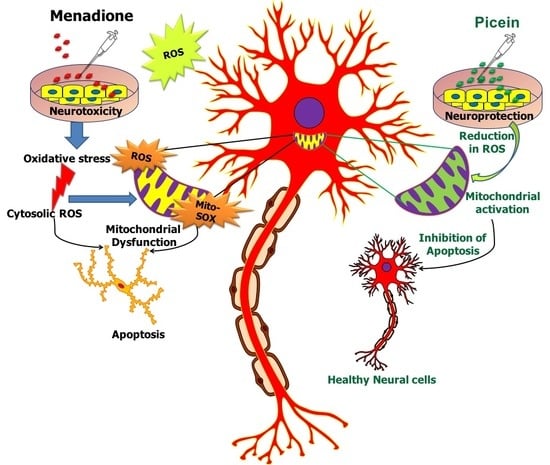
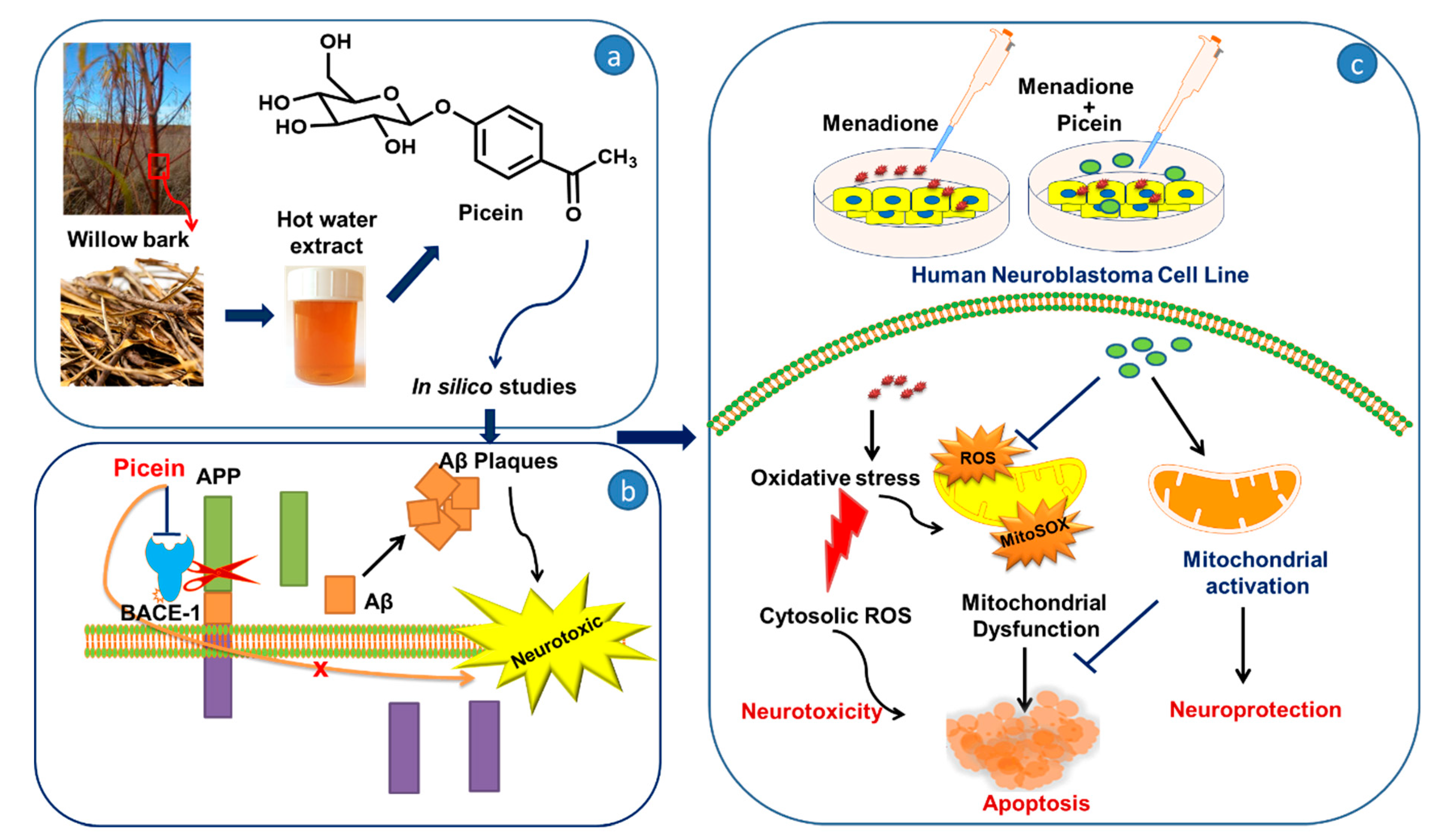
Plant-Derived Natural Biomolecule Picein Attenuates Menadione Induced Oxidative Stress on Neuroblastoma Cell Mitochondria
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Similarity Search
2.2. Testing of Lipinski’s Rule of 5, Generation of 3D Model of Protein and Ligands and Docking Studies
2.3. Identification of Putative Biomolecular Target and Protein-Protein Interaction Analysis
2.4. Mammalian Cell Culture
2.5. Experimental Design for In Vitro Studies
2.6. Mitochondrial Activity (MTT) Assay
2.7. Reactive Oxygen Species (ROS) Formation
2.8. Mitochondrial Superoxide Production
2.9. Live Cell Imaging
2.10. Statistical Analysis
3. Results
3.1. Chemical Similarity Search
3.2. Identification of Putative Biomolecular Target and Protein-Protein Interaction
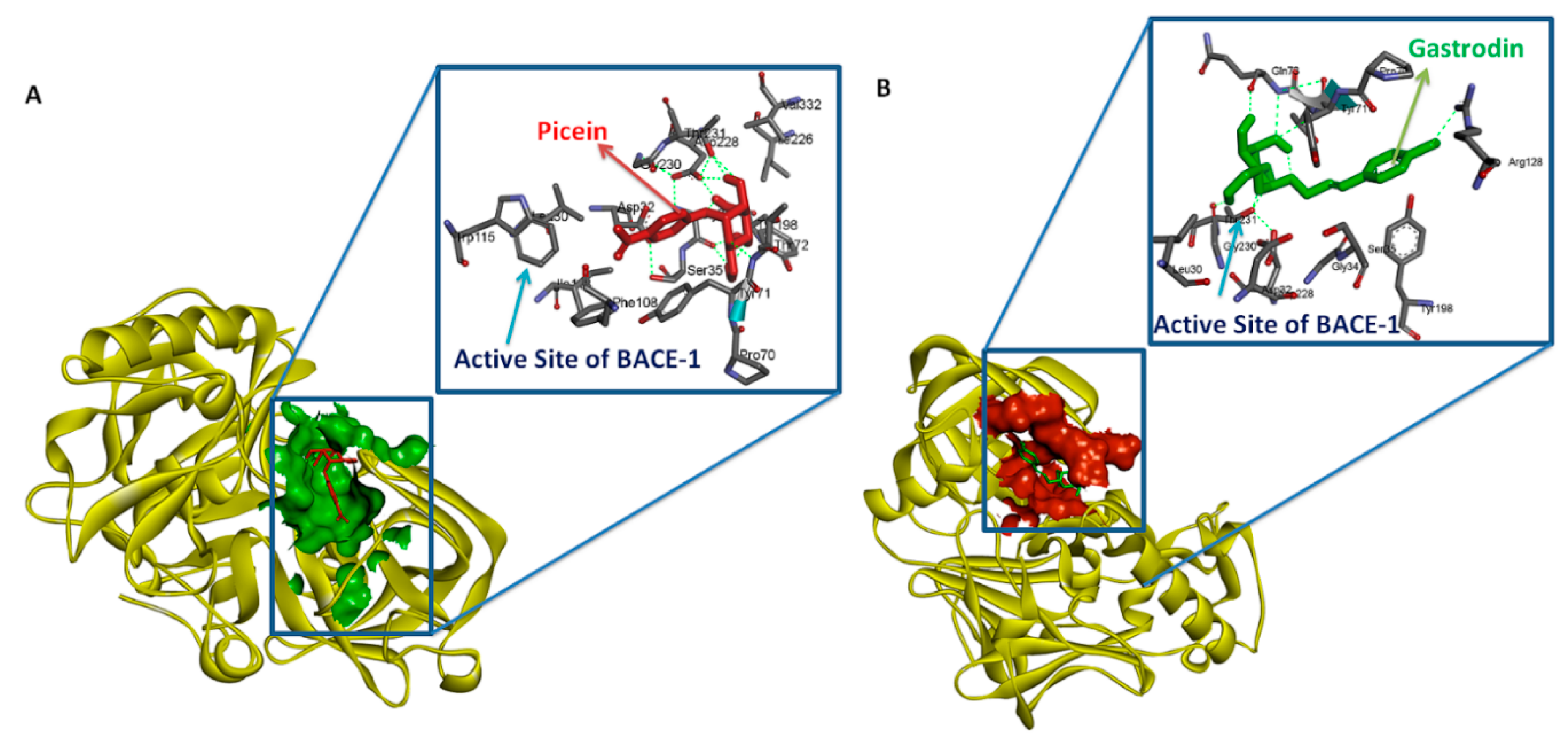
3.3. Testing of Lipinski’s Rule of 5 and Docking Analysis
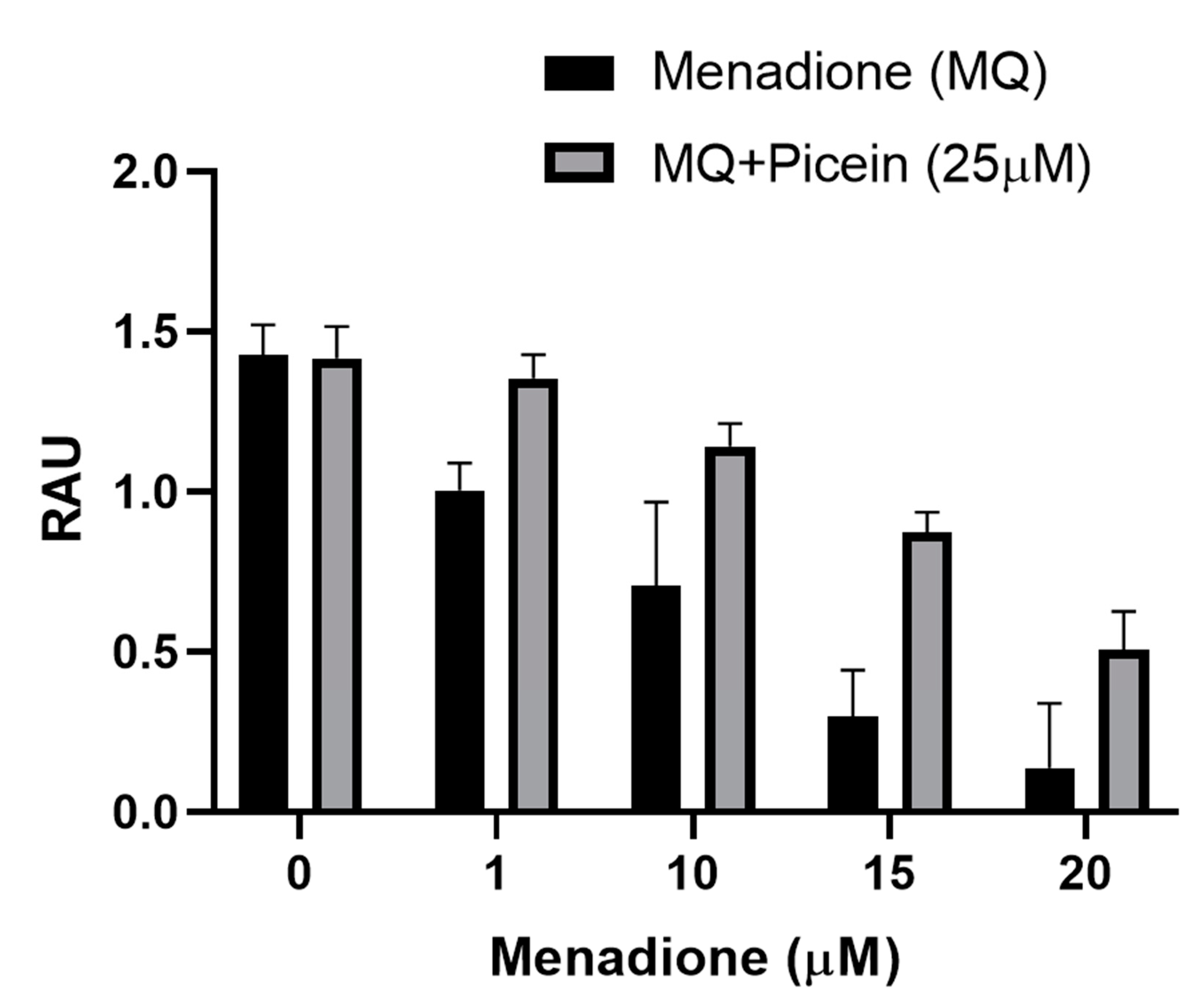
3.4. Mitochondrial Activity (MTT)
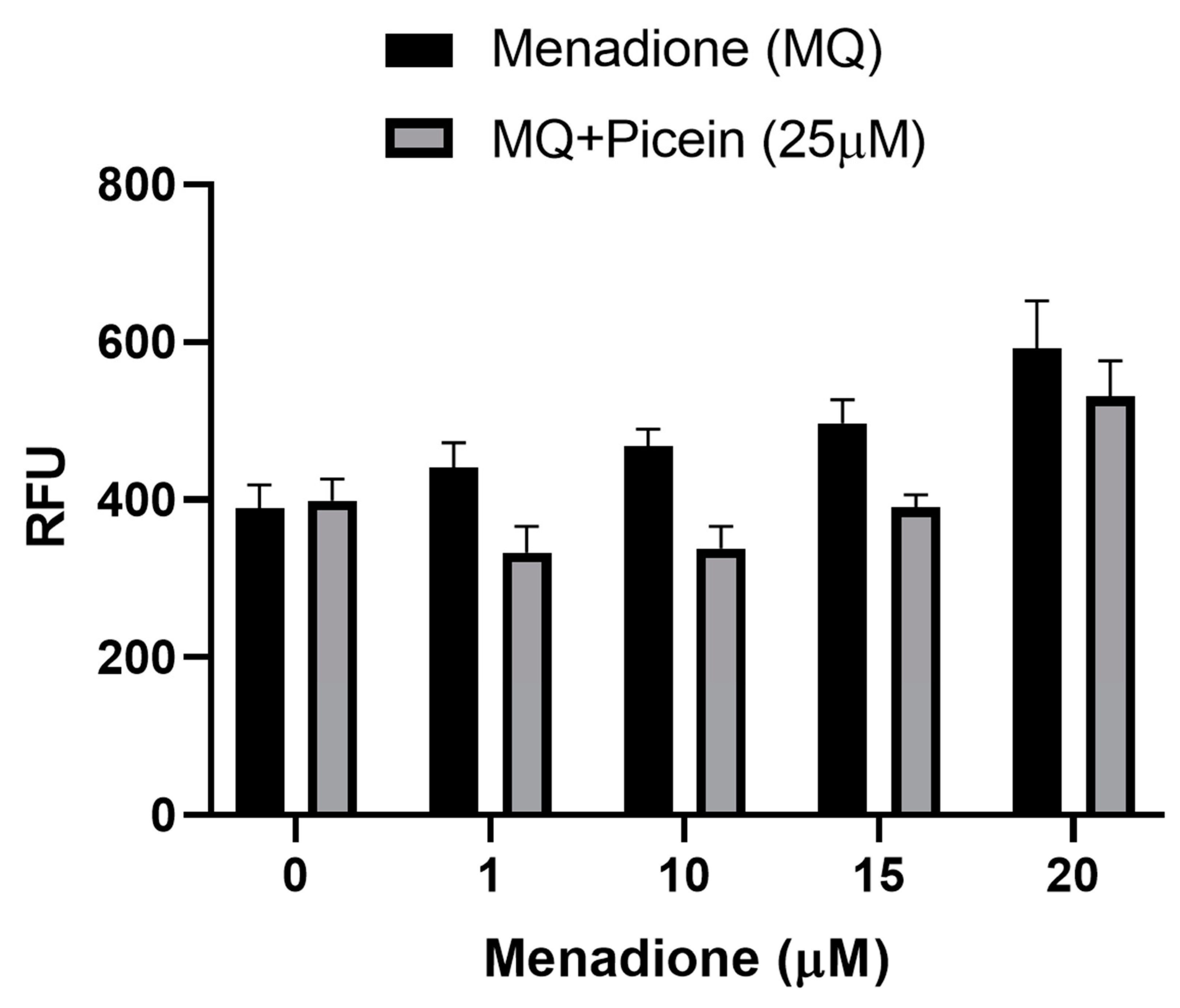
3.5. Reactive Oxygen Species
3.6. Mitochondrial Superoxide Production
3.7. Live Cell Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer disease |
| Aβ | Amyloid beta |
| APP | Amyloid-beta precursor protein |
| BACE-1 | Beta secretase enzyme |
| CHIPPI | CHimeric protein-protein interaction server |
| DNA | Deoxyribonucleic acid |
| DMEM | Dulbecco’s modified Eaglés medium |
| DMSO | Dimethyl sulfoxide |
| DCFH-DA | 2′,7′-dichlorofluorescein diacetate |
| PBS | Phosphate buffer saline |
| FBS | Foetal bovine serum |
| HPLC | High performance liquid chromatography |
| KEGG | Kyoto encyclopedia of genes and genomes |
| MTT | Thiazolyl blue tetrazolium bromide |
| MQ | Menadione |
| MGL Tool | Molecular graphics laboratory tool |
| MitoSOX | Mitochondrial superoxide |
| PD | Parkinson disease |
| PPI | Protein-protein interaction |
| RFU | Relative fluorescence units |
| RAU | Relative absorbance units |
| RCSB | Research collaboratory for structural bioinformatics protein data bank |
| ROS | Reactive oxygen species |
| SH-SY5Y | Neuroblastoma cell |
| SIMCOMP | SIMilar COMPound. |
References
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Aseervatham, G.S.B.; Sivasudha, T.; Jeyadevi, R. Environmental factors and unhealthy lifestyle influence oxidative stress in humans—An overview. Environ. Sci. Pollut. Res. 2013, 20, 4356–4369. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic Cermak, A.M.; Pavicic, I.; Trosic, I. Oxidative stress response in SH-SY5Y cells exposed to short-term 1800 MHz radiofrequency radiation. J. Environ. Sci. Health Tox Hazard. Subst. Environ. Eng. 2018, 53, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.N.; Jetti, R.; Kesari, K.K.; Kumar, R.S.; Nayak, S.B.; Bhat, P.G. Radiofrequency electromagnetic radiation-induced behavioral changes and their possible basis. Environ. Sci. Pollut. Res. Int. 2019, 26, 30693–30710. [Google Scholar] [CrossRef] [PubMed]
- Di Monte, D.; Bellomo, G.; Thor, H.; Nicotera, P.; Orrenius, S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch. Biochem. Biophys. 1984, 235, 343–350. [Google Scholar] [CrossRef]
- Luukkonen, J.; Liimatainen, A.; Höytö, A.; Juutilainen, J.; Naarala, J. Pre-exposure to 50 Hz magnetic fields modifies menadione-induced genotoxic effects in Human SH-SY5Y neuroblastoma cells. PLoS ONE. 2011, 6, e18021. [Google Scholar] [CrossRef]
- Martínez, M.A.; Rodríguez, J.L.; Lopez-Torres, B.; Martínez, M.; Martínez-Larrañaga, M.R.; Maximiliano, J.E.; Anadón, A.; Ares, I. Use of humanneuroblastomaSH-SY5Ycells to evaluate glyphosate-induced effects on oxidative stress, neuronal development and cell death signaling pathways. Environ. Int. 2020, 35, 105414. [Google Scholar] [CrossRef]
- Loor, G.; Kondapalli, J.; Schriewer, J.M.; Chandel, N.S.; Vanden, H.T.L.; Schumacker, P.T. Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radic. Biol. Med. 2010, 49, 1925–1936. [Google Scholar] [CrossRef]
- Criddle, D.N.; Gillies, S.; Baumgartner-Wilson, H.K. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J. Biol. Chem. 2006, 281, 40485–40492. [Google Scholar] [CrossRef]
- Thor, H.; Smith, M.T.; Hartzell, P.; Bellomo, G.; Jewell, S.A.; Orrenius, S. The metabolism of menadione (2-methyl-1.4-naphthoquinone) by isolated hepatocytes. J. Biol. Chem. 1982, 257, 12419–12425. [Google Scholar]
- Fahn, S.; Cohen, G. The oxidant stress hypothesis in Parkinson’s disease: Evidence supporting it. Ann. Neurol. 1992, 32, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Balazs, L.; Leon, M. Evidence of an oxidative challenge in the Alzheimer’s brain. Neurochem. Res. 1994, 19, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.A.; Omar, R.A.; Kim, K.S.; Robakis, N.K. Immunohistochemical evidence of antioxidant stress in Alzheimer’s disease. Am. J. Pathol. 1992, 140, 621–628. [Google Scholar]
- Smith, C.D.; Carney, J.M.; Starke-Reed, P.E.; Oliver, C.N.; Stadtman, E.R.; Floyd, R.A.; Markesbery, W.R. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1991, 88, 10540–10543. [Google Scholar] [CrossRef] [PubMed]
- Volcier, L.; Crino, P.B. Involvement of free radicals in dementia of the Alzheimer’s type: A hypothesis. Neurobiol. Aging 1990, 11, 567–571. [Google Scholar] [CrossRef]
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, M. World Alzheimer Report 2015: The Global Impact of Dementia. An. Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Qiu, C.; Kivipelto, M.; von Strauss, E. Epidemiology of Alzheimer’s disease: Occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. 2009, 11, 111–128. [Google Scholar] [PubMed]
- Dou, J.; Xu, W.; Koivisto, J.J.; Mobley, J.K.; Padmakshan, D.; Kögler, M.; Xu, C.; Willför, S.; Ralph, J.; Vuorinen, T. Characteristics of Hot Water Extracts from the Bark of Cultivated Willow (Salix sp.). ACS Sustain. Chem. Eng. 2018, 6, 5566–5573. [Google Scholar] [CrossRef]
- Poljsak, B.; Milisav, I. Aging, oxidative stress and antioxidants. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Morales-González, J.A., Ed.; Intech: Rijeka, Croatia, 2013; pp. 331–353. [Google Scholar]
- Gligoric, E.; Igic, R.; Suvajdžic, L.; Grujic-Letic, N. Species of the Genus Salix, L.: Biochemical Screening and Molecular Docking Approach to Potential Acetylcholinesterase Inhibitors. Appl. Sci. 2019, 9, 1842. [Google Scholar] [CrossRef]
- Chen, D.; Fan, J.; Wang, P.; Zhu, L.; Jin, Y.; Peng, Y.; Du, S. Isolation, identification and antioxidative capacity of water-soluble phenylpropanoid compounds from Rhodiola crenulate. Food Chem. 2012, 134, 2126–2133. [Google Scholar] [CrossRef]
- Luthria, D.L.; Jones, A.D.; Donovan, J.L.; Waterhouse, A.L. Waterhouse, GC-MS Determination of Catechin and Epicatechin Levels in Human Plasma. J. High. Resolut. Chromatogr. 1997, 20, 621–623. [Google Scholar] [CrossRef]
- Mahdi, J.G. Medicinal potential of willow: A chemical perspective of aspirin discovery. J. Saudi Chem. Soc. 2010, 14, 317–322. [Google Scholar] [CrossRef]
- Chiang, M.F.; Liu, W.K.; Yen, S.H. Reversible heat stress-related loss of phosphorylated Alzheimer-type epitopes in tau proteins of human neuroblastoma cells. J. Neurosci. 1993, 13, 4854–4860. [Google Scholar] [CrossRef] [PubMed]
- Ko, L.; Sheu, K.F.R.; Young, O.; Thaler, H.; Blass, J.P. Expression of cultured human neuroblastoma cells of epitopes associated with affected neurons in Alzheimer’s disease. Am. J. Pathol. 1990, 136, 867–879. [Google Scholar] [PubMed]
- Ko, L.; Liu, W.K.; Georgieff, I.S.; Yen, S.H. Modulated induction of tau proteins in cultured human neuroblastoma cells. Brain Res. 1996, 707, 256–265. [Google Scholar] [CrossRef]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef]
- Sorce, S.; Krause, K.H. NOX enzymes in the central nervous system: From signaling to disease. Antioxid. Redox Signal. 2009, 11, 2481–2504. [Google Scholar] [CrossRef]
- Loukogeorgakis, S.P.; van den Berg, M.J.; Sofat, R.; Nitsch, D.; Charakida, M.; Haiyee, B.; de Groot, E.; MacAllister, R.J.; Kuijpers, T.W.; Deanfield, J.E. Role of NADPH oxidase in endothelial ischemia/reperfusion injury in humans. Circulation 2010, 121, 2310–2316. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Peng, M.; Meng, H.; Ma, H.; Cai, P.; Xu, Y.; Zhao, Q.; Si, G. A Review on Central Nervous System Effects of Gastrodin. Front. Pharmacol. 2018, 9, 24. [Google Scholar] [CrossRef]
- Hu, Y.; Li, C.; Shen, W. Gastrodin alleviates memory deficits and reduces neuropathology in a mouse model of Alzheimer’s disease. Neuropathology 2014, 34, 370–377. [Google Scholar] [CrossRef]
- Li, M.; Qian, S. Gastrodin protects neural progenitor cells against Amyloid β (1-42)-induced neurotoxicity and improves hippocampal neurogenesis in Amyloid β (1-42)-injected mice. J. Mol. Neurosci. 2016, 60, 21–32. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhou, S.F.; Wang, Q.; Guo, J.N.; Liang, H.M.; Deng, J.B.; He, W.Y. Gastrodin suppresses BACE1 expression under oxidative stress condition via inhibition of the PKR/eIF2alpha pathway in Alzheimer’s disease. Neuroscience 2016, 325, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kim, I.S.; More, S.V.; Kim, B.W.; Bahk, Y.Y.; Choi, D.K. Gastrodin protects apoptotic dopaminergic neurons in a toxin-induced Parkinson’s disease model, Evid. Based Complement. Alternat. Med. 2013, 2013, 514095. [Google Scholar]
- Li, C.; Chen, X.; Zhang, N.; Song, Y.; Mu, Y. Gastrodin inhibits neuroinflammation in rotenone-induced Parkinson’s disease model rats. Neural. Regen. Res. 2012, 7, 325–331. [Google Scholar] [PubMed]
- Xi, X.; Ren, X.Q. The protective effect of gastrodin injection on Parkinson’s disease rats. Chin. J. Gerontol. 2016, 36, 4996–4997. [Google Scholar]
- Sun, R.Z.; Zhou, C.H.; Xue, S.S.; Wang, H.N.; Peng, Z.W.; Zhang, Y.H. The effect of gastrodin on the depressive-like behavior and the expression of IL-1β and IL-6 in CUS rats. Chin. J. Neuroanat. 2017, 33, 221–224. [Google Scholar]
- Lee, B.; Sur, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Gastrodin reversed the traumatic stress-induced depressed-like symptoms in rats. J. Nat. Med. 2016, 70, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Naka, N.; Kanehisa, M.; Goto, S. SIMCOMP/SUBCOMP: Chemical structure search servers for network analyses. Nucleic Acids Res. 2010, 38, W652–W656. [Google Scholar] [CrossRef]
- Verma, S.S.; Rai, V.; Awasthee, N.; Dhasmana, A.; Rajalaksmi, D.S.; Nair, M.S.; Gupta, S.C. Isodeoxyelephantopin, a Sesquiterpene Lactone Induces ROS Generation, Suppresses NF-κB Activation, Modulates LncRNA Expression and Exhibit Activities Against Breast Cancer. Sci. Rep. 2019, 9, 17980. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Dhasmana, A.; Jamal, Q.M.; Gupta, R.; Siddiqui, M.H.; Kesari, K.K.; Wadhwa, G.; Khsn, S.; Haque, S.; Lohani, M. Titanium dioxide nanoparticles provide protection against polycyclic aromatic hydrocarbon BaP and chrysene-induced perturbation of DNA repair machinery: A computational biology approach. Biotechnol. Appl. Biochem. 2016, 63, 497–513. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Juutilainen, J.; Luukkonen, J.; Naarala, J. Induction of micronuclei and superoxide production in neuroblastoma and glioma cell lines exposed to weak 50 Hz magnetic fields. J. R. Soc. Interface 2016, 13, 20150995. [Google Scholar] [CrossRef] [PubMed]
- Tomkova, S.; Misuth, M.; Lenkavska, L.; Miskovsky, P.; Huntosova, V. In vitro identification of mitochondrial oxidative stress production by time-resolved fluorescence imaging of glioma cells. Acta Mol. Cell Res. 2018, 1865, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Choi, H.J.; Zhu, B.T. Rapid generation of mitochondrial superoxide induces mitochondrion-dependent but caspase-independent cell death in hippocampal neuronal cells that morphologically resembles necroptosis. Toxicol. Appl. Pharmacol. 2012, 262, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Halilovic, A.; Schmedt, T.; Benischke, A.S.; Hamill, C. Menadione-Induced DNA Damage Leads to Mitochondrial Dysfunction and Fragmentation During Rosette Formation in Fuchs Endothelial Corneal Dystrophy. Antioxid. Redox Signal. 2016, 24, 1072–1083. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2015. [Google Scholar]
- Feng, X.Z.; Chen, Y.W.; Yang, J.S. Studies on constituents of Tian-ma. Acta Chim. Sin. 1979, 37, 175–182. [Google Scholar]
- Zhou, J.; Yang, Y.B.; Yang, J.R. The chemistry of Gastrodia elata BL. Acta Chim. Sin. 1979, 37, 183–189. [Google Scholar]
- Luo, L.; Kim, S.W.; Lee, H.K.; Kim, I.D.; Lee, H.; Lee, J.K. Gastrodin exerts robust neuroprotection in the postischemic brain via its protective effect against Zn2+-toxicity and its anti-oxidative effects in astrocytes. Anim. Cells Syst. 2018, 22, 429–437. [Google Scholar] [CrossRef]
- Cole, S.L.; Vassar, R. The Basic Biology of BACE1: A Key Therapeutic Target for Alzheimer’s Disease. Curr. Genom. 2007, 8, 509–530. [Google Scholar]
- Andorf, C.M.; Honavar, V.; Sen, T.Z. Predicting the Binding Patterns of Hub Proteins: A Study Using Yeast Protein Interaction Networks. PLoS ONE 2013, 8, e56833. [Google Scholar] [CrossRef]
- Yu, H.; Kim, P.M.; Sprecher, E.; Trifonov, V.; Gerstein, M. The Importance of Bottlenecks in Protein Networks: Correlation with Gene Essentiality and Expression Dynamics. PLoS Comput. Biol. 2007, 3, e59. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Lee, H.C.; Lee, J.J.; Lee, M.N.; Hong, M.J.; Park, Y.S.; Lee, H.D.; Choi, N.; Kim, Y.G.; Lee Ko, J.S. Effects of combined radiofrequency radiation exposure on levels of reactive oxygen species in neuronal cells. J. Radiat. Res. 2014, 55, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Gogal, R.M.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H] thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
- Malich, G.; Markovic, B.; Winder, C. The sensitivity and specificity of the MTS tetrazolium assay for detecting in vitro cytotocity of 20 chemicals using human cell lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef]
- Pasricha, R.; Sachdev, D. Biological Characterization of Nanofiber Composites. In Nanofiber Composites for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2017; pp. 157–196. [Google Scholar]
- Chuang, Y.Y.; Chen, Y.; Gadisetti, V.R.; Chandramouli, J.A.; Cook, D.; Coffin, M.H.; Tsai, W.; DeGraff, H.; Yan, S.; Zhao, A.; et al. Gene expression after treatment with hydrogen peroxide, menadione, or t-butyl hydroperoxide in breast cancer cells, Cancer Res. Cancer Res. 2002, 62, 6246–6254. [Google Scholar] [PubMed]
- Luo, X.; Pitkanen, S.; Kassovska-Bratinova, S.; Robinson, B.H.; Lehotay, D.C. Excessive formation of hydroxyl radicals and aldehydic lipid peroxidation products in cultured skin fibroblasts from patients with complex I deficiency. J. Clin. Investig. 1997, 99, 2877–2882. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Satoh, K.; Hakeda, Y.; Kumegawa, M. Apoptosis-inducing activity of vitamin C and vitamin K. Cell Mol. Biol. 2000, 46, 129–143. [Google Scholar]
- Grishko, V.; Solomon, M.; Wilson, G.L.; LeDoux, S.P.; Gillespie, M.N. Oxygen radical-induced mitochondrial DNA damage and repair in pulmonary vascular endothelial cell phenotypes. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, L1300–L1308. [Google Scholar] [CrossRef]
- Kesari, K.K.; Luukkonen, J.; Juutilainen, J.; Naarala, J. Genomic instability induced by 50Hz magnetic fields is a dynamically evolving process not blocked by antioxidant treatment. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 794, 46–51. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Yoshihiro, K.; Haskó, G.; Pacher, P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem. Biophys Res. Commun. 2007, 358, 203–208. [Google Scholar] [CrossRef]
- Ghanian, Z.; Konduri, G.G.; Audi, S.H.; Camara, A.K.S.; Ranji, M. Quantitative optical measurement of mitochondrial superoxide dynamics in pulmonary artery endothelial cells. J. Innov. Opt. Health Sci. 2018, 11, 1750018. [Google Scholar] [CrossRef] [PubMed]
- Naarala, J.; Kesari, K.K.; McClure, I.; Chavarriaga, C.; Juutilainen, J.; Martino, C.F. Direction-Dependent Effects of Combined Static and ELF Magnetic Fields on Cell Proliferation and Superoxide Radical Production. Biomed. Res. Int. 2017, 2017, 5675086. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, J.; Höytö, A.; Viluksela, M.; Juutilainen, J.; Naarala, J. TCDD-induced mitochondrial superoxide production does not lead to mitochondrial degeneration or genomic instability in human SH-SY5Y neuroblastoma cells. Toxicol. In Vitro 2017, 44, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.; Kuida, K.; Johnson, E.M. Jr Caspase inhibition extends the commitment to neuronal death beyond cytochrome c release to the point of mitochondrial depolarization. J. Cell Biol. 2000, 150, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.M.; Kim, H.J.; Lee, Z.W.; Kim, S.J.; Kim, S.I.; Paik, S.G.; Ha, K.S. Real-time measurement of intracellular reactive oxygen species using mito tracker orange (CMH(2)TMRos). Biosci. Rep. 2001, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]



| S. No. | Chemical Name | Chemical Structure | Similarity Score |
|---|---|---|---|
| 1 | Picein | 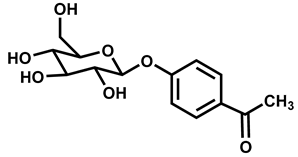 | 1.0 |
| 2 | Gastrodin | 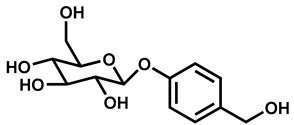 | 0.92 |
| S. No. | Rece-Ptor | Ligand | Binding Energy Kcal/Mol | Ki | Hydrophobic Interaction | Hydrophilic Interaction | Length of H Bond (Ǻ) |
|---|---|---|---|---|---|---|---|
| 1 | BACE1 | Gastrodin | −5.78 Kcal/Mol | 57.49 µM | Leu30, Asp32, Gly34, Ser35, Gln73, Pro70, Tyr71, Thr72, Arg128, Tyr198, Asp228, Thr231, Gly230 | BACE1:THR72:N-Gastrodin:O20 | 2.93955 |
| BACE1:GLN73:N-Gastrodin:O20 | 3.03446 | ||||||
| BACE1:ARG128:NH2-Gastrodin:O1 | 2.77309 | ||||||
| BACE1:THR231:OG1-Gastrodin:O22 | 2.91867 | ||||||
| Gastrodin:H42-BACE1:THR231:OG1 | 2.23271 | ||||||
| Gastrodin:H36-BACE1:GLY230:O | 2.18995 | ||||||
| Gastrodin:H38-BACE1:GLN73:O | 1.8119 | ||||||
| BACE1:THR72:N-Gastrodin:O20 | 2.93955 | ||||||
| BACE1:GLN73:N-Gastrodin:O20 | 2.77309 | ||||||
| BACE1:ARG128:NH2-Gastrodin:O1 | 3.03446 | ||||||
| BACE1:THR231:OG1-Gastrodin:O22 | 2.91867 | ||||||
| Gastrodin:H42-BACE1:THR231:OG1 | 2.23271 | ||||||
| Gastrodin:H36-BACE1:GLY230:O | 1.8119 | ||||||
| Gastrodin:H38-BACE1:GLN73:O | 2.18995 | ||||||
| 2 | BACE1 | Picein | −5.94 Kcal/Mol | 44.03 µM | Ser35, Leu30, Asp32, Gly34, Pro70, Thr72, Phe108, Trp115, Tyr71, Ile118, Tyr198, Thr231, Ile226, Asp228, Gly230, Val332 | BACE1:THR72:N-Picein:O12 | 3.03172 |
| BACE1:THR231:OG1-Picein:O21 | 2.55481 | ||||||
| Picein:H39-BACE1:ASP228:OD2 | 2.20644 | ||||||
| Picein:H39-BACE1:THR231:OG1 | 1.96118 | ||||||
| Picein:H37-BACE1:ASP228:OD2 | 1.74178 | ||||||
| Picein:H33-BACE1:GLY34:O | 2.24183 | ||||||
| Picein:H35-BACE1:GLY34:O | 1.87567 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesari, K.K.; Dhasmana, A.; Shandilya, S.; Prabhakar, N.; Shaukat, A.; Dou, J.; Rosenholm, J.M.; Vuorinen, T.; Ruokolainen, J. Plant-Derived Natural Biomolecule Picein Attenuates Menadione Induced Oxidative Stress on Neuroblastoma Cell Mitochondria. Antioxidants 2020, 9, 552. https://doi.org/10.3390/antiox9060552
Kesari KK, Dhasmana A, Shandilya S, Prabhakar N, Shaukat A, Dou J, Rosenholm JM, Vuorinen T, Ruokolainen J. Plant-Derived Natural Biomolecule Picein Attenuates Menadione Induced Oxidative Stress on Neuroblastoma Cell Mitochondria. Antioxidants. 2020; 9(6):552. https://doi.org/10.3390/antiox9060552
Chicago/Turabian StyleKesari, Kavindra Kumar, Anupam Dhasmana, Shruti Shandilya, Neeraj Prabhakar, Ahmed Shaukat, Jinze Dou, Jessica M. Rosenholm, Tapani Vuorinen, and Janne Ruokolainen. 2020. "Plant-Derived Natural Biomolecule Picein Attenuates Menadione Induced Oxidative Stress on Neuroblastoma Cell Mitochondria" Antioxidants 9, no. 6: 552. https://doi.org/10.3390/antiox9060552
APA StyleKesari, K. K., Dhasmana, A., Shandilya, S., Prabhakar, N., Shaukat, A., Dou, J., Rosenholm, J. M., Vuorinen, T., & Ruokolainen, J. (2020). Plant-Derived Natural Biomolecule Picein Attenuates Menadione Induced Oxidative Stress on Neuroblastoma Cell Mitochondria. Antioxidants, 9(6), 552. https://doi.org/10.3390/antiox9060552