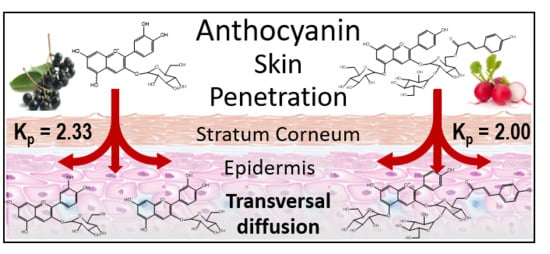
Ex Vivo and In Vivo Assessment of the Penetration of Topically Applied Anthocyanins Utilizing ATR-FTIR/PLS Regression Models and HPLC-PDA-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Porcine Skin Tissue
2.3. Subject Recruitment
2.4. Formulations
2.5. Tape Stripping Procedures
2.6. Analysis of Removed Tape Strips
2.6.1. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy
2.6.2. HPLC-PDA-MS Analysis
2.7. Chemometrics
2.8. Protein Quantification
2.9. Determination of Anthocyanin Penetration Profiles
3. Results and Discussion
3.1. Characterization of the Anthocyanins in Elderberry and Red Radish Lipstick Formulations
3.2. Detection and Quantification of Anthocyanins in Dermal Tissues
3.3. Characterization of ATR-FTIR Spectral Analysis
3.4. Determination of Anthocyanin Release and Penetration Kinetic Parameters
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Anthocyanin(s) |
| ATR-FTIR | Attenuated total reflectance-Fourier transform infrared |
| HPLC-MS | High-performance liquid chromatography-mass spectrometry |
| Kp | Permeability coefficient |
| MW | Molecular weight |
| PLS | Partial least squares |
| PDA | Photodiode array |
| SC | Stratum corneum |
Appendix A
Determination of Anthocyanin Penetration Profiles
References
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Andersen, Ø.M.; Jordheim, M. Basic Anthocyanin Chemistry and Dietary Sources. In Anthocyanins in Health and Disease; Wallace, T.C., Giusti, M.M., Eds.; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2014; pp. 13–90. [Google Scholar]
- Gee, J.M.; Johnson, I.T. Polyphenolic compounds: Interactions with the gut and implications for human health. Curr. Med. Chem. 2001, 8, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Desnita, R.; Wahdaningsih, S.; Hervianti, S. Span 60 as surfactant of topical microemulsion of purple sweet potato (Ipomoea batatas L.) ethanol extract and antioxidant activity test using dpph method. Int. J. PharmTech Res. 2016, 9, 198–203. [Google Scholar]
- Desnita, R.; Veronika, M.; Wahdaningsih, S. Topical microemulsion’s formulation of purple sweet potato (Ipomoea batatas L.) ethanol extract as antioxidant by using various concentration of span. Int. J. PharmTech Res. 2016, 9, 234–239. [Google Scholar]
- Chan, C.; Lien, C.; Lai, Y.; Huang, C.; Liao, W.C. Influence of purple sweet potato extracts on the UV absorption properties of a cosmetic cream. J. Cosmet. Sci. 2010, 61, 333–341. [Google Scholar] [PubMed]
- Bae, J.Y.; Lim, S.S.; Kim, S.J.; Choi, J.S.; Park, J.; Ju, S.M.; Han, S.J.; Kang, I.J.; Kang, Y.H. Bog blueberry anthocyanins alleviate photoaging in ultraviolet-B irradiation-induced human dermal fibroblasts. Mol. Nutr. Food Res. 2009, 53, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Park, H.B.; Kim, Y.M.; Chung, J., II; Shin, S.C.; Lee, W.S.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from Black Soybean Seed Coats Inhibit UVB-Induced Inflammatory Cylooxygenase-2 Gene Expression and PGE 2 Production through Regulation of the Nuclear Factor- K B and Phosphatidylinositol 3-Kinase/Akt Pathway. J. Agric. Food Chem. 2008, 56, 8969–8974. [Google Scholar] [CrossRef]
- Hsu, C.; Chou, S.; Huang, P.; Mong, M.; Wang, C.-K.; Hsueh, Y.; Jhan, J.-K. Crude ethanol extracts from grape seeds and peels exhibit anti-tyrosinase activity. J. Cosmet. Sci. 2012, 63, 225–232. [Google Scholar]
- Crisan, M.; David, L.; Moldovan, B.; Vulcu, A.; Dreve, S.; Perde-schrepler, M.; Tatomir, C.; Filip, G.; Bolfa, P. New nanomaterials for the improvment of psoriatic lesions. J. Mater. Chem. B 2013, 1, 3152–3158. [Google Scholar] [CrossRef]
- Kim, M.J.; Choung, S.Y. Mixture of polyphenols and anthocyanins from vaccinium uliginosum L. Alleviates DNCB-induced atopic dermatitis in NC/Nga mice. Evid.-Based Complement. Altern. Med. 2012, 2012, 461989. [Google Scholar] [CrossRef]
- Brown, M.; Turner, R.; Lim, S.T. Topical Product Formulation Development. In Transdermal and Topical Drug Delivery: Principles and Practice; Benson, H.A.E., Watkinson, A.C., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. Biopharm. 2001, 52, 103–112. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Hasenkopf, K.; Eisner, P.; Kerscher, M. Release and in vitro skin permeation of polyphenols from cosmetic emulsions. Int. J. Cosmet. Sci. 2013, 35, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Frauen, M.; Rode, T.; Rapp, C.; Steinhart, H. Determination of green-tea catechins in cosmetic formulations and in in-vitro skin extracts by high-performance liquid chromatography coupled with electrospray ionization mass spectrometry. Chromatographia 2002, 55, 43–48. [Google Scholar] [CrossRef]
- Marquele, F.D.; Oliveira, A.R.M.; Bonato, P.S.; Lara, M.G.; Fonseca, M.J.V. Propolis extract release evaluation from topical formulations by chemiluminescence and HPLC. J. Pharm. Biomed. Anal. 2006, 41, 461–468. [Google Scholar] [CrossRef]
- Kitagawa, S.; Yoshii, K.; Morita, S.; Teraoka, R. Efficient Topical Delivery of Chlorogenic Acid by an Oil-in-Water Microemulsion to Protect Skin against UV-Induced Damage. Chem. Pharm. Bull. 2011, 59, 793–796. [Google Scholar] [CrossRef]
- Dal Belo, S.E.; Gaspar, L.R.; Maia Campos, P.M.B.G.; Marty, J.P. Skin penetration of epigallocatechin-3-gallate and quercetin from green tea and Ginkgo biloba extracts vehiculated in cosmetic formulations. Skin Pharmacol. Physiol. 2009, 22, 299–304. [Google Scholar] [CrossRef]
- Batchelder, R.J.; Calder, R.J.; Thomas, C.P.; Heard, C.M. In vitro transdermal delivery of the major catechins and caffeine from extract of Camellia sinensis. Int. J. Pharm. 2004, 283, 45–51. [Google Scholar] [CrossRef]
- Plundrich, N.; Grace, M.H.; Raskin, I.; Lila, M.A. Bioactive polyphenols from muscadine grape and blackcurrant stably concentrated onto protein-rich matrices for topical applications. Int. J. Cosmet. Sci. 2013, 35, 394–401. [Google Scholar] [CrossRef]
- Montanari, J.; Vera, M.; Mensi, E.; Morilla, M.; Romero, E. Nanoberries for topical delivery of antioxidants. J. Cosmet. Sci. 2013, 64, 469–481. [Google Scholar]
- Westfall, A.; Giusti, M.M. Color profiles and stability of acylated and nonacylated anthocyanins as novel pigment sources in a lipstick model: A viable alternative to synthetic colorants. J. Cosmet. Sci. 2017, 68, 233–244. [Google Scholar]
- Westfall, A.; Sigurdson, G.T.; Giusti, M.M. Antioxidant, UV Protection, and Antiphotoaging Properties of Anthocyanin-Pigmented Lipstick Formulations. J. Cosmet. Sci. 2019, 70, 63–76. [Google Scholar] [PubMed]
- Barone, S.; Cohen, I.; Schlossman, M. Monograph Number 8: Lipstick Technology; Linda, R., Ed.; Society of Cosmetic Chemists: New York, NY, USA, 2002. [Google Scholar]
- Saad, P.; Flach, C.R.; Walters, R.M.; Mendelsohn, R. Infrared spectroscopic studies of sodium dodecyl sulphate permeation and interaction with stratum corneum lipids in skin. Int. J. Cosmet. Sci. 2012, 34, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Weigmann, H.J.; Lindemann, U.; Antoniou, C.; Tsikrikas, G.N.; Stratigos, A.I.; Katsambas, A.; Sterry, W.; Lademann, J. UV/VIS absorbance allows rapid, accurate, and reproducible mass determination of corneocytes removed by tape stripping. Skin Pharmacol. Appl. Skin Physiol. 2003, 16, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Pig ear skin ex vivo as a model for in vivo dermatopharmacokinetic studies in man. Pharm. Res. 2006, 23, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Wiedersberg, S.; Nicoli, S. Skin Permeation Assessment: Tape Stripping. In Transdermal and Topical Drug Delivery: Principles and Practice; Benson, H., Watkinson, A.C., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Jacobi, U.; Toll, R.; Audring, H.; Sterry, W.; Lademann, J. The porcine snout—An in vitro model for human lips? Exp. Dermatol. 2005, 14, 96–102. [Google Scholar] [CrossRef]
- Weigmann, H.J.; Lademann, J.; Schanzer, S.; Lindemann, U.; von Pelchrzim, R.; Schaefer, H.; Sterry, W.; Shah, V. Correlation of the Local Distribution of Topically Applied Substances Inside the Stratum Corneum Determined by Tape-Stripping to Differences in Bioavailability. Skin Pharmacol. Appl. Skin Physiol. 2001, 12, 98–102. [Google Scholar] [CrossRef]
- Pirot, F.; Kalia, Y.N.; Stinchcomb, A.L.; Keating, G.; Bunge, A.; Guy, R.H. Characterization of the permeability barrier of human skin in vivo. Proc. Natl. Acad. Sci. USA 1997, 94, 1562–1567. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Başer, K.H.C. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization of red radish anthocyanins. J. Food Sci. 1996, 61, 322–326. [Google Scholar] [CrossRef]
- Barry, A.M. Encapsulation, Color Stability, and Distribution of Anthocyanins from Purple Corn (Zea mays L.), Blueberry (Vaccinium sp.), and Red Radish (Raphanus sativus) in a Cold-Setting Pectin-Alginate Gel. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2013. [Google Scholar]
- Hadgraft, J.; Lane, M.E. Skin Permeation: Spectroscopic Methods. In Transdermal and Topical Drug Delivery: Principles and Practice; Benson, H.A., Watkinson, A.C., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Pasieczna-patkowska, S.; Olejnik, T. Analysis of cosmetic products using different IR spectroscopy techniques. Ann. Univ. Mariae Curie-Sklodowska Lubin-Pol. 2013, 68, 95–106. [Google Scholar] [CrossRef]
- He, J.; Rodriguez-Saona, L.; Giusti, M.M. Midinfrared Spectroscopy for Juice Authentication s Rapid Differentiation of Commercial Juices. J. Agric. Food Chem. 2007, 55, 4443–4452. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley and Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Cavadid, A.S. Multicomponent Quality Control Analysis for the Tomato Industry Using Portable Mid-Infrared (MIR) Spectroscopy. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2014. [Google Scholar]
- Parks, J.; Cleck, R.; Bunge, A. Chemical release from topical formulations across synthetic membranes: Infinite dose. J. Pharm. Sci. 1997, 86, 187–192. [Google Scholar] [CrossRef]
- Fitzpatrick, D.; Corish, J.; Hayes, B. Modelling skin permeability in risk assessment- the future. Chemosphere 2004, 55, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Arct, J.; Oborska, A.; Mojski, M.; Binkowska, A. Common cosmetic hydrophilic ingredients as penetration modifiers of flavonoids. Int. J. Cosmet. Sci. 2002, 24, 357–367. [Google Scholar] [CrossRef]
- Trommer, H.; Neubert, R.H.H. Overcoming the stratum corneum: The modulation of skin penetration. A review. Skin Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef]
- Lane, M.E.; Santos, P.; Watkinson, A.C.; Hadgraft, J. Passive Skin Permeation Enhancement. In Transdermal and Topical Drug Delivery: Principles and Practice; Benson, H., Watkinson, A.C., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bojanowski, K. Hypodermal delivery of cosmetic actives for improved facial skin morphology and functionality. Int. J. Cosmet. Sci. 2013, 35, 562–567. [Google Scholar] [CrossRef]
- Goto, T. Structure stability and color variation of natural anthocyanins. Prog. Chem. Nat. Prod. 1987, 52, 113–158. [Google Scholar]
- Figueiredo, P.; Elhabiri, M.; Toki, K.; Saito, N.; Dangles, O.; Brouillard, R. New aspects of anthocyanin complexation. Intramolecular copigmentation as a means for colour loss? Phytochemistry 1996, 41, 301–308. [Google Scholar] [CrossRef]
- Goto, T.; Kondo, T. Structure and molecular stacking of anthocyanins—Flower color variation. Angew. Chem. Int. Ed. Engl. 1991, 30, 17–33. [Google Scholar] [CrossRef]
- Giusti, M. Elucidation of the structure and conformation of red radish (Raphanus sativus) anthocyanins using one-and two-dimensional nuclear magnetic resonance techniques. J. Agric. Food Chem. 1998, 46, 4858–4863. [Google Scholar] [CrossRef]
- Giusti, M.M.; Rodríguez-Saona, L.E.; Wrolstad, R.E. Molar absorptivity and color characteristics of acylated and non- acylated pelargonidin-based anthocyanins. J. Agric. Food Chem. 1999, 47, 4631–4637. [Google Scholar] [CrossRef] [PubMed]
- Flynn, G.L. Physiochemical determinants of skin absorption. In Principles of Route-to-Route Exrapolation for Risk Assessment; Gerrity, T., Henry, C., Eds.; Elsevier: New York, NY, USA, 1990. [Google Scholar]
- Gee, C.M.; Nicolazzo, J.A.; Watkinson, A.C.; Finnin, B.C. Assessment of the lateral diffusion and penetration of topically applied drugs in humans using a novel concentric tape stripping design. Pharm. Res. 2012, 29, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Hwang, T.L.; Huang, Y.L.; Fang, C.L. Enhancement of the transdermal delivery of catechins by liposomes incorporating anionic surfactants and ethanol. Int. J. Pharm. 2006, 310, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, T.M.; Johnson, T.; Peterson, N.; Homer, L.; Walts, D.; Johnson, N.; Cunningham, J. Combination glutathione and anthocyanins as an alternative for skin care during external-beam radiation. Am. J. Surg. 2005, 189, 627–631. [Google Scholar] [CrossRef]
- Afaq, F.; Zaid, M.A.; Khan, N.; Dreher, M.; Mukhtar, H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009, 18, 553–561. [Google Scholar] [CrossRef]
- Jm, H.; Hc, K.; Ct, L.; Es, K. Inhibitory effect of liposome-encapsulated anthocyanin on melanogenesis in human melanocytes. Pharm Biol 2013, 51, 941–947. [Google Scholar]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Dermatopharmacokinetic prediction of topical drug bioavailability in vivo. J. Investig. Dermatol. 2007, 127, 887–894. [Google Scholar] [CrossRef]
- Roberts, M.; Anissimov, Y.; Gonsalvez, R. Mathematical models in percutaneous absorption. In Percutaneous Absorption, Drugs, Cosmetics, Mechanisms, Methodology; Bronaugh, R., Maibach, H.I., Eds.; Dekker: New York, NY, USA, 1999; pp. 3–55. [Google Scholar]
- Higuchi, W. Analysis of data on the medicament release from ointments. J. Pharmacol. Sci. 1962, 51, 802–804. [Google Scholar] [CrossRef]


 ), and PLS-R of FTIR spectra in vivo (
), and PLS-R of FTIR spectra in vivo ( ) and ex vivo (
) and ex vivo ( ). Means are shown as the average of triplicate samples from six participants. The lines are the best fit for Equation (A1) used to determine permeability and diffusivity of the formulas.
). Means are shown as the average of triplicate samples from six participants. The lines are the best fit for Equation (A1) used to determine permeability and diffusivity of the formulas.
 ), and PLS-R of FTIR spectra in vivo (
), and PLS-R of FTIR spectra in vivo ( ) and ex vivo (
) and ex vivo ( ). Means are shown as the average of triplicate samples from six participants. The lines are the best fit for Equation (A1) used to determine permeability and diffusivity of the formulas.
). Means are shown as the average of triplicate samples from six participants. The lines are the best fit for Equation (A1) used to determine permeability and diffusivity of the formulas.
| Peak | tR (min) | Source | [M+] | Anthocyanin |
|---|---|---|---|---|
| 1 | 8.3 | Elderberry | 581, 287 | Cy-3-samb |
| 2 | 14.4 | Elderberry | 449, 287 | Cy-3-glu |
| 3 | 23.6 | Red radish | 933, 271 | Pg-3-fer-soph-5-glu |
| 4 | 24.5 | Red radish | 989, 271 | Pg-3-cou-soph-5-mal-glu |
| 5 | 26.5 | Red radish | 1019, 271 | Pg-3-fer-soph-5-mal-glu |
| Release from Lipstick Formulations | |||
|---|---|---|---|
| Formula | Kr a × 102 (cm h−1) | KH b × 102 (cm h−0.5) | DV c × 103 (cm2 h−1) |
| Elderberry | 5.80 ± 0.11 * | 7.33 ± 0.03 * | 4.22 ± 0.02 * |
| Red Radish | 4.04 ± 0.13 * | 5.12 ± 0.06 * | 2.06 ± 0.04 * |
| Formula | Test System | K a | D/L2 b (h−1) |
|---|---|---|---|
| Elderberry | Ex vivo | 0.229 ± 0.02 | 0.142 ± 0.05 |
| In vivo HPLC | 0.224 ± 0.01 | 0.142 ± 0.04 | |
| In vivo FTIR | 0.224 ±0.02 | 0.144 ± 0.06 | |
| Red Radish | Ex vivo | 0.204 ± 0.01 | 0.136 ± 0.07 |
| In vivo HPLC | 0.209 ± 0.03 | 0.146 ± 0.02 | |
| In vivo FTIR | 0.207 ± 0.03 | 0.138 ± 0.01 |
| Formula | Test System | Kp × 104 a (cm h−1) | Jss b (μg cm2 h−1) |
|---|---|---|---|
| Elderberry | Ex vivo | 2.39 ± 0.59* | 3.34 ± 0.11* |
| In vivo HPLC | 2.33 ± 0.72 | 3.26 ± 0.04 | |
| In vivo FTIR | 2.36 ± 0.37 | 3.30 ± 0.09 | |
| Red Radish | Ex vivo | 2.02 ± 0.41* | 2.93 ± 0.04* |
| In vivo HPLC | 2.00 ± 0.68 | 3.04 ± 0.05 | |
| In vivo FTIR | 2.09 ± 0.34 | 2.88 ± 0.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westfall, A.; Sigurdson, G.T.; Rodriguez-Saona, L.E.; Giusti, M.M. Ex Vivo and In Vivo Assessment of the Penetration of Topically Applied Anthocyanins Utilizing ATR-FTIR/PLS Regression Models and HPLC-PDA-MS. Antioxidants 2020, 9, 486. https://doi.org/10.3390/antiox9060486
Westfall A, Sigurdson GT, Rodriguez-Saona LE, Giusti MM. Ex Vivo and In Vivo Assessment of the Penetration of Topically Applied Anthocyanins Utilizing ATR-FTIR/PLS Regression Models and HPLC-PDA-MS. Antioxidants. 2020; 9(6):486. https://doi.org/10.3390/antiox9060486
Chicago/Turabian StyleWestfall, Alexandra, Gregory T. Sigurdson, Luis E. Rodriguez-Saona, and M. Mónica Giusti. 2020. "Ex Vivo and In Vivo Assessment of the Penetration of Topically Applied Anthocyanins Utilizing ATR-FTIR/PLS Regression Models and HPLC-PDA-MS" Antioxidants 9, no. 6: 486. https://doi.org/10.3390/antiox9060486
APA StyleWestfall, A., Sigurdson, G. T., Rodriguez-Saona, L. E., & Giusti, M. M. (2020). Ex Vivo and In Vivo Assessment of the Penetration of Topically Applied Anthocyanins Utilizing ATR-FTIR/PLS Regression Models and HPLC-PDA-MS. Antioxidants, 9(6), 486. https://doi.org/10.3390/antiox9060486