Protective Effects of Myricetin on Benzo[a]pyrene-Induced 8-Hydroxy-2′-Deoxyguanosine and BPDE-DNA Adduct
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Animals and Housing
2.4. Cell Viability Assay
2.5. BPDE-DNA Adduct Formation Analysis
2.6. Quantification of DNA Damage via 8-Hydroxydeoxyguanosine
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
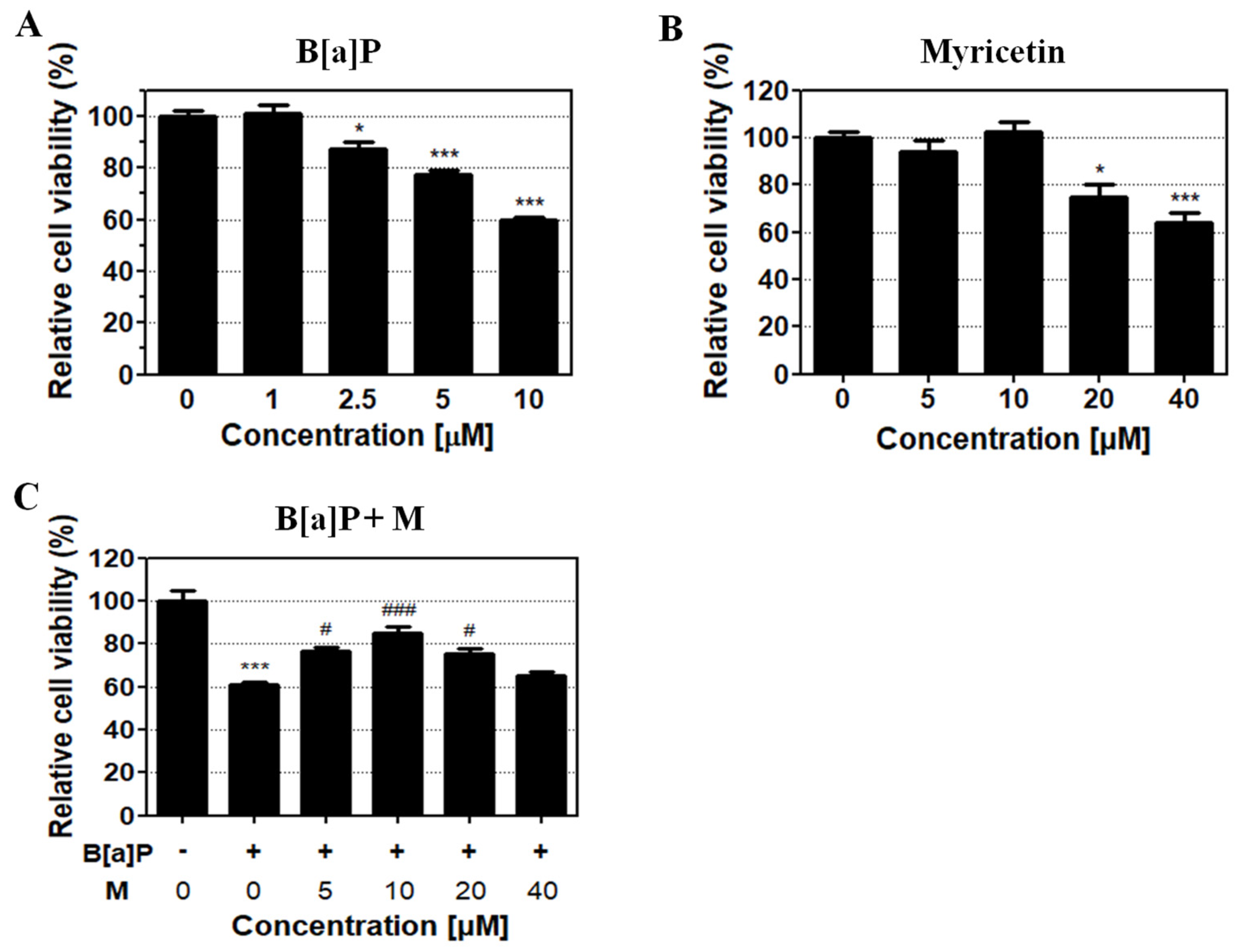
3.1. Protective Effect of Myricetin against B[a]P-Induced Toxicity
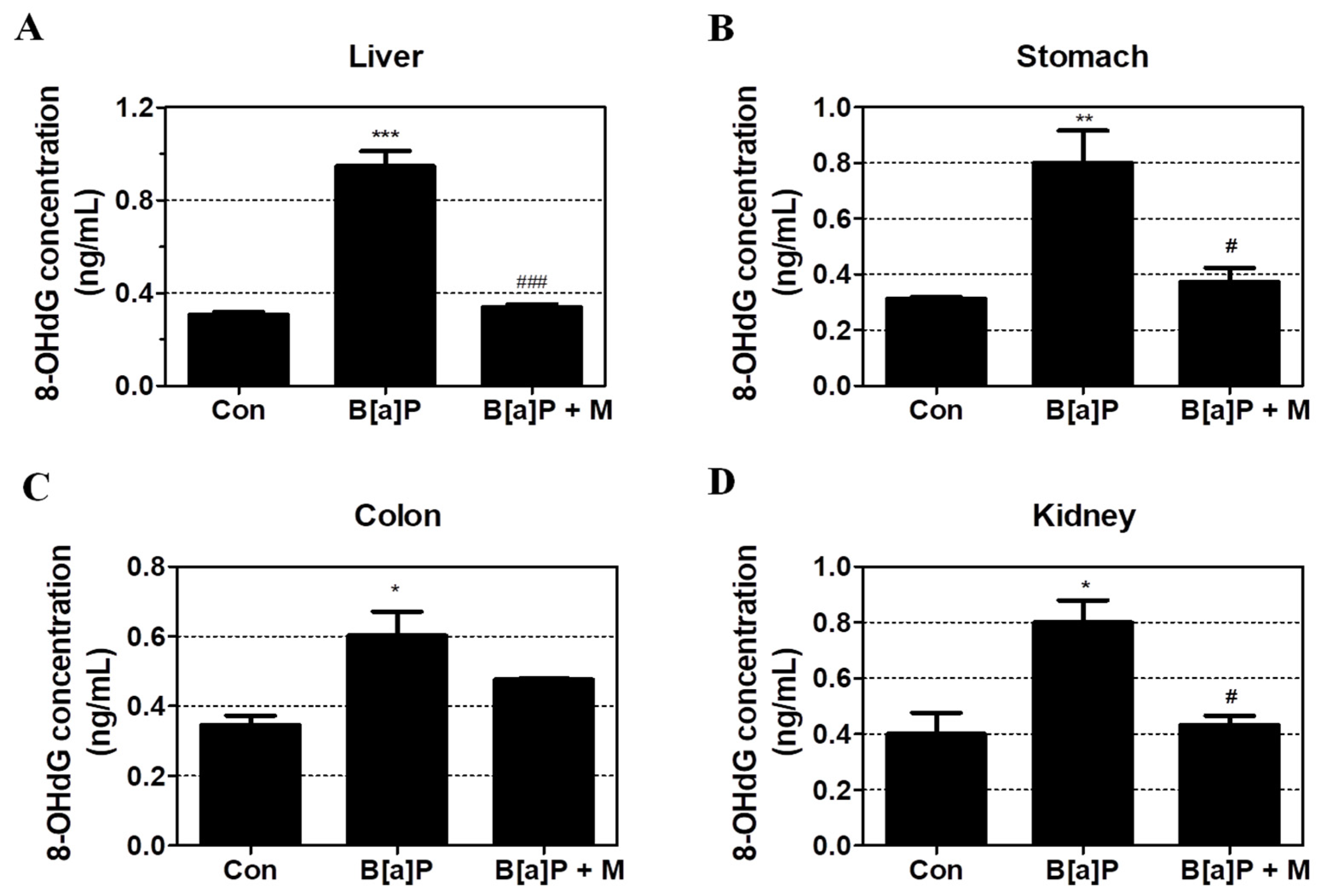
3.2. Protective Effects of Myricetin against B[a]P-Induced Oxidative DNA Damage
3.3. Inhibition Effect of Myricetin against BPDE-DNA Adduct Formation
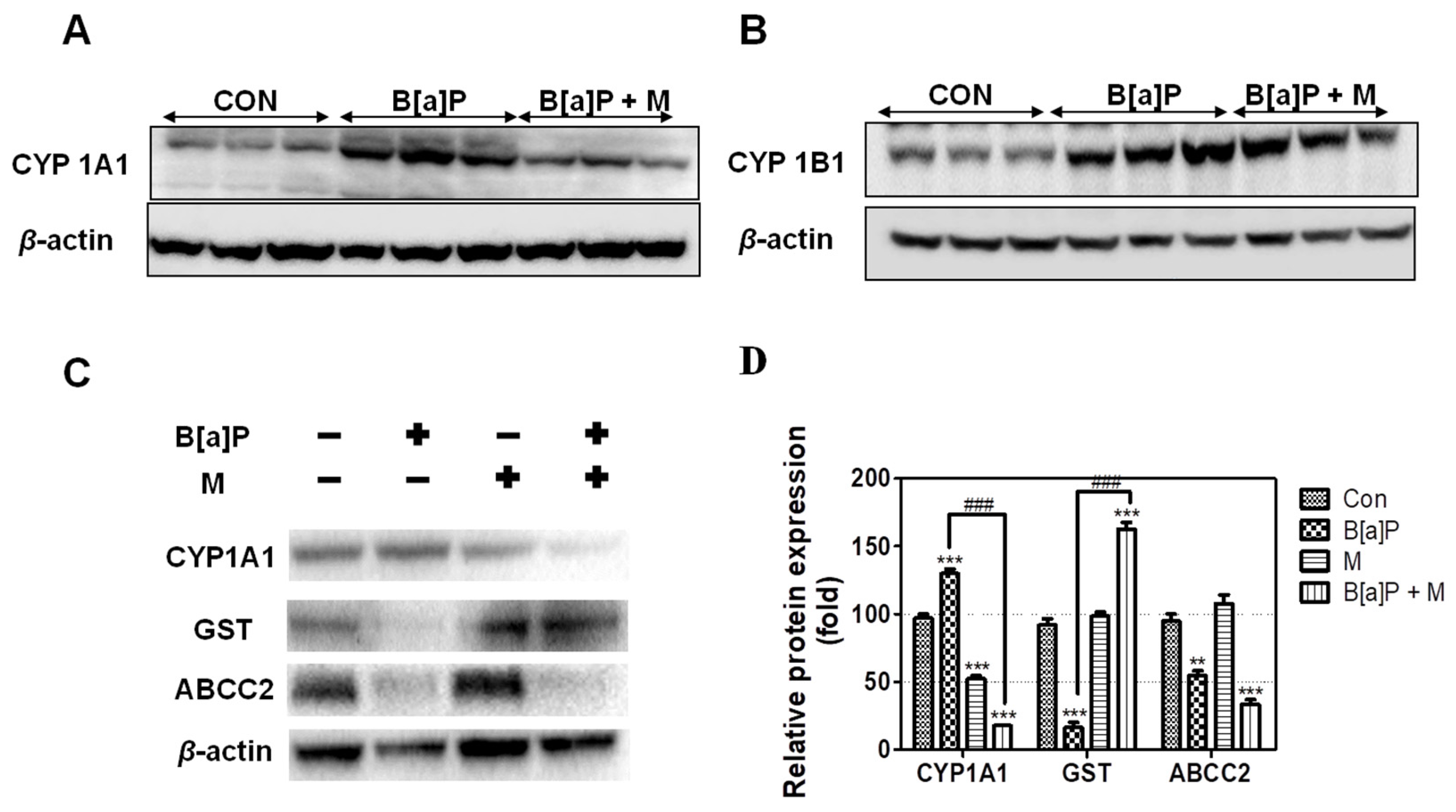
3.4. Regulatory Effect of Myricetin on the Expression of Phase I, II, and III Enzyme
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fasulo, S.; Marino, S.; Mauceri, A.; Maisano, M.; Giannetto, A.; D’Agata, A.; Parrino, V.; Minutoli, R.; De Domenico, E. A multibiomarker approach in Coris julis living in a natural environment. Ecotoxicol. Environ. Saf. 2010, 73, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Hattemer-Frey, H.A.; Travis, C.C. Benzo-a-pyrene: Environmental partitioning and human exposure. Toxicol. Ind. Health 1991, 7, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Alomirah, H.; Al-Zenki, S.; Al-Hooti, S.; Zaghloul, S.; Sawaya, W.; Ahmed, N.; Kannan, K. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control 2011, 22, 2028–2035. [Google Scholar] [CrossRef]
- Sinha, R.; Kulldorff, M.; Gunter, M.J.; Strickland, P.; Rothman, N. Dietary benzo[a]pyrene intake and risk of colorectal adenoma. Cancer Epidem. Biomar. 2005, 14, 2030–2034. [Google Scholar] [CrossRef] [Green Version]
- Ba, Q.; Li, J.; Huang, C.; Qiu, H.; Li, J.; Chu, R.; Zhang, W.; Xie, D.; Wu, Y.; Wang, H. Effects of benzo[a]pyrene exposure on human hepatocellular carcinoma cell angiogenesis, metastasis, and NF-kappaB signaling. Environ. Health Perspect. 2015, 123, 246–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Zhang, J.; Nie, J.H.; Duan, J.C.; Shi, Y.F.; Feng, L.; Yang, X.Z.; An, Y.; Sun, Z.W. The chronic effect of amorphous silica nanoparticles and benzo [a] pyrene co-exposure at low dose in human bronchial epithelial BEAS-2B cells. Toxicol. Res. UK 2019, 8, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Gelboin, H.V. Benzo [alpha] pyrene metabolism, activation and carcinogenesis: Role and regulation of mixed-function oxidases and related enzymes. Physiol. Rev. 1980, 60, 1107–1166. [Google Scholar] [CrossRef] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Hodek, P.; Koblihova, J.; Kizek, R.; Frei, E.; Arlt, V.M.; Stiborova, M. The relationship between DNA adduct formation by benzo [a] pyrene and expression of its activation enzyme cytochrome P450 1A1 in rat. Environ. Toxicol. Pharmacol. 2013, 36, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Phillips, D.H.; Venitt, S. DNA and protein adducts in human tissues resulting from exposure to tobacco smoke. Int. J. Cancer 2012, 131, 2733–2753. [Google Scholar] [CrossRef]
- Fang, A.H.; Smith, W.A.; Vouros, P.; Gupta, R.C. Identification and characterization of a novel benzo [a] pyrene-derived DNA adduct. Biochem. Biophys. Res. Commun. 2001, 281, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.; Hodgson, E. Metabolism of Toxicants. In A Textbook of Modern Toxicology; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Zhang, Y. Phase II Enzymes. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Perumal Vijayaraman, K.; Muruganantham, S.; Subramanian, M.; Shunmugiah, K.P.; Kasi, P.D. Silymarin attenuates benzo(a)pyrene induced toxicity by mitigating ROS production, DNA damage and calcium mediated apoptosis in peripheral blood mononuclear cells (PBMC). Ecotoxicol. Environ. Saf. 2012, 86, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, Y.; Chen, M.; Huang, M.; Harvey, R.G.; Blair, I.A.; Penning, T.M. Detoxication of structurally diverse polycyclic aromatic hydrocarbon (PAH) o-quinones by human recombinant catechol-O-methyltransferase (COMT) via O-methylation of PAH catechols. J. Biol. Chem. 2011, 286, 25644–25654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Mangal, D.; Frey, A.J.; Harvey, R.G.; Blair, I.A.; Penning, T.M. Aryl hydrocarbon receptor facilitates DNA strand breaks and 8-oxo-2′-deoxyguanosine formation by the aldo-keto reductase product benzo [a] pyrene-7,8-dione. J. Biol. Chem. 2009, 284, 29725–29734. [Google Scholar] [CrossRef] [Green Version]
- Hessel, S.; John, A.; Seidel, A.; Lampen, A. Multidrug resistance-associated proteins are involved in the transport of the glutathione conjugates of the ultimate carcinogen of benzo [a] pyrene in human Caco-2 cells. Arch. Toxicol. 2013, 87, 269–280. [Google Scholar] [CrossRef]
- Guo, B.; Xu, Z.; Yan, X.; Buttino, I.; Li, J.; Zhou, C.; Qi, P. Novel ABCB1 and ABCC Transporters Are Involved in the Detoxification of Benzo (α) pyrene in Thick Shell Mussel, Mytilus coruscus. Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Kranz, J.; Hessel, S.; Aretz, J.; Seidel, A.; Petzinger, E.; Geyer, J.; Lampen, A. The role of the efflux carriers Abcg2 and Abcc2 for the hepatobiliary elimination of benzo [a] pyrene and its metabolites in mice. Chem. Biol. Interact. 2014, 224, 36–41. [Google Scholar] [CrossRef]
- Dutta, K.; Ghosh, D.; Nazmi, A.; Kumawat, K.L.; Basu, A. A common carcinogen benzo[a]pyrene causes neuronal death in mouse via microglial activation. PLoS ONE 2010, 5, e9984. [Google Scholar] [CrossRef]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122–146. [Google Scholar] [CrossRef] [Green Version]
- Adeneye, A.A. Hypoglycemic and hypolipidemic effects of methanol seed extract of Citrus paradisi Macfad (Rutaceae) in alloxan-induced diabetic Wistar rats. Nig. Q. J. Hosp. Med. 2008, 18, 211–215. [Google Scholar] [CrossRef]
- Feng, J.; Chen, X.; Wang, Y.; Du, Y.; Sun, Q.; Zang, W.; Zhao, G. Myricetin inhibits proliferation and induces apoptosis and cell cycle arrest in gastric cancer cells. Mol. Cell Biochem. 2015, 408, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Rajavel, T.; Habtemariam, S.; Nabavi, S.F.; Nabavi, S.M. Molecular mechanisms underlying anticancer effects of myricetin. Life Sci. 2015, 142, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.E.; Haza, A.I.; Arranz, N.; Garcia, A.; Morales, P. Dietary polyphenols protect against N-nitrosamines and benzo (a) pyrene-induced DNA damage (strand breaks and oxidized purines/pyrimidines) in HepG2 human hepatoma cells. Eur. J. Nutr. 2008, 47, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lv, J.; Zhang, W.; Zhang, L. Dietary exposure estimates of 16 polycyclic aromatic hydrocarbons (PAHs) in Xuanwei and Fuyuan, counties in a high lung cancer incidence area in China. J. Environ. Monit. 2012, 14, 886–892. [Google Scholar] [CrossRef]
- Martorell, I.; Nieto, A.; Nadal, M.; Perelló, G.; Marcé, R.M.; Domingo, J.L. Human exposure to polycyclic aromatic hydrocarbons (PAHs) using data from a duplicate diet study in Catalonia, Spain. Food Chem. Toxicol. 2012, 50, 4103–4108. [Google Scholar] [CrossRef]
- Saunders, C.R.; Shockley, D.C.; Knuckles, M.E. Behavioral effects induced by acute exposure to benzo (a) pyrene in F-344 rats. Neurotox. Res. 2001, 3, 557–579. [Google Scholar] [CrossRef]
- Shi, Z.; Dragin, N.; Miller, M.L.; Stringer, K.F.; Johansson, E.; Chen, J.; Uno, S.; Gonzalez, F.J.; Rubio, C.A.; Nebert, D.W. Oral benzo [a] pyrene-induced cancer: Two distinct types in different target organs depend on the mouse Cyp1 genotype. Int. J. Cancer 2010, 127, 2334–2350. [Google Scholar] [CrossRef] [Green Version]
- Jayakumar, J.K.; Nirmala, P.; Praveen Kumar, B.A.; Kumar, A.P. Evaluation of protective effect of myricetin, a bioflavonoid in dimethyl benzanthracene-induced breast cancer in female Wistar rats. South Asian J. Cancer 2014, 3, 107–111. [Google Scholar] [CrossRef]
- Dimitriou, K.; Kassomenos, P. The influence of specific atmospheric circulation types on PM10-bound benzo (a) pyrene inhalation related lung cancer risk in Barcelona, Spain. Environ. Int. 2018, 112, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Ismail, I.M.I.; Khoder, M.; Shamy, M.; Alghamdi, M.; Al Khalaf, A.; Costa, M. Polycyclic aromatic hydrocarbons (PAHs) in the settled dust of automobile workshops, health and carcinogenic risk evaluation. Sci. Total Environ. 2017, 601–602, 478–484. [Google Scholar] [CrossRef]
- Roh, T.; Kwak, M.Y.; Kwak, E.H.; Kim, D.H.; Han, E.Y.; Bae, J.Y.; Bang du, Y.; Lim, D.S.; Ahn, I.Y.; Jang, D.E.; et al. Chemopreventive mechanisms of methionine on inhibition of benzo (a) pyrene-DNA adducts formation in human hepatocellular carcinoma HepG2 cells. Toxicol. Lett. 2012, 208, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, A.L.; Naidu, R. Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: A review of the microbial degradation of benzo [a] pyrene. Int. Biodeterior. Biodegrad. 2000, 45, 57–88. [Google Scholar] [CrossRef]
- Baird, W.M.; Hooven, L.A.; Mahadevan, B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ. Mol. Mutagen. 2005, 45, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Y.M.; Zhang, P.Y. Protective effects of curcumin and quercetin during benzo (a) pyrene induced lung carcinogenesis in mice. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1736–1743. [Google Scholar]
- Shahid, A.; Ali, R.; Ali, N.; Hasan, S.K.; Bernwal, P.; Afzal, S.M.; Vafa, A.; Sultana, S. Modulatory effects of catechin hydrate against genotoxicity, oxidative stress, inflammation and apoptosis induced by benzo (a) pyrene in mice. Food Chem. Toxicol. 2016, 92, 64–74. [Google Scholar] [CrossRef]
- Jee, S.C.; Kim, M.; Sung, J.S. Modulatory Effects of Silymarin on Benzo [a] pyrene-Induced Hepatotoxicity. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Zheng, X.Y.; Ye, J.; Wu, T.T.; Wang, J.; Chen, W. Potential anticancer activity of myricetin in human T24 bladder cancer cells both in vitro and in vivo. Nutr. Cancer 2012, 64, 599–606. [Google Scholar] [CrossRef]
- Bertin, R.; Chen, Z.; Marin, R.; Donati, M.; Feltrinelli, A.; Montopoli, M.; Zambon, S.; Manzato, E.; Froldi, G. Activity of myricetin and other plant-derived polyhydroxyl compounds in human LDL and human vascular endothelial cells against oxidative stress. Biomed. Pharmacother. 2016, 82, 472–478. [Google Scholar] [CrossRef]
- Su, H.M.; Feng, L.N.; Zheng, X.D.; Chen, W. Myricetin protects against diet-induced obesity and ameliorates oxidative stress in C57BL/6 mice. J. Zhejiang Univ. Sci. B 2016, 17, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.B.; Mishra, N.; Rizvi, S.I. Protective role of myricetin on markers of oxidative stress in human erythrocytes subjected to oxidative stress. Nat. Prod. Commun. 2009, 4, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Rehman, M.U.; Rather, I.A. Myricetin Abrogates Cisplatin-Induced Oxidative Stress, Inflammatory Response, and Goblet Cell Disintegration in Colon of Wistar Rats. Plants 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penning, T.M.; Burczynski, M.E.; Hung, C.F.; McCoull, K.D.; Palackal, N.T.; Tsuruda, L.S. Dihydrodiol dehydrogenases and polycyclic aromatic hydrocarbon activation: Generation of reactive and redox active o-quinones. Chem. Res. Toxicol. 1999, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Nishimura, S.; Kurokawa, Y.; Hayashi, Y. Oral administration of the renal carcinogen, potassium bromate, specifically produces 8-hydroxydeoxyguanosine in rat target organ DNA. Carcinogenesis 1987, 8, 1959–1961. [Google Scholar] [CrossRef]
- Genies, C.; Maitre, A.; Lefebvre, E.; Jullien, A.; Chopard-Lallier, M.; Douki, T. The extreme variety of genotoxic response to benzo[a]pyrene in three different human cell lines from three different organs. PLoS ONE 2013, 8, e0078356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, T.; Jennen, D.; van Delft, J.; van Herwijnen, M.; Kyrtoupolos, S.; Kleinjans, J. New insights into BaP-induced toxicity: Role of major metabolites in transcriptomics and contribution to hepatocarcinogenesis. Arch. Toxicol. 2016, 90, 1449–1458. [Google Scholar] [CrossRef] [Green Version]
- Uppstad, H.; Ovrebo, S.; Haugen, A.; Mollerup, S. Importance of CYP1A1 and CYP1B1 in bioactivation of benzo [a] pyrene in human lung cell lines. Toxicol. Lett. 2010, 192, 221–228. [Google Scholar] [CrossRef]
- Briede, J.J.; Godschalk, R.W.; Emans, M.T.; De Kok, T.M.; Van Agen, E.; Van Maanen, J.; Van Schooten, F.J.; Kleinjans, J.C. In vitro and in vivo studies on oxygen free radical and DNA adduct formation in rat lung and liver during benzo[a]pyrene metabolism. Free Radic. Res. 2004, 38, 995–1002. [Google Scholar] [CrossRef]
- Divi, R.L.; Lindeman, T.L.; Shockley, M.E.; Keshava, C.; Weston, A.; Poirier, M.C. Correlation between CYP1A1 transcript, protein level, enzyme activity and DNA adduct formation in normal human mammary epithelial cell strains exposed to benzo [a] pyrene. Mutagenesis 2014, 29, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Torii, Y.; Uchida, K.; Nakamura, Y.; Hara, Y.; Osawa, T. Black tea polyphenols, theaflavins, prevent cellular DNA damage by inhibiting oxidative stress and suppressing cytochrome P450 1A1 in cell cultures. J. Agric. Food Chem. 2002, 50, 213–220. [Google Scholar] [CrossRef]
- Sulc, M.; Indra, R.; Moserova, M.; Schmeiser, H.H.; Frei, E.; Arlt, V.M.; Stiborova, M. The impact of individual cytochrome P450 enzymes on oxidative metabolism of benzo [a] pyrene in human livers. Environ. Mol. Mutagen. 2016, 57, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Frova, C. Glutathione transferases in the genomics era: New insights and perspectives. Biomol. Eng. 2006, 23, 149–169. [Google Scholar] [CrossRef]
- Cai, Y.; Pan, L.; Miao, J. In vitro study of the effect of metabolism enzymes on benzo (a) pyrene-induced DNA damage in the scallop Chlamys farreri. Environ. Toxicol. Pharmacol. 2016, 42, 92–98. [Google Scholar] [CrossRef] [PubMed]
- van Zanden, J.J.; de Mul, A.; Wortelboer, H.M.; Usta, M.; van Bladeren, P.J.; Rietjens, I.M.; Cnubben, N.H. Reversal of in vitro cellular MRP1 and MRP2 mediated vincristine resistance by the flavonoid myricetin. Biochem. Pharmacol. 2005, 69, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Woo, E.R.; Choi, J.S. Effects of myricetin on the bioavailability of carvedilol in rats. Pharm. Biol. 2012, 50, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, N.D.; Hardwick, R.N.; Brouwer, K.L.R. Role of Hepatic Efflux Transporters in Regulating Systemic and Hepatocyte Exposure to Xenobiotics. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 509–535. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Li, L.; Thornton, C.; Carvalho, P.; Avery, B.A.; Willett, K.L. Simultaneous determination of benzo [a] pyrene and eight of its metabolites in Fundulus heteroclitus bile using ultra-performance liquid chromatography with mass spectrometry. J. Chromatogr. B 2008, 863, 141–149. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jee, S.-C.; Kim, M.; Kim, K.S.; Kim, H.-S.; Sung, J.-S. Protective Effects of Myricetin on Benzo[a]pyrene-Induced 8-Hydroxy-2′-Deoxyguanosine and BPDE-DNA Adduct. Antioxidants 2020, 9, 446. https://doi.org/10.3390/antiox9050446
Jee S-C, Kim M, Kim KS, Kim H-S, Sung J-S. Protective Effects of Myricetin on Benzo[a]pyrene-Induced 8-Hydroxy-2′-Deoxyguanosine and BPDE-DNA Adduct. Antioxidants. 2020; 9(5):446. https://doi.org/10.3390/antiox9050446
Chicago/Turabian StyleJee, Seung-Cheol, Min Kim, Kyeong Seok Kim, Hyung-Sik Kim, and Jung-Suk Sung. 2020. "Protective Effects of Myricetin on Benzo[a]pyrene-Induced 8-Hydroxy-2′-Deoxyguanosine and BPDE-DNA Adduct" Antioxidants 9, no. 5: 446. https://doi.org/10.3390/antiox9050446
APA StyleJee, S.-C., Kim, M., Kim, K. S., Kim, H.-S., & Sung, J.-S. (2020). Protective Effects of Myricetin on Benzo[a]pyrene-Induced 8-Hydroxy-2′-Deoxyguanosine and BPDE-DNA Adduct. Antioxidants, 9(5), 446. https://doi.org/10.3390/antiox9050446




