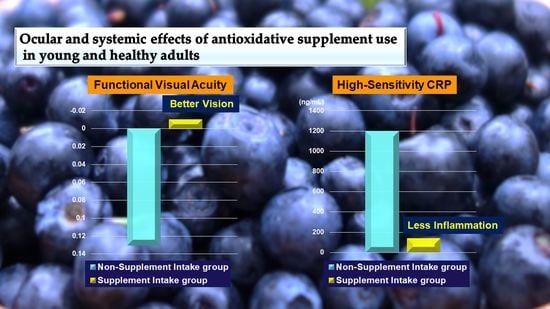
Ocular and Systemic Effects of Antioxidative Supplement Use in Young and Healthy Adults: Real-World Cross-Sectional Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ophthalmologic Examinations
2.3. Blood Sample Test
2.4. Ingestion Frequency Investigation
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Michikawa, T.; Ishida, S.; Nishiwaki, Y.; Kikuchi, Y.; Tsuboi, T.; Hosoda, K.; Ishigami, A.; Iwasawa, S.; Nakano, M.; Takebayashi, T. Serum antioxidants and age-related macular degeneration among older Japanese. Asia Pac. J. Clin. Nutr. 2009, 18, 1–7. [Google Scholar]
- Kassoff, A.; Kassoff, J.; Buehler, J.; Eglow, M.; Kaufman, F.; Mehu, M.; Kieval, S.; Mairs, M.; Graig, B.; Quattrocchi, A.; et al. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar]
- Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.; Ferris, F.L.; Elman, M.; Antoszyk, A.; Ruby, A.; Orth, D.; Bressler, S.; et al. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar]
- Jaschinski, W. The proximity-fixation-disparity curve and the preferred viewing distance at a visual display as an indicator of near vision fatigue. Optom. Vis. Sci. 2002, 79, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Yang, X.B.; Liu, L.Q. Comparative study on visual fatigue caused by watching liquid crystal display and projection display. Chin. J. Ophthalmol. 2019, 55, 595–600. [Google Scholar]
- Ozawa, Y.; Kawashima, M.; Inoue, S.; Inagaki, E.; Suzuki, A.; Ooe, E.; Kobayashi, S.; Tsubota, K. Bilberry extract supplementation for preventing eye fatigue in video display terminal workers. J. Nutr. Health Aging 2015, 19, 548–554. [Google Scholar] [CrossRef]
- Serban, M.C.; Sahebkar, A.; Dragan, S.; Stoichescu-Hogea, G.; Ursoniu, S.; Andrica, F.; Banach, M. A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations. Clin. Nutr. 2016, 35, 842–851. [Google Scholar] [CrossRef]
- Harman, D. A Antioxidant supplements: Effects on disease and aging in the United States population. J. Am. Aging Assoc. 2000, 23, 25–31. [Google Scholar] [CrossRef][Green Version]
- Minami, S.; Nagai, N.; Suzuki, M.; Kurihara, T.; Sonobe, H.; Watanabe, K.; Shinoda, H.; Takagi, H.; Tsubota, K.; Ozawa, Y. Spatial-sweep steady-state pattern electroretinography can detect subtle differences in visual function among healthy adults. Sci. Rep. 2019, 9, 18119. [Google Scholar] [CrossRef]
- Goto, E.; Yagi, Y.; Matsumoto, Y.; Tsubota, K. Impaired functional visual acuity of dry eye patients. Am. J. Ophthalmol. 2002, 133, 181–186. [Google Scholar] [CrossRef]
- Nishi, Y.; Shinoda, H.; Uchida, A.; Koto, T.; Mochimaru, H.; Nagai, N.; Tsubota, K.; Ozawa, Y. Detection of early visual impairment in patients with epiretinal membrane. Acta Ophthalmol. 2013, 91, e353–e357. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Nagai, N.; Suzuki, M.; Shinoda, H.; Uchida, A.; Mochimaru, H.; Izumi-Nagai, K.; Sasaki, M.; Tsubota, K.; Ozawa, Y. Functional Visual Acuity in Age-Related Macular Degeneration. Optom. Vis. Sci. 2016, 93, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Asato, T.; Minami, S.; Suzuki, M.; Shinoda, H.; Kurihara, T.; Sonobe, H.; Watanabe, K.; Uchida, A.; Ban, N.; et al. Correlation between Macular Pigment Optical Density and Neural Thickness and Volume of the Retina. Nutrients 2020, 12, 888. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Murakami, K.; Livingstone, M.B.E.; Sasaki, S. Thirteen-Year Trends in Dietary Patterns among Japanese Adults in the National Health and Nutrition Survey 2003–2015: Continuous Westernization of the Japanese Diet. Nutrients 2018, 10, 994. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zheng, Y.; Wang, P.; Zhang, Y. The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 375. [Google Scholar]
- ELDerawi, W.A.; Naser, I.A.; Taleb, M.H.; Abutair, A.S. The Effects of Oral Magnesium Supplementation on Glycemic Response among Type 2 Diabetes Patients. Nutrients 2018, 11, 44. [Google Scholar]
- Pickworth, C.K.; Deichert, D.A.; Corroon, J.; Bradley, R.D. Randomized controlled trials investigating the relationship between dietary pattern and high-sensitivity C-reactive protein: A systematic review. Nutr. Rev. 2019, 77, 363–375. [Google Scholar] [CrossRef]
- Zhang, E.; Gao, M.; Gao, J.; Xiao, J.; Li, X.; Zhao, H.; Wang, J.; Zhang, N.; Wang, S.; Liu, Y. Inflammatory and Hematological Indices as Simple, Practical Severity Predictors of Microdysfunction Following Coronary Intervention: A Systematic Review and Meta-Analysis. Angiology 2020, 71, 349–359. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, D.; Wang, M.; Zhao, F.; Han, X.; Qi, Y.; Liu, J. Association Between Circulating Oxidized LDL and Atherosclerotic Cardiovascular Disease: A Meta-analysis of Observational Studies. Can. J. Cardiol. 2017, 33, 1624–1632. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Morelli, N.R.; Scavuzzi, B.M.; Miglioranza, L.; Lozovoy, M.A.B.; Simão, A.N.C.; Dichi, I. Metabolic syndrome components are associated with oxidative stress in overweight and obese patients. Arch. Endocrinol. Metab. 2018, 62, 309–318. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Heidari-Bakavoli, A.R.; Shoeibi, S.; Mirhafez, S.R.; Moohebati, M.; Esmaily, H.; Ghazavi, H.; Saberi Karimian, M.; Parizadeh, S.M.; Mohammadi, M.; et al. Association of Serum hs-CRP Levels With the Presence of Obesity, Diabetes Mellitus, and Other Cardiovascular Risk Factors. J. Clin. Lab. Anal. 2016, 30, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Harford, K.A.; Reynolds, C.M.; McGillicuddy, F.C.; Roche, H.M. Fats, inflammation and insulin resistance: Insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc. Nutr. Soc. 2011, 70, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Mitta, V.P.; Christen, W.G.; Glynn, R.J.; Semba, R.D.; Ridker, P.M.; Rimm, E.B.; Hankinson, S.E.; Schaumberg, D.A. C-reactive protein and the incidence of macular degeneration: Pooled analysis of 5 cohorts. JAMA Ophthalmol. 2013, 131, 507–513. [Google Scholar] [CrossRef]
- Keenan, T.D.; Chew, E.Y. Association between C-Reactive Protein and Age-Related Macular Degeneration: Les Liaisons Dangereuses. JAMA Ophthalmol. 2017, 135, 916–917. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, S.; Boccardi, V.; Hosseini, B.; Taghizadeh, M.; Hamedifard, Z. A Meta-analysis of Randomized Control Trials: The Impact of Vitamin C Supplementation on Serum CRP and Serum hs-CRP Concentrations. Curr. Pharm. Des. 2018, 24, 3520–3528. [Google Scholar] [CrossRef]
- Kaido, M.; Dogru, M.; Ishida, R.; Tsubota, K. Concept of functional visual acuity and its applications. Cornea 2007, 26, 29–35. [Google Scholar] [CrossRef]
- Sasaki, S.; Kobayashi, M.; Tsugane, S. Validity of a self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I: Comparison with dietary records for food groups. J. Epidemiol. 2003, 13, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tsubono, Y.; Kobayashi, M.; Sasaki, S.; Tsugane, S. Validity and reproducibility of a self-administered food frequency questionnaire used in the baseline survey of the JPHC Study Cohort I. J. Epidemiol. 2003, 13, 125–133. [Google Scholar] [CrossRef]
- Kosehira, M.; Machida, N.; Kitaichi, N. A 12-Week-Long Intake of Bilberry Extract (Vaccinium myrtillus L.) Improved Objective Findings of Ciliary Muscle Contraction of the Eye: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial. Nutrients 2020, 12, 600. [Google Scholar] [CrossRef] [PubMed]
- Nakaishi, H.; Matsumoto, H.; Tominaga, S.; Hirayama, M. Effects of black current anthocyanoside intake on dark adaptation and VDT work-induced transient refractive alteration in healthy humans. Altern. Med. Rev. 2000, 5, 553–562. [Google Scholar] [PubMed]
- Riva, A.; Togni, S.; Franceschi, F.; Kawada, S.; Inaba, Y.; Eggenhoffner, R.; Giacomelli, L. T The effect of a natural, standardized bilberry extract (Mirtoselect®) in dry eye: A randomized, double blinded, placebo-controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2518–2525. [Google Scholar] [PubMed]
- Kawabata, F.; Tsuji, T. Effects of dietary supplementation with a combination of fish oil, bilberry extract, and lutein on subjective symptoms of asthenopia in humans. Biomed. Res. 2011, 32, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Negishi, K.; Dogru, M.; Saiki, M.; Tsubota, K. Improvement of functional visual acuity after cataract surgery in patients with good pre- and postoperative spectacle-corrected visual acuity. J. Refract. Surg. 2009, 25, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, T.H.; Yamaguchi, T.; Negishi, K.; Kaido, M.; Matsumoto, Y.; Ishida, R.; Kojima, T.; Ibrahim, O.M.; Saiki, M.; Dogru, M.; et al. Functional visual acuity after neodymium: YAG laser capsulotomy in patients with posterior capsule opacification and good visual acuity preoperatively. J. Cataract Refract. Surg. 2011, 37, 258–264. [Google Scholar] [CrossRef]
- Kaido, M.; Dogru, M.; Yamada, M.; Sotozono, C.; Kinoshita, S.; Shimazaki, J.; Tsubota, K. Functional visual acuity in Stevens-Johnson syndrome. Am. J. Ophthalmol. 2006, 142, 917–922. [Google Scholar] [CrossRef]
- Tanaka, M.; Takano, Y.; Dogru, M.; Toda, I.; Asano-Kato, N.; Komai-Hori, Y.; Tsubota, K. Effect of preoperative tear function on early functional visual acuity after laser in situ keratomileusis. J. Cataract Refract. Surg. 2004, 30, 2311–2315. [Google Scholar] [CrossRef]
- Ishioka, M.; Kato, N.; Takano, Y.; Shimazaki, J.; Tsubota, K. The quantitative detection of blurring of vision after eyedrop instillation using a functional visual acuity system. Acta Ophthalmol. 2009, 87, 574–575. [Google Scholar] [CrossRef]
- Kaido, M.; Uchino, M.; Kojima, T.; Dogru, M.; Tsubota, K. Effects of diquafosol tetrasodium administration on visual function in short break-up time dry eye. J. Ocul. Pharmacol. Ther. 2013, 29, 595–603. [Google Scholar] [CrossRef]
- Yang, C.H.; Albietz, J.; Harkin, D.G.; Kimlin, M.G.; Schmid, K.L. Impact of oral vitamin D supplementation on the ocular surface in people with dry eye and/or low serum vitamin D. Cont. Lens Anterior Eye 2018, 41, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Agirbasli, M.; Tanrikulu, A.; Acar Sevim, B.; Azizy, M.; Bekiroglu, N. Total cholesterol-to-high-density lipoprotein cholesterol ratio predicts high-sensitivity C-reactive protein levels in Turkish children. J. Clin. Lipidol. 2015, 9, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Bohula, E.A.; Giugliano, R.P.; Leiter, L.A.; Verma, S.; Park, J.G.; Sever, P.S.; Lira Pineda, A.; Honarpour, N.; Wang, H.; Murphy, S.A.; et al. Inflammatory and Cholesterol Risk in the FOURIER Trial. Circulation 2018, 138, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Esteve, E.; Ricart, W.; Fernandez-Real, J.M. Dyslipidemia and inflammation: An evolutionary conserved mechanism. Clin. Nutr. 2005, 24, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Sarinnapakorn, V.; Wanicagool, W. Association between hs-CRP and Hba1c in overweight type 2 diabetic female patients. J. Med. Assoc. Thai. 2013, 96, S54–S58. [Google Scholar]
- Jonas, J.B.; Wei, W.B.; Xu, L.; Wang, Y.X. Systemic inflammation and eye diseases. The Beijing Eye Study. PLoS ONE 2018, 13, e0204263. [Google Scholar] [CrossRef]
- Yasuma, T.R.; Nakamura, M.; Nishiguchi, K.M.; Kikuchi, M.; Kaneko, H.; Niwa, T.; Hamajima, N.; Terasaki, H. Elevated C-reactive protein levels and ARMS2/HTRA1 gene variants in subjects without age-related macular degeneration. Mol. Vis. 2010, 16, 2923–2930. [Google Scholar]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Noda, K.; Imamura, Y.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1433–1439. [Google Scholar] [CrossRef]
- Miyake, S.; Takahashi, N.; Sasaki, M.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: Cellular and molecular mechanism. Lab. Investig. 2012, 92, 102–109. [Google Scholar] [CrossRef]
- Narimatsu, T.; Negishi, K.; Miyake, S.; Hirasawa, M.; Osada, H.; Kurihara, T.; Tsubota, K.; Ozawa, Y. Blue light-induced inflammatory marker expression in the retinal pigment epithelium-choroid of mice and the protective effect of a yellow intraocular lens material in vivo. Exp. Eye Res. 2015, 132, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Kamoshita, M.; Toda, E.; Osada, H.; Narimatsu, T.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci. Rep. 2016, 6, 30226. [Google Scholar] [CrossRef] [PubMed]
- Osada, H.; Okamoto, T.; Kawashima, H.; Toda, E.; Miyake, S.; Nagai, N.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Neuroprotective effect of bilberry extract in a murine model of photo-stressed retina. PLoS ONE 2017, 12, e0178627. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Kawashima, H.; Osada, H.; Toda, E.; Homma, K.; Nagai, N.; Imai, Y.; Tsubota, K.; Ozawa, Y. Dietary Spirulina Supplementation Protects Visual Function From Photostress by Suppressing Retinal Neurodegeneration in Mice. Transl. Vis. Sci. Technol. 2019, 8, 20. [Google Scholar] [CrossRef]
- Wilson, L.R.; Tripkovic, L.; Hart, K.H.; Lanham-New, S.A. Vitamin D deficiency as a public health issue: Using vitamin D2 or vitamin D3 in future fortification strategies. Proc. Nutr. Soc. 2017, 76, 392–399. [Google Scholar] [CrossRef]
- Di Rosa, M.; Malaguarnera, L.; Nicolosi, A.; Sanfilippo, C.; Mazzarino, C.; Pavone, P.; Berretta, M.; Cosentino, S.; Cacopardo, B.; Pinzone, M.R.; et al. Vitamin D3: An. ever green molecule. Front. Biosci. 2013, 5, 247–260. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef]
- Sheerah, H.A.; Eshak, E.S.; Cui, R.; Imano, H.; Iso, H.; Tamakoshi, A. Relationship Between Dietary Vitamin D and Deaths From Stroke and Coronary Heart Disease: The Japan Collaborative Cohort Study. Stroke 2018, 49, 454–457. [Google Scholar] [CrossRef]
| Factors | n = 27 |
|---|---|
| Age | 26.6 ± 0.8 |
| Sex (male %) | 9 (33) |
| BCVA (LogMAR) | −0.08 ± 0.00 |
| FVA score | 0.08 ± 0.03 |
| VMR | 0.90 ± 0.02 |
| Contrast VA 6% | 0.28 ± 0.03 |
| Contrast VA 12% | 0.13 ± 0.03 |
| Spherical Equivalent (Diopters) | −2.95 ± 0.65 |
| Axial Length (mm) | 24.91 ± 0.28 |
| IOP (mmHg) | 13.5 ± 0.4 |
| Smoking habit (%) | 5 (19) |
| Drinking habit (%) | 21 (78) |
| Snacking habit (%) | 19 (70) |
| Exercise habit (%) | 10 (37) |
| Antioxidative supplement intake (%) | 11 (41) |
| Duration of antioxidative supplement intake (Months) (range) | 4.44 ± 1.89 (0–36) |
| Participants | Contents |
|---|---|
| 1 | Resveratrol (Santa Berry extract) 4 mg |
| 2 | Anthocyanin (Bilberry extract) 160 mg, Multivitamin, Zinc 7 mg, Vitamin D 10.5 μg |
| 3 | Bifidobacteria, Malic acid |
| 4 | Anthocyanin (Bilberry extract) 160 mg, Bifidobacteria, Multivitamin |
| 5 | Anthocyanin (Bilberry extract) 160 mg |
| 6 | Anthocyanin (Bilberry extract) 160 mg |
| 7 | Vitamin C 2000 mg |
| 8 | Anthocyanin (Bilberry extract) 160 mg, Lutein 10 mg |
| 9 | Anthocyanin (Bilberry extract) 160 mg, Multivitamin, Zinc 3.5 mg |
| 10 | Anthocyanin (Bilberry extract) 160 mg, Multivitamin, Rose oil |
| 11 | Bifidobacteria, Theanine 200 mg |
| Factors | Non-Supplement Intake Group (n = 16) | Supplement Intake Group (n = 11) | p |
|---|---|---|---|
| Age | 26.69 ± 0.93 | 32.57 ± 3.62 | 0.837 |
| Sex (male, eyes %) † | 4 (25) | 5 (46) | 0.411 |
| BCVA § | −0.08 ± 0.00 | −0.08 ± 0.00 | 1.000 |
| FVA score | 0.13 ± 0.03 | −0.01 ± 0.02 | 0.004 ** |
| VMR | 0.87 ± 0.02 | 0.96 ± 0.01 | 0.007 ** |
| Contrast VA 6% | 0.28 ± 0.04 | 0.27 ± 0.04 | 0.834 |
| Contrast VA 12% | 0.12 ± 0.04 | 0.13 ± 0.04 | 0.863 |
| Spherical Equivalent (D) | −3.26 ± 0.98 | −2.51 ± 0.65 | 0.585 |
| Axial length (mm) | 25.06 ± 0.36 | 24.69 ± 0.42 | 0.529 |
| IOP | 13.85 ± 0.63 | 13.53 ± 0.70 | 0.287 |
| OCT data | |||
| MV (IRL) | 3.99 ± 0.07 | 3.95 ± 0.07 | 0.691 |
| MV (ORL) | 4.38 ± 0.04 | 4.42 ± 0.05 | 0.527 |
| MV (RPE) | 0.38 ± 0.00 | 0.38 ± 0.04 | 0.863 |
| Smoking habit (%) † | 1 (6) | 4 (36) | 0.125 |
| Drinking habit (%) † | 11 (69) | 10 (91) | 0.350 |
| Snacking habit (%) † | 10 (63) | 9 (82) | 0.405 |
| Exercise habit (%) † | 6 (38) | 4 (36) | 1.000 |
| Duration of antioxidative supplement intake (Months) (range) | 0.19 ± 0.10 (0–1) | 10.64 ± 4.03 (2–36) | 0.004 ** |
| Factors | Non-Supplement Intake Group (n = 16) | Supplement Intake Group (n = 11) | p |
|---|---|---|---|
| HDL (mg/dL) | 61.31 ± 4.77 | 62.27 ± 4.65 | 0.891 |
| LDL (mg/dL) | 111.94 ± 8.15 | 93.18 ± 5.22 | 0.065 |
| LDL/ HDL ratio | 2.02 ± 0.22 | 1.59 ± 0.15 | 0.159 |
| MDA-LDL (U/L) | 91.75 ± 7.66 | 79.18 ± 7.87 | 0.278 |
| TG (mg/dL) | 109.50 ± 20.17 | 90.36 ± 13.16 | 0.482 |
| T-Cho (mg/dL) | 191.50 ± 6.30 | 168.64 ± 6.28 | 0.020 * |
| HbA1c (NGSP) (%) | 5.33 ± 0.09 | 5.13 ± 0.04 | 0.048 * |
| AST (GOT) (U/L) | 23.31 ± 4.89 | 16.73 ± 1.60 | 0.289 |
| ALT (GPT) (U/L) | 36.81 ± 17.44 | 16.91 ± 3.93 | 0.361 |
| γ-GTP (U/L) | 29.06 ± 7.40 | 22.64 ± 4.24 | 0.511 |
| WBC (μL) | 6137.50 ± 337.25.64 | 6000.00 ± 463.39 | 0.808 |
| RBC (×104/μL) | 467.69 ± 12.55 | 479.45 ± 11.90 | 0.522 |
| Hb (g/dL) | 13.83 ± 0.46 | 14.28 ± 0.34 | 0.471 |
| HT (%) | 41.76 ± 1.14 | 43.42 ± 0.97 | 0.309 |
| MCV (fL) | 89.46 ± 1.48 | 90.68 ± 1.24 | 0.560 |
| MCH (pg) | 29.59 ± 0.61 | 29.84 ± 0.39 | 0.761 |
| MCHC (%) | 33.02 ± 0.28 | 32.90 ± 0.18 | 0.752 |
| Plt (×104/μL) | 26.34 ± 1.30 | 29.40 ± 1.29 | 0.118 |
| hs-CRP (ng/mL) | 1197.38 ± 436.07 | 134.44 ± 40.53 | 0.034 * |
| Factors | r | p |
|---|---|---|
| HDL (mg/dL) | −0.389 | 0.045 * |
| LDL (mg/dL) | 0.625 | <0.001 ** |
| LDL/HDL ratio | 0.748 | <0.001 ** |
| MDA-LDL (U/L) | 0.468 | 0.014 * |
| TG (mg/dL) | 0.243 | 0.222 |
| T-Cho (mg/dL) | 0.511 | 0.006 ** |
| HbA1c (NGSP) (%) | 0.374 | 0.054 |
| AST(GOT) (U/L) | 0.376 | 0.053 |
| ALT(GPT) (U/L) | 0.412 | 0.033 * |
| γ-GTP (U/L) | 0.585 | 0.001 ** |
| Factors | Non-Supplement Intake Group (n = 16) | Supplement Intake Group (n = 11) | p |
|---|---|---|---|
| Energy (kcal/day) | 1712.40 ± 183.28 | 1635.25 ± 254.15 | 0.802 |
| Protein (g/day) | 60.45 ± 6.49 | 51.58 ± 10.41 | 0.453 |
| Total fat (g/day) | 58.25 ± 6.84 | 51.33 ± 12.35 | 0.602 |
| Carbohydrate (g/day) | 221.65 ± 27.20 | 192.53 ± 27.87 | 0.475 |
| Retinol (mg/day) | 382.07 ± 126.67 | 459.74 ± 170.71 | 0.712 |
| Retinol Equivalent (μgRAE) | 553.47 ± 135.05 | 548.98 ± 175.03 | 0.984 |
| Vitamin D (μg/day) | 6.64 ± 1.19 | 3.37 ± 0.73 | 0.047 * |
| SFA (g/day) | 18.43 ± 2.41 | 15.71 ± 3.91 | 0.537 |
| MUFA (g/day) | 21.82 ± 2.41 | 20.48 ± 5.28 | 0.801 |
| PUFA (g/day) | 11.21 ± 1.27 | 9.54 ± 1.98 | 0.463 |
| Cholesterol (mg/day) | 373.72 ± 86.81 | 233.87 ± 52.59 | 0.231 |
| n-3PUFA (g/day) | 1.89 ± 0.28 | 1.52 ± 0.35 | 0.407 |
| n-6 PUFA (g/day) | 9.28 ± 1.00 | 7.99 ± 1.63 | 0.482 |
| Lycopene (mg/day) | 2959.97 ± 1016.85 | 2511.61 ± 1064.93 | 0.769 |
| α-carotene (μg/day) | 264.76 ± 74.70 | 122.18 ± 37.19 | 0.149 |
| β-carotene (μg/day) | 1422.06 ± 318.29 | 891.53 ± 176.60 | 0.210 |
| α-tocopherol (mg/day) | 5.90 ± 0.74 | 4.68 ± 0.88 | 0.302 |
| β-tocopherol (mg/day) | 0.32 ± 0.03 | 0.27 ± 0.06 | 0.490 |
| γ-tocopherol (mg/day) | 8.25 ± 1.15 | 7.47 ± 1.92 | 0.713 |
| δ-tocopherol (mg/day) | 1.82 ± 0.27 | 1.55 ± 0.39 | 0.562 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minami, S.; Nagai, N.; Suzuki, M.; Uchida, A.; Shinoda, H.; Tsubota, K.; Ozawa, Y. Ocular and Systemic Effects of Antioxidative Supplement Use in Young and Healthy Adults: Real-World Cross-Sectional Data. Antioxidants 2020, 9, 487. https://doi.org/10.3390/antiox9060487
Minami S, Nagai N, Suzuki M, Uchida A, Shinoda H, Tsubota K, Ozawa Y. Ocular and Systemic Effects of Antioxidative Supplement Use in Young and Healthy Adults: Real-World Cross-Sectional Data. Antioxidants. 2020; 9(6):487. https://doi.org/10.3390/antiox9060487
Chicago/Turabian StyleMinami, Sakiko, Norihiro Nagai, Misa Suzuki, Atsuro Uchida, Hajime Shinoda, Kazuo Tsubota, and Yoko Ozawa. 2020. "Ocular and Systemic Effects of Antioxidative Supplement Use in Young and Healthy Adults: Real-World Cross-Sectional Data" Antioxidants 9, no. 6: 487. https://doi.org/10.3390/antiox9060487
APA StyleMinami, S., Nagai, N., Suzuki, M., Uchida, A., Shinoda, H., Tsubota, K., & Ozawa, Y. (2020). Ocular and Systemic Effects of Antioxidative Supplement Use in Young and Healthy Adults: Real-World Cross-Sectional Data. Antioxidants, 9(6), 487. https://doi.org/10.3390/antiox9060487