Baicalein Inhibits Benzo[a]pyrene-Induced Toxic Response by Downregulating Src Phosphorylation and by Upregulating NRF2-HMOX1 System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cell Proliferation Assay
2.4. Treatment of Cells for RNA and Protein Extraction
2.5. RNA Extraction and Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
2.6. siRNA Transfection
2.7. ROS Measurement by Flow Cytometry
2.8. Western Blotting
2.9. Extraction of Cytoplasmic and Nuclear Protein
2.10. Immunocytochemistry
2.11. Statistical Analysis
3. Results
3.1. BAI Reduces BaP-Induced CYP1A1 Expression in Keratinocytes
3.2. Baicalein Activates NRF2-HMOX1 Antioxidative Pathway
3.3. Baicalein Inhibits BaP-Induced ROS Production in Keratinocytes
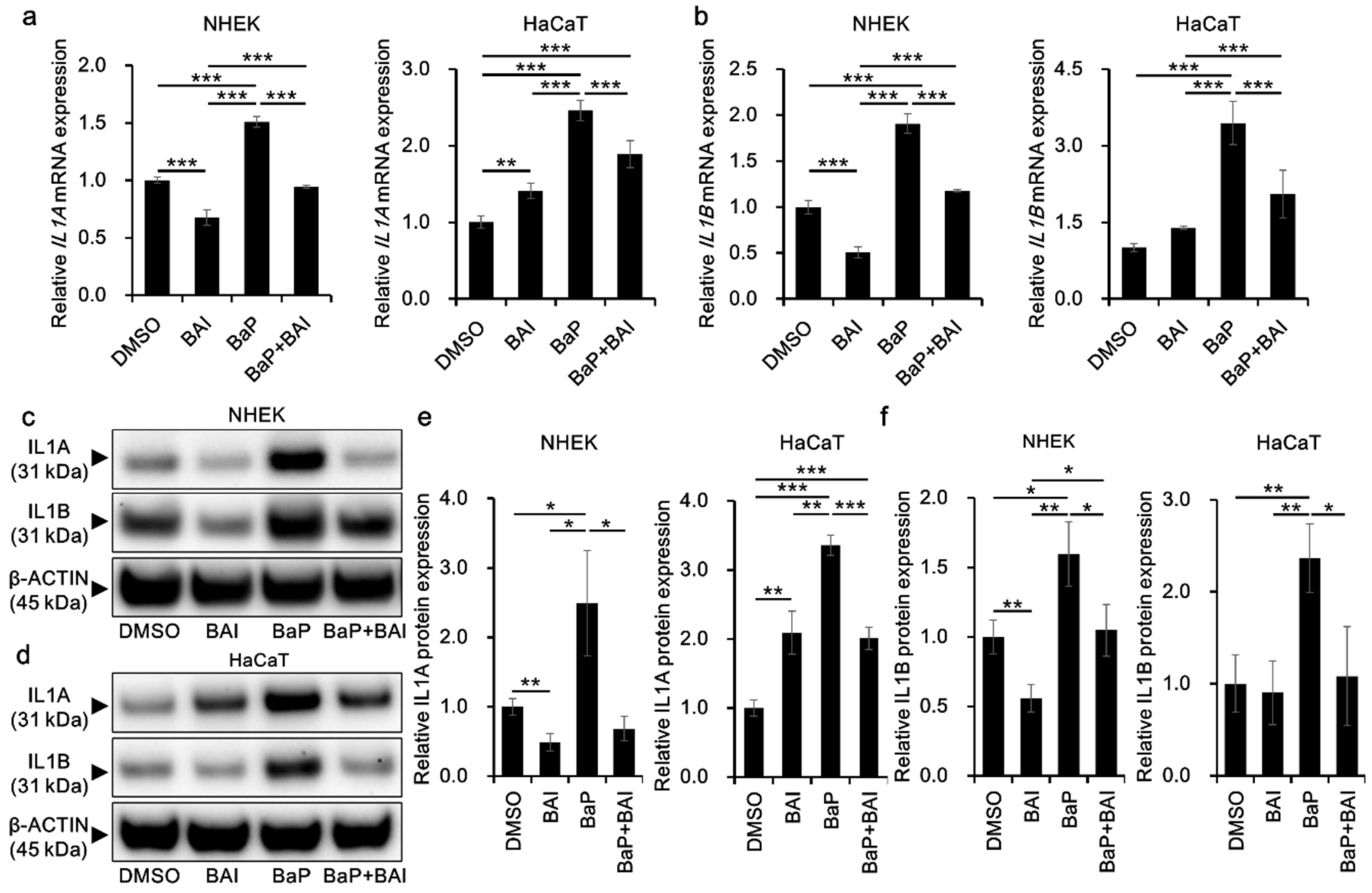
3.4. BaP-Induced Proinflammatory Cytokine Expression Is Attenuated by Baicalein
3.5. BaP-Induced Src Phosphorylation and AHR Nuclear Translocation Are Inhibited by Baicalein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Peng, F.; Tsuji, G.; Zhang, J.Z.; Chen, Z.; Furue, M. Potential role of PM2.5 in melanogenesis. Environ. Int. 2019, 132, 105063. [Google Scholar] [CrossRef]
- Furue, M.; Tsuji, G. Chloracne and hyperpigmentation caused by exposure to hazardous aryl hydrocarbon receptor ligands. Int. J. Environ. Res. Public Health 2019, 16, 4864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petry, T.; Schmid, P.; Schlatter, C. The use of toxic equivalency factors in assessing occupational and environmental health risk associated with exposure to airborne mixtures of Polycyclic Aromatic Hydrocarbons (PAHs). Chemosphere 1996, 32, 639–648. [Google Scholar] [CrossRef]
- Lawther, P.J.; Walter, R.E. Coal fires, industrial emissions and motor vehicles as sources of environmental carcinogens. IARC Sci. Publ. 1976, 13, 27–40. [Google Scholar]
- Bausinger, J.; Schütz, P.; Piberger, A.N.; Speit, G. Further characterization of Benzo[a]pyrene Diol-epoxide (BPDE)-induced comet assay effects. Mutagenesis 2016, 31, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Köhle, C.; Bock, K.W. Coordinate regulation of phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochem. Pharmacol. 2007, 73, 1853–1862. [Google Scholar] [CrossRef]
- Tsuji, G.; Takahara, M.; Uchi, H.; Takeuchi, S.; Mitoma, C.; Moroi, Y.; Furue, M. An environmental contaminant, Benzo[a]pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J. Dermatol. Sci. 2011, 62, 42–49. [Google Scholar] [CrossRef]
- Esser, C.; Bargen, I.; Weighardt, H.; Haarmann-Stemmann, T.; Krutmann, J. Functions of the aryl hydrocarbon receptor in the skin. Semin. Immunopathol. 2013, 35, 677–691. [Google Scholar] [CrossRef]
- Esser, C.; Rannug, A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 2015, 67, 259–279. [Google Scholar] [CrossRef] [Green Version]
- Furue, M.; Takahara, M.; Nakahara, T.; Uchi, H. Role of AhR/ARNT system in skin homeostasis. Arch. Dermatol. Res. 2014, 306, 769–779. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, P.; Garmyn, M.; Van Laethem, A. The aryl hydrocarbon receptor: An illuminating effector of the UVB response. Sci. STKE 2007, 403, pe49. [Google Scholar] [CrossRef] [PubMed]
- Barouki, R.; Coumoul, X.; Fernandez-Salguero, P.M. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007, 581, 3608–3615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enan, E.; Matsumura, F. Identification of c-Src as the integral component of the cytosolic ah receptor complex, transducing the signal of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) through the protein phosphorylation pathway. Biochem. Pharmacol. 1996, 52, 1599–1612. [Google Scholar] [CrossRef]
- Fritsche, E.; Schäfer, C.; Calles, C.; Bernsmann, T.; Bernshausen, T.; Wurm, M.; Hübenthal, U.; Cline, J.E.; Hajimiragha, H.; Schroeder, P.; et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc. Natl. Acad. Sci. USA 2007, 104, 8851–8856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazlauskas, A.; Sundström, S.; Poellinger, L.; Pongratz, I. The Hsp90 chaperone complex regulates intracellular localization of the dioxin receptor. Mol. Cell. Biol. 2001, 21, 2594–2607. [Google Scholar] [CrossRef] [Green Version]
- Lees, M.J.; Peet, D.J.; Whitelaw, M.L. Defining the role for XAP2 in stabilization of the dioxin receptor. J. Biol. Chem. 2003, 278, 35878–35888. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Soshilov, A.; Denison, M.S. Role of the Per/Arnt/Sim domains in ligand-dependent transformation of the aryl hydrocarbon receptor. J. Biol. Chem. 2008, 283, 32995–33005. [Google Scholar] [CrossRef] [Green Version]
- Stockinger, B.; Di Meglio, P.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–443. [Google Scholar] [CrossRef]
- Emi, Y.; Ikushiro, S.; Iyanagi, T. Xenobiotic responsive element-mediated transcriptional activation in the UDP-glucuronosyltransferase family 1 gene complex. J. Biol. Chem. 1996, 271, 3952–3958. [Google Scholar] [CrossRef] [Green Version]
- Favreau, L.V.; Pickett, C.B. Transcriptional regulation of the rat NAD(P)H: Quinone reductase gene. identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J. Biol. Chem. 1991, 266, 4556–4561. [Google Scholar] [PubMed]
- Fujisawa-Sehara, A.; Sogawa, K.; Yamane, M.; Fujii-Kuriyama, Y. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: A similarity to glucocorticoid regulatory elements. Nucleic Acids Res. 1987, 15, 4179–4191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rushmore, T.H.; King, R.G.; Paulson, K.E.; Pickett, C.B. Regulation of glutathione S-transferase Ya subunit gene expression: Identification of a unique xenobiotic-responsive element controlling inducible expression by planar aromatic compounds. Proc. Natl. Acad. Sci. USA 1990, 87, 3826–3830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anandasadagopan, S.K.; Singh, N.M.; Raza, H.; Bansal, S.; Selvaraj, V.; Singh, S.; Chowdhury, A.R.; Leu, N.A.; Avadhani, N.G. β-naphthoflavone-induced mitochondrial respiratory damage in cyp1 knockout mouse and in cell culture systems: Attenuation by resveratrol treatment. Oxidative Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopf, P.G.; Walker, M.K. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol. Appl. Pharmacol. 2010, 245, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Uchi, H.; Hashimoto-Hachiya, A.; Furue, M. Tryptophan photoproduct FICZ upregulates IL1A, IL1B, and IL6 expression via oxidative stress in keratinocytes. Oxidative Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Tamaki, A.; Hayashi, H.; Nakajima, H.; Takii, T.; Katagiri, D.; Miyazawa, K.; Hirose, K.; Onozaki, K. Polycyclic aromatic hydrocarbon increases mRNA level for interleukin 1 beta in human fibroblast-like synoviocyte line via aryl hydrocarbon receptor. Biol. Pharm. Bull. 2004, 27, 407–410. [Google Scholar] [CrossRef] [Green Version]
- Ueng, T.H.; Hung, C.C.; Kuo, M.L.; Chan, P.K.; Hu, S.H.; Yang, P.C.; Chang, L.W. Induction of fibroblast growth factor-9 and interleukin-1alpha gene expression by motorcycle exhaust particulate extracts and Benzo[a]pyrene in human lung adenocarcinoma cells. Toxicol. Sci. 2005, 87, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Lavieri, R.; Rubartelli, A.; Carta, S. Redox stress unbalances the inflammatory cytokine network: Role in autoinflammatory patients and healthy subjects. J. Leukoc. Biol. 2016, 99, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Domercq, M.; Perez-Samartin, A.; Aparicio, D.; Alberdi, E.; Pampliega, O.; Matute, C. P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia 2010, 58, 730–740. [Google Scholar] [CrossRef]
- Onami, K.; Kimura, Y.; Ito, Y.; Yamauchi, T.; Yamasaki, K.; Aiba, S. Nonmetal haptens induce ATP release from keratinocytes through opening of pannexin hemichannels by reactive oxidative species. J. Investig. Dermatol. 2014, 134, 1951–1960. [Google Scholar] [CrossRef] [Green Version]
- Haarmann-Stemmann, T.; Esser, C.; Krutmann, J. The janus-faced role of aryl hydrocarbon receptor signaling in the skin: Consequences for prevention and treatment of skin disorders. J. Investig. Dermatol. 2015, 135, 2572–2576. [Google Scholar] [CrossRef] [Green Version]
- Furue, M.; Uchi, H.; Mitoma, C.; Hashimoto-Hachiya, A.; Chiba, T.; Ito, T.; Nakahara, T.; Tsuji, G. Antioxidants for healthy skin: The emerging role of aryl hydrocarbon receptors and nuclear factor-erythroid 2-related factor-2. Nutrients 2017, 9, 223. [Google Scholar] [CrossRef]
- Nakahara, T.; Mitoma, C.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Uchi, H.; Yan, X.; Hachisuka, J.; Chiba, T.; Esaki, H.; et al. Antioxidant Opuntia ficus-indica extract activates AHR-NRF2 signaling and upregulates filaggrin and loricrin expression in human keratinocytes. J. Med. Food 2015, 18, 1143–1149. [Google Scholar] [CrossRef]
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Uchi, H.; Takahara, M.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. Antioxidant soybean tar glyteer rescues T-helper-mediated downregulation of filaggrin expression via aryl hydrocarbon receptor. J. Dermatol. 2015, 42, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Takahara, M.; Uchi, H.; Matsuda, T.; Chiba, T.; Takeuchi, S.; Yasukawa, F.; Moroi, Y.; Furue, M. Identification of ketoconazole as an AhR-Nrf2 activator in cultured human keratinocytes: The basis of its anti-inflammatory effect. J. Investig. Dermatol. 2012, 132, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Van den Bogaard, E.H.; Bergboer, J.G.; Vonk-Bergers, M.; van Vlijmen-Willems, I.M.; Hato, S.V.; van der Valk, P.G.; Schröder, J.M.; Joosten, I.; Zeeuwen, P.L.; Schalkwijk, J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Investig. 2013, 123, 917–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissonnette, R.; Poulin, Y.; Zhou, Y.; Tan, J.; Hong, H.C.; Webster, J.; Ip, W.; Tang, L.; Lyle, M. Efficacy and safety of topical WBI-1001 in patients with mild to severe atopic dermatitis: Results from a 12-Week, multicentre, randomized, placebo-controlled double-blind trial. Br. J. Dermatol. 2012, 166, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Bolduc, C.; Maari, C.; Nigen, S.; Webster, J.M.; Tang, L.; Lyle, M. Efficacy and safety of topical WBI-1001 in patients with mild to moderate psoriasis: Results from a randomized double-blind placebo-controlled, phase II trial. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int. J. Mol. Sci. 2019, 20, 5424. [Google Scholar] [CrossRef] [Green Version]
- Robbins, K.; Bissonnette, R.; Maeda-Chubachi, T.; Ye, L.; Peppers, J.; Gallagher, K.; Kraus, J.E. Phase 2, Randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of plaque psoriasis. J. Am. Acad. Dermatol. 2019, 80, 714–721. [Google Scholar] [CrossRef]
- Smith, S.H.; Jayawickreme, C.; Rickard, D.J.; Nicodeme, E.; Bui, T.; Simmons, C.; Coquery, C.M.; Neil, J.; Pryor, W.M.; Mayhew, D.; et al. Tapinarof is a natural ahr agonist that resolves skin inflammation in mice and humans. J. Investig. Dermatol. 2017, 137, 2110–2119. [Google Scholar] [CrossRef] [Green Version]
- Zang, Y.N.; Jiang, D.L.; Cai, L.; Chen, X.; Wang, Q.; Xie, Z.W.; Liu, Y.; Zhang, C.Y.; Jing, S.; Chen, G.H.; et al. Use of a dose-response model to guide future clinical trial of benvitimod cream to treat mild and moderate psoriasis. Int. J. Clin. Pharmacol. Ther. 2016, 54, 87–95. [Google Scholar] [CrossRef]
- Liau, P.R.; Wu, M.S.; Lee, C.K. Inhibitory effects of Scutellaria baicalensis root extract on linoleic acid hydroperoxide-induced lung mitochondrial lipid peroxidation and antioxidant activities. Molecules 2019, 24, 2143. [Google Scholar] [CrossRef] [Green Version]
- Oshima, N.; Narukawa, Y.; Hada, N.; Kiuchi, F. Quantitative analysis of anti-inflammatory activity of orengedokuto: Importance of combination of flavonoids in inhibition of PGE2 production in mouse macrophage-like cell line J774.1. J. Nat. Med. 2013, 67, 281–288. [Google Scholar] [CrossRef]
- Wang, Y.S.; Cho, J.G.; Hwang, E.S.; Yang, J.E.; Gao, W.; Fang, M.Z.; Zheng, S.D.; Yi, T.H. Enhancement of protective effects of radix scutellariae on UVB-induced photo damage in human HaCaT keratinocytes. Appl. Biochem. Biotechnol. 2018, 184, 1073–1093. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Bae, J.S. Anti-inflammatory effects of baicalin, baicalein, and wogonin in vitro and in vivo. Inflammation 2015, 38, 110–125. [Google Scholar] [CrossRef]
- Lu, Y.; Joerger, R.; Wu, C. Study of the chemical composition and antimicrobial activities of ethanolic extracts from roots of Scutellaria baicalensis Georgi. J. Agric. Food Chem. 2011, 59, 10934–10942. [Google Scholar] [CrossRef]
- Lin, H.; Hao, Y.; Wan, X.; He, J.; Tong, Y. Baicalein inhibits cell development, metastasis and EMT and induces apoptosis by regulating ERK signaling pathway in osteosarcoma. J. Recept. Signal. Transduct. 2020, 40, 49–57. [Google Scholar] [CrossRef]
- De Oliveiar, M.R.; Nabavi, S.F.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Nabavi, S.M. The effect of baicalein and baicalin on mitochondrial function and dynamics: A review. Pharmacol. Res. 2015, 100, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, K.; Nagasawa, H.; Kogure, K. Effect of orengedoku-to on cerebral vascular accident. Pharma Med. 1988, 6, 33–37. [Google Scholar]
- Ohta, Y.; Kobayashi, T.; Nishida, K.; Sasaki, E.; Ishiguro, I. Preventive effect of oren-gedoku-to (Huanglian-Jie-Du-Tang) extract on the development of stress-induced acute gastric mucosal lesions in rats. J. Ethnopharmacol. 1999, 67, 377–384. [Google Scholar] [CrossRef]
- Wang, L.M.; Mineshita, S. Preventive effects of unsei-in and oren-gedoku-to, chinese traditional medicines, against rat paw oedema and abdominal constriction in mice. J. Pharm. Pharmacol. 1996, 48, 327–331. [Google Scholar] [CrossRef]
- Ito, H. Application of oren-gedoku-to to many illnesses in japanese oriental medicine kampo. Newest Ther. 2001, 10, 243–246. [Google Scholar]
- Zhang, S.; Qin, C.; Safe, S.H. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: Effects of structure and cell context. Environ. Health Perspect. 2003, 111, 1877–1882. [Google Scholar] [CrossRef]
- Chan, H.Y.; Chen, Z.Y.; Tsang, D.S.; Leung, L.K. Baicalein inhibits DMBA-DNA adduct formation by modulating CYP1A1 and CYP1B1 activities. Biomed. Pharmacother. 2002, 56, 269–275. [Google Scholar] [CrossRef]
- Uchi, H.; Yasumatsu, M.; Morino-Koga, S.; Mitoma, C.; Furue, M. Inhibition of aryl hydrocarbon receptor signaling and induction of NRF2-mediated antioxidant activity by cinnamaldehyde in human keratinocytes. J. Dermatol. Sci. 2017, 85, 36–43. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell. Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Cha, H.J.; Hong, S.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; Kim, B.W.; Joen, Y.J.; Choi, Y.Y. Protective effect of phloroglucinol on oxidative stress-induced DNA damage and apoptosis through activation of the Nrf2/HO-1 signaling pathway in HaCaT human keratinocytes. Mar. Drugs. 2019, 17, 225. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Oh, J.; Anerilla, J.N.; Kim, H.Y.; Kim, J.S.; Kim, J.S. Grape peel extract and resveratrol inhibit wrinkle formation in mice model through activation of Nrf2/HO-1 signaling pathway. J. Food Sci. 2019, 84, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Park, S.H.; Yoo, J.A.; Kwon, K.; Kim, J.W.; Oh, S.W.; Park, S.J.; Kim, J.; Yu, E.; Han, B.S.; et al. Antagonizing effects of Clematis apiifolia DC. extract against Benzo[a]pyrene-induced damage to human keratinocytes. Oxidative Med. Cell. Longev. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Godschalk, R.W.L.; van Schooten, F.J. Inflammation and the chemical carcinogen Benzo[a]pyrene: Partners in crime. Mutat. Res. 2017, 774, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Dertinger, S.D.; Nazarenko, D.A.; Silverstone, A.E.; Gasiewicz, T.A. Aryl hydrocarbon receptor signaling plays a significant role in mediating Benzo[a]pyrene- and cigarette smoke condensate-induced cytogenetic damage in vivo. Carcinogenesis 2001, 22, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, Y.; Nakatsuru, Y.; Ichinose, M.; Takahashi, Y.; Kume, H.; Mimura, J.; Fujii-Kuriyama, Y.; Ishikawa, T. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 779–782. [Google Scholar] [CrossRef] [Green Version]
- Mimura, J.; Yamashita, K.; Nakamura, K.; Morita, M.; Takagi, T.N.; Nakao, K.; Ema, M.; Sogawa, K.; Yasuda, M.; Katsuki, M.; et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the ah (Dioxin) receptor. Genes Cells 1997, 2, 645–654. [Google Scholar] [CrossRef]
- Naveenkumar, C.; Raghunandakumar, S.; Asokkumar, S.; Binuclara, J.; Rajan, B.; Premkumar, T.; Devaki, T. Mitigating role of baicalein on lysosomal enzymes and xenobiotic metabolizing enzyme status during lung carcinogenesis of swiss albino mice induced by Benzo(a)pyrene. Fundam. Clin. Pharmacol. 2014, 28, 310–322. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, B.; Sun, J.; Lu, L.; Liu, L.; Qiu, J.; Li, Q.; Yan, C.; Jiang, S.; Mohammadtursun, N.; et al. Scutellaria flavonoids effectively inhibit the malignant phenotypes of non-small cell lung cancer in an Id1-dependent manner. Int. J. Biol. Sci. 2019, 15, 1500–1513. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Tian, F.; Ying, W.; Qian, X. Quantitative proteomics reveal the anti-tumour mechanism of the carbohydrate recognition domain of Galectin-3 in hepatocellular carcinoma. Sci. Rep. 2017, 7, 5189. [Google Scholar] [CrossRef] [Green Version]
- Mohebati, A.; Guttenplan, J.B.; Kochhar, A.; Zhao, Z.L.; Kosinska, W.; Subbaramaiah, K.; Dannenberg, A.J. Hydrocarvon receptor-mediated activation of CYP1A1 and CYP1B1 transcription and mutagenesis. Cancer Prev. Res. 2012, 5, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Oh, M.C.; Piao, M.J.; Fernando, P.M.; Han, X.; Madduma Hewage, S.R.; Park, J.E.; Ko, M.S.; Jung, U.; Kim, I.G.; Hyun, J.W. Baicalein protects human skin cells against ultraviolet B-induced oxidative stress. Biomol. Ther. 2016, 24, 616–622. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Sumiyoshi, M. Effects of baicalein and wogonin isolated from Scutellaria baicalensis roots on skin damage in acute UVB-irradiated hairless mice. Eur. J. Pharmacol. 2011, 661, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Zhang, R.; Piao, M.J.; Chae, S.; Kim, H.S.; Park, J.H.; Jung, K.S.; Hyun, J.W. Baicalein inhibits oxidative stress-induced cellular damage via antioxidant effects. Toxicol. Ind. Health 2012, 28, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shi, G.; Wand, J.; Zhang, D.; Pan, Y.; Dou, H.; Hou, Y. Baicalein ameliorates pristine-induced lupus nephritis via activating Nrf2/HO-1 in myeloid-derived suppressor cells. Arthritis Res. Ther. 2019, 21, 105. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Li, S.; Zhu, L.; Guo, S.; Yi, X.; Cui, T.; Hey, Y.; Chang, Y.; Liu, B.; Li, C.; et al. Baicalein protects human vitiligo melanocytes from oxidative stress through axtivation of NF-E2-related factor 2 (NRF2) signaling pathway. Free Radic. Biol. Med. 2018, 129, 492–503. [Google Scholar] [CrossRef]
- Mangano, K.; Cavalli, E.; Manmmana, S.; Basile, M.S.; Caltabiano, R.; Pesce, S.; Puleo, S.; Atanasov, A.G.; Magro, G.; Niciletti, F.; et al. Involvement of the Nrf2/HO-1/CO axis and therapeutic intervention with the CO-releasing molecule CORM-A1, in a murine model of autoimmune hepatitis. J. Cell Physiol. 2018, 233, 4156–4165. [Google Scholar] [CrossRef]
- Fagone, P.; Mangano, K.; Mammana, S.; Cavalli, E.; Marco, R.D.; Barcellona, M.L.; Salvatorelli, L.; Magro, G.; Nicoletti, F. Carbon monoxide-releasing molecule-A1 (CORM-A1) improves clinical signs of experimental autoimmune uveoretinitis (EAU) in rats. Clin. Immunol. 2015, 157, 198–204. [Google Scholar] [CrossRef]
- Chora, A.A.; Fontoura, P.; Cunha, A.; Pais, T.F.; Cardoso, S.; Ho, P.P.; Lee, L.Y.; Sobel, R.A.; Steinman, L.; Soares, M.P. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J. Clin. Investig. 2007, 117, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Fagone, P.; Mangano, K.; Coco, M.; Perciavalle, V.; Garotta, G.; Romao, C.C.; Nicoletti, F. Therapeutic potential of carbon monoxide in multiple sclerosis. Clin. Exp. Immunol. 2012, 167, 179–187. [Google Scholar] [CrossRef]
- Mackern-Oberti, J.P.; Llanos, C.; Carreño, L.J.; Riquelme, S.A.; Jacobelli, S.H.; Anegon, I.; Kalergis, A.M. Carbon monoxide exposure improves immune function in lupus-prone mice. Immunology 2013, 140, 123–132. [Google Scholar] [CrossRef]
- Qin, S.; Deng, F.; Wu, W.; Jiang, L.; Yamashiro, T.; Yano, S.; Hou, D.X. Baicalein modulates Nrf2/Keap1 system in both Keap1-dependent and Keap1-independent mechanisms. Arch. Biochem. Biophys. 2014, 559, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Cui, W.; Li, G.; Yuan, S.; Xu, D.; Hoi, M.P.M.; Lin, Z.; Dou, J.; Han, Y.; Lee, S.M.Y. Baicalein protects against 6-OHDA-induced neurotoxicity through activation of Keap1/Nrf2/HO-1 and involving PKCα and PI3K/AKT signaling pathways. J. Agric. Food Chem. 2012, 60, 8171–8182. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, Y.; Ito, T.; Tsuji, G.; Furue, M. Baicalein Inhibits Benzo[a]pyrene-Induced Toxic Response by Downregulating Src Phosphorylation and by Upregulating NRF2-HMOX1 System. Antioxidants 2020, 9, 507. https://doi.org/10.3390/antiox9060507
Tanaka Y, Ito T, Tsuji G, Furue M. Baicalein Inhibits Benzo[a]pyrene-Induced Toxic Response by Downregulating Src Phosphorylation and by Upregulating NRF2-HMOX1 System. Antioxidants. 2020; 9(6):507. https://doi.org/10.3390/antiox9060507
Chicago/Turabian StyleTanaka, Yuka, Takamichi Ito, Gaku Tsuji, and Masutaka Furue. 2020. "Baicalein Inhibits Benzo[a]pyrene-Induced Toxic Response by Downregulating Src Phosphorylation and by Upregulating NRF2-HMOX1 System" Antioxidants 9, no. 6: 507. https://doi.org/10.3390/antiox9060507
APA StyleTanaka, Y., Ito, T., Tsuji, G., & Furue, M. (2020). Baicalein Inhibits Benzo[a]pyrene-Induced Toxic Response by Downregulating Src Phosphorylation and by Upregulating NRF2-HMOX1 System. Antioxidants, 9(6), 507. https://doi.org/10.3390/antiox9060507




