporter isoforms. Endocrinology 1990, 127, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Zakłos-Szyda, M.; Pawlik, N. Japanese quince (Chaenomeles japonica L.) fruit polyphenolic extract modulates carbohydrate metabolism in HepG2 cells via AMP-activated protein kinase. Acta Biochim. Pol. 2018, 65, 67–78. [Google Scholar] [CrossRef] [PubMed]
- De, C.; Palacio, J.R.; Martínez, P.; Morros, A. Effect of oxidative stress on plasma membrane fluidity of THP-1 induced macrophages. BBA-Biomembr. 2013, 1828, 357–364. [Google Scholar]
- Mojic, M.; Ajdz, V.; Spasojevic, I.; Bulatovic, M.; Spasojevic, S.; Mijatovic, V.; Spasojevic, I.; Ivan, S.M.V.M. Membrane fluidity, invasiveness and dynamic phenotype of metastatic prostate cancer cells after treatment with soy isoflavones. J. Membr. Biol. 2013, 246, 307–314. [Google Scholar]
- Drucker, D.J.; Jin, T.; Asa, S.L.; Young, T.A.; Brubaker, P.L. Activation of proglucagon gene transcription by protein kinase-A in a novel mouse enteroendocrine cell line. Mol. Endocrinol. 1994, 8, 1646–1655. [Google Scholar]
- Yang, Y.; Jiang, G.; Zhang, P.; Fan, J. Programmed cell death and its role in inflammation. Mil. Med. Res. 2015. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Ekbatan, S.S.; Li, X.; Ghorbani, M.; Azadi, B. Chlorogenic acid and its microbial metabolites exert anti-proliferative effects, s-phase cell-cycle arrest and apoptosis in human colon cancer Caco-2 cells. Int. J. Mol. Sci. 2018, 19, 723. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Huang, C.; Mansai, H.A.A.; Wei, W.; Zhang, X.; Li, X.; Liu, S.; Yang, S. Puerarin promotes MIN6 cell survival by reducing cellular reactive oxygen species. Mol. Med. Rep. 2018, 17, 7281–7286. [Google Scholar] [CrossRef]
- Ježek, P.; Jabůrek, M.; Holendová, B.; Plecitá-Hlavatá, L. Fatty acid-stimulated insulin secretion vs. lipotoxicity. Molecules 2018, 23, 1483. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Cumaoǧlu, A.; Rackova, L.; Stefek, M.; Kartal, M.; Maechler, P.; Karasu, Ç. Effects of olive leaf polyphenols against H2O2 toxicity in insulin secreting β-cells. Acta Biochim. Pol. 2011, 58, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Zakłos-Szyda, M.; Budryn, G. The effects of Trifolium pratenese L. sprouts’ phenolic compounds on cell growth andd migration of MDA-MB-231, MCF-7 and HUVEC cells. Nutrients 2020, 12, 257. [Google Scholar]
- Altun, M.L.; Özbek, H.; Çitoǧlu, G.S.; Yilmaz, B.S.; Bayram, I.; CengIz, N. Hepatoprotective and hypoglycemic activities of Viburnum opulus L. Turkish J. Pharm. Sci. 2010, 7, 35–48. [Google Scholar]
- Sequeira, I.R.; Poppitt, S.D. Unfolding novel mechanisms of polyphenol flavonoids for better glycaemic control: Targeting pancreatic islet amyloid polypeptide (IAPP). Nutrients 2017, 9, 788. [Google Scholar] [CrossRef]
- Tkhe, K.F.; Klymchenko, A.S.; Collot, M. Recent Advances in fluorescent probes for lipid droplets. Materials 2018, 11, 1768. [Google Scholar]
- Nemecz, M.; Constantin, A.; Dumitrescu, M.; Alexandru, N.; Filippi, A.; Tanko, G.; Georgescu, A. The distinct effects of palmitic and oleic acid on pancreatic beta cell function: The elucidation of associated mechanisms and effector. Molecules 2019, 9, 1554. [Google Scholar] [CrossRef]
- Plötz, T.; Hartmann, M.; Lenzen, S.; Elsner, M. The role of lipid droplet formation in the protection of unsaturated fatty acids against palmitic acid induced lipotoxicity to rat insulin-producing cells. Nutr. Metab. 2016, 13, 16. [Google Scholar]
- Yang, L.; Wei, J.; Sheng, F.; Li, P. Attenuation of palmitic acid–induced lipotoxicity by chlorogenic acid through activation of SIRT1 in hepatocytes. Mol. Nutr. Food Res. 2019, 63, 1801432. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, L.; Zhang, H.; Wu, G.; Zhang, Z.; Lv, J. Chlorogenic acid against palmitic acid in endoplasmic reticulum stress-mediated apoptosis resulting in protective effect of primary rat hepatocytes. Lipids Health Dis. 2018, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tousch, D.; Lajoix, A.D.; Hosy, E.; Azay-Milhau, J.; Ferrare, K.; Jahannault, C.; Cros, G.; Petit, P. Chicoric acid, a new compound able to enhance insulin release and glucose uptake. Biochem. Biophys. Res. Commun. 2008, 377, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.K.; Takii, M.; Kojima, Y.; Yokosawa, H.; Ishikawa, T. Structure-dependent inhibitory effects of green tea catechins on insulin secretion from pancreatic β-cells. Biol. Pharm. Bull. 2015, 38, 476–481. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oliveira, V.B.; Araújo, R.L.B.; Eidenberger, T.; Brandão, M.G.L. Chemical composition and inhibitory activities on dipeptidyl peptidase IV and pancreatic lipase of two underutilized species from the Brazilian Savannah: Oxalis cordata A.St.-Hil. and Xylopia aromatica (Lam.) Mart. Food Res. Int. 2018, 105, 989–995. [Google Scholar] [CrossRef]
- Parasassi, T.; Krasnowska, E.K.; Bagatolli, L.; Gratton, E. Laurdan and prodan as polarity-sensitive fluorescent membrane probes. J. Fluoresc. 1998, 8, 365–373. [Google Scholar] [CrossRef]
- Maulucci, G.; Cohen, O.; Daniel, B.; Sansone, A.; Petropoulou, P.I.; Filou, S.; Spyridonidis, A.; Pani, G.; De Spirito, M.; Chatgilialoglu, C.; et al. Fatty acid-related modulations of membrane fluidity in cells: Detection and implications. Free Radic. Res. 2016, 50, S40–S50. [Google Scholar] [CrossRef]
- Pérez-Hernández, I.H.; Domínguez-Fuentes, J.M.; Palomar-Morales, M.; Zazueta-Mendizabal, A.C.; Baiza-Gutman, A.; Mejía-Zepeda, R. Liver mitochondrial membrane fluidity at early development of diabetes and its correlation with the respiration. J. Bioenerg. Biomembr. 2017, 49, 231–239. [Google Scholar] [CrossRef]
- Ionescu, D.; Marginǎ, D.; Ilie, M.; Iftime, A.; Ganea, C. Quercetin and epigallocatechin-3-gallate effect on the anisotropy of model membranes with cholesterol. Food Chem. Toxicol. 2013, 61, 94–100. [Google Scholar] [CrossRef]
- Phan, H.T.T.; Yoda, T.; Chahal, B.; Morita, M.; Takagi, M.; Vestergaard, C. Biochimica et Biophysica Acta Structure-dependent interactions of polyphenols with a biomimetic membrane system. BBA-Biomembr. 2014, 1838, 2670–2677. [Google Scholar] [CrossRef]
- Zhao, W.; Prijic, S.; Urban, B.C.; Tisza, M.J.; Zuo, Y.; Li, L.; Tan, Z.; Chen, X.; Mani, S.A.; Chang, J.T. Candidate antimetastasis drugs suppress the metastatic capacity of breast cancer cells by reducing membrane fluidity. Cancer Res. 2016, 76, 2037–2050. [Google Scholar] [CrossRef]
- Margina, D.; Gradinaru, D.; Manda, G.; Neagoe, I.; Ilie, M. Membranar effects exerted in vitro by polyphenols–quercetin, epigallocatechin gallate and curcumin–on HUVEC and Jurkat cells, relevant for diabetes mellitus. Food Chem. Toxicol. 2013, 61, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Rashidi, R.; Shafiee-Nick, R. Flavonoids for preserving pancreatic beta cell survival and function: A mechanistic review. Biomed. Pharmacother. 2019, 111, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Ashworth, H.A.; Bahm, Q.; Erickson, J.; Shinkle, A.; Vu, M.P.; Woodbury, D.; Bell, J.D. Differential detection of phospholipid fluidity, order, and spacing by fluorescence spectroscopy of bis-pyrene, prodan, nystatin, and merocyanine 540. Biophys. J. 2006, 91, 4091–4101. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Yuan, H.; Liu, M.; Hu, J. 1H-NMR study of the effect of acetonitrile on the interaction of ibuprofen with human serum albumin. J. Pharm. Biomed. Sci. 2002, 30, 151–159. [Google Scholar] [CrossRef]
- Sinisi, V.; Forzato, C.; Cefarin, N.; Navarini, L.; Berti, F. Interaction of chlorogenic acids and quinides from coffee with human serum albumin. Food Chem. 2015, 168, 332–340. [Google Scholar] [CrossRef]
- Strugała, P.; Cyboran-Mikołajczyk, S.; Dudra, A.; Mizgier, P.; Kucharska, A.Z.; Olejniczak, T.; Gabrielska, J. Biological Activity of Japanese Quince Extract and Its Interactions with Lipids, Erythrocyte Membrane, and Human Albumin. J. Membr. Biol. 2016, 249, 393–410. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Segura-Carretero, A.; del Mar Contreras, M. Phenolic compounds as natural and multifunctional anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam. Repub. Iran 2017, 31, 886–892. [Google Scholar] [CrossRef]
- Romana, B.S.; Chela, H.; Dailey, F.E.; Nassir, F.; Tahan, V. Non-Alcoholic Fatty Pancreas Disease (NAFPD): A Silent Spectator or the Fifth Component of Metabolic Syndrome? A Literature Review. Endocr. Metab. Immune. Disord. Drug. Targets. 2018, 18, 547–554. [Google Scholar] [CrossRef]
- Noushmehr, H.; D’Amico, E.; Farilla, L.; Hui, H.; Wawrowsky, K.A.; Mlynarski, W.; Doria, A.; Abumrad, N.A.; Perfetti, R. Fatty acid translocase (FAT/CD36) is localized on insulin-containing granules in human pancreatic beta-cells and mediates fatty acid effects on insulin secretion. Diabetes 2005, 54, 472–481. [Google Scholar] [CrossRef]
- Chen, E.; Tsai, T.H.; Li, L.; Saha, P.; Chan, L.; Chang, B.H. PLIN2 is a Key Regulator of the Unfolded Protein Response and Endoplasmic Reticulum Stress Resolution in Pancreatic β Cells. Sci. Rep. 2017, 19, 40855. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Bae, G.D.; Baek, D.J.; Park, E.-Y.; Jun, H.-S. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 384. [Google Scholar] [CrossRef] [PubMed]










| Phenolic Compounds | FJ | PRF |
|---|---|---|
| mg/g of Juice | mg/g of Extract | |
| Flavanols | 1.21 ± 0.01 | 100.74 ± 0.10 |
| Procyanidin B1 | 0.31 ± 0.00 | 29.76 ± 0.05 |
| (+)-Catechin | 0.68 ± 0.01 | 53.18 ± 0.03 |
| Procyanidin B2 | 0.22 ± 0.00 | 17.79 ± 0.02 |
| Hydroxycinnamic Acids (HCA) | 7.97 ± 0.02 | 645.85 ± 2.00 |
| Neochlorogenic acid | n.d. | 0.36 ± 0.00 |
| Chlorogenic acid | 7.97 ± 0.02 | 645.49 ± 2.00 |
| Flavonols | 0.01 ± 0.00 | 2.35 ± 0.00 |
| Rutin | 0.01 ± 0.00 | 1.09 ± 0.00 |
| Isorhamnetin | 0.002 ± 0.000 | 0.12 ± 0.00 |
| Isorhamnetin 3-O-rutinoside | 0.01 ± 0.00 | 0.84 ± 0.00 |
| Quercetin | n.d. | 0.30 ± 0.00 |
| Anthocyanins | 1.12 ± 0.00 | 78.06 ± 0.68 |
| Cyanidin-3-O-sambubioside | 0.85 ± 0.00 | 55.78 ± 0.69 |
| Cyanidin-3-O-glucoside | 0.20 ± 0.01 | 17.08 ± 0.01 |
| Cyanidin-3-O-rutinoside | 0.06 ± 0.00 | 5.21 ± 0.01 |
| Phenolics Total | 10.32 ± 0.03 | 827.00 ± 1.68 |
| Samples | I470/I396 |
|---|---|
| cells | 0.514 ± 0.010 |
| cells + FJ | 0.408 ± 0.016 |
| cells | 0.508 ± 0.015 |
| cells + PRF | 0.441 ± 0.022 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakłos-Szyda, M.; Kowalska-Baron, A.; Pietrzyk, N.; Drzazga, A.; Podsędek, A. Evaluation of Viburnum opulus L. Fruit Phenolics Cytoprotective Potential on Insulinoma MIN6 Cells Relevant for Diabetes Mellitus and Obesity. Antioxidants 2020, 9, 433. https://doi.org/10.3390/antiox9050433
Zakłos-Szyda M, Kowalska-Baron A, Pietrzyk N, Drzazga A, Podsędek A. Evaluation of Viburnum opulus L. Fruit Phenolics Cytoprotective Potential on Insulinoma MIN6 Cells Relevant for Diabetes Mellitus and Obesity. Antioxidants. 2020; 9(5):433. https://doi.org/10.3390/antiox9050433
Chicago/Turabian StyleZakłos-Szyda, Małgorzata, Agnieszka Kowalska-Baron, Nina Pietrzyk, Anna Drzazga, and Anna Podsędek. 2020. "Evaluation of Viburnum opulus L. Fruit Phenolics Cytoprotective Potential on Insulinoma MIN6 Cells Relevant for Diabetes Mellitus and Obesity" Antioxidants 9, no. 5: 433. https://doi.org/10.3390/antiox9050433
APA StyleZakłos-Szyda, M., Kowalska-Baron, A., Pietrzyk, N., Drzazga, A., & Podsędek, A. (2020). Evaluation of Viburnum opulus L. Fruit Phenolics Cytoprotective Potential on Insulinoma MIN6 Cells Relevant for Diabetes Mellitus and Obesity. Antioxidants, 9(5), 433. https://doi.org/10.3390/antiox9050433