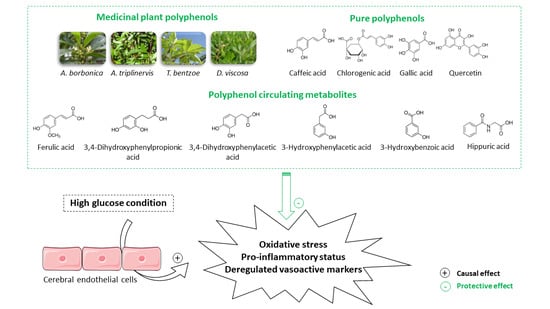
Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Identification of Polyphenols from Medicinal Plant Extracts
2.2. Evaluation of Free Radical-Scavenging and Reducing Capacities of Medicinal Plant Extracts
2.3. Endothelial Cell Culture
2.4. Evaluation of Cell Viability and Mitochondrial Metabolic Activity
2.5. Measurement of Intracellular ROS Levels
2.6. Determination of Antioxidant SOD Activity
2.7. Measurement of Intracellular NO Levels
2.8. Evaluation of NFκB/SEAP Activity
2.9. Quantification of Cytokine Secretion
2.10. Evaluation of Gene Expression
2.11. Statistical Analysis
3. Results
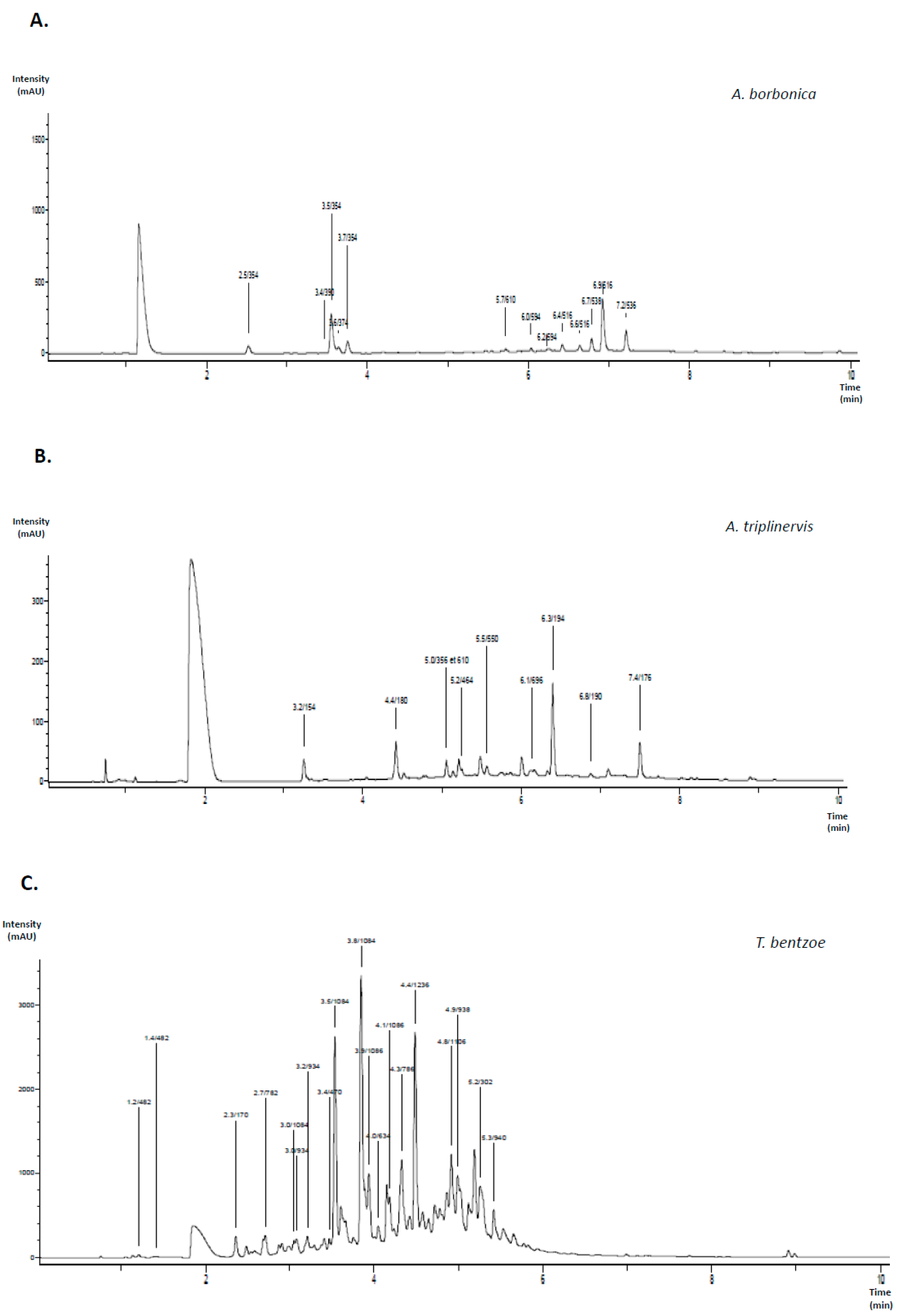
3.1. Characterization of Polyphenols Extracted from Medicinal Plants and Evaluation of Their Free Radical-Scavenging and Reducing Activities
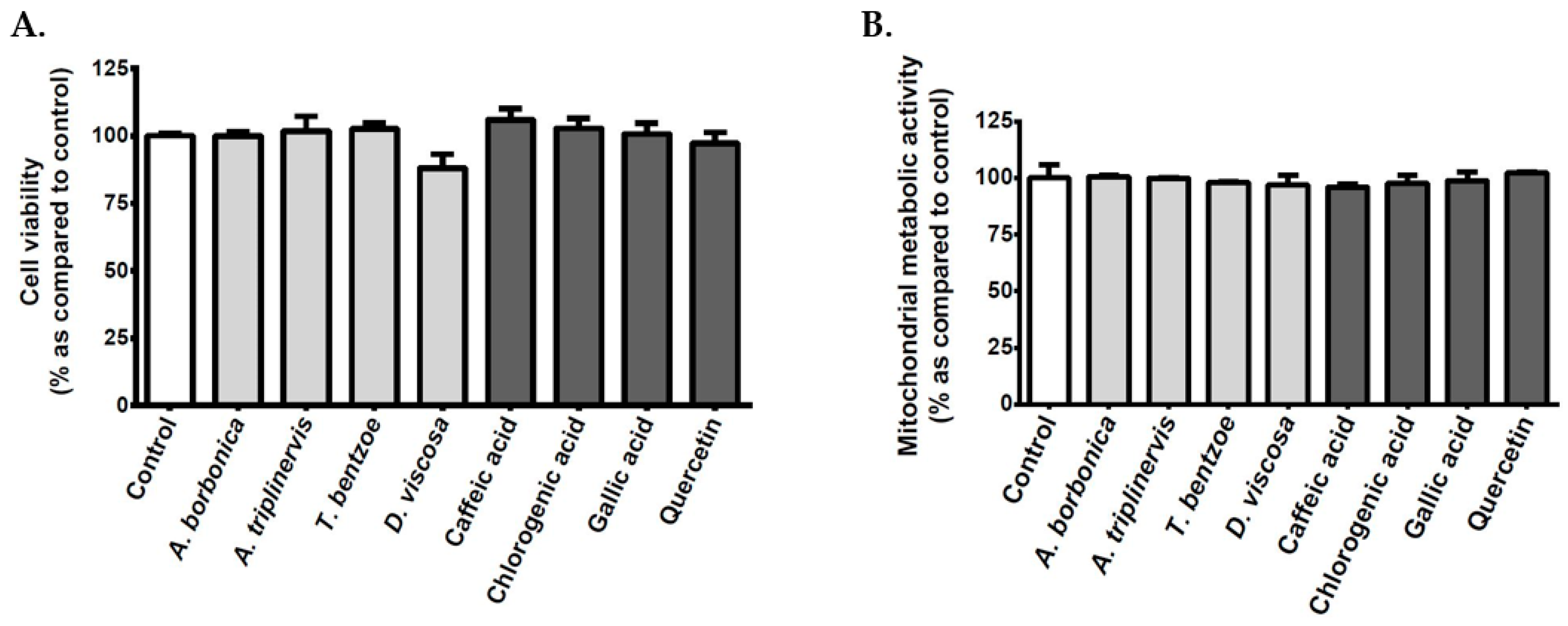
3.2. Effect of Polyphenols on the Viability of Cerebral Endothelial Cells
3.3. Effect of Polyphenols on Intracellular ROS Levels on Cerebral Endothelial Cells in Hyperglycemic Condition
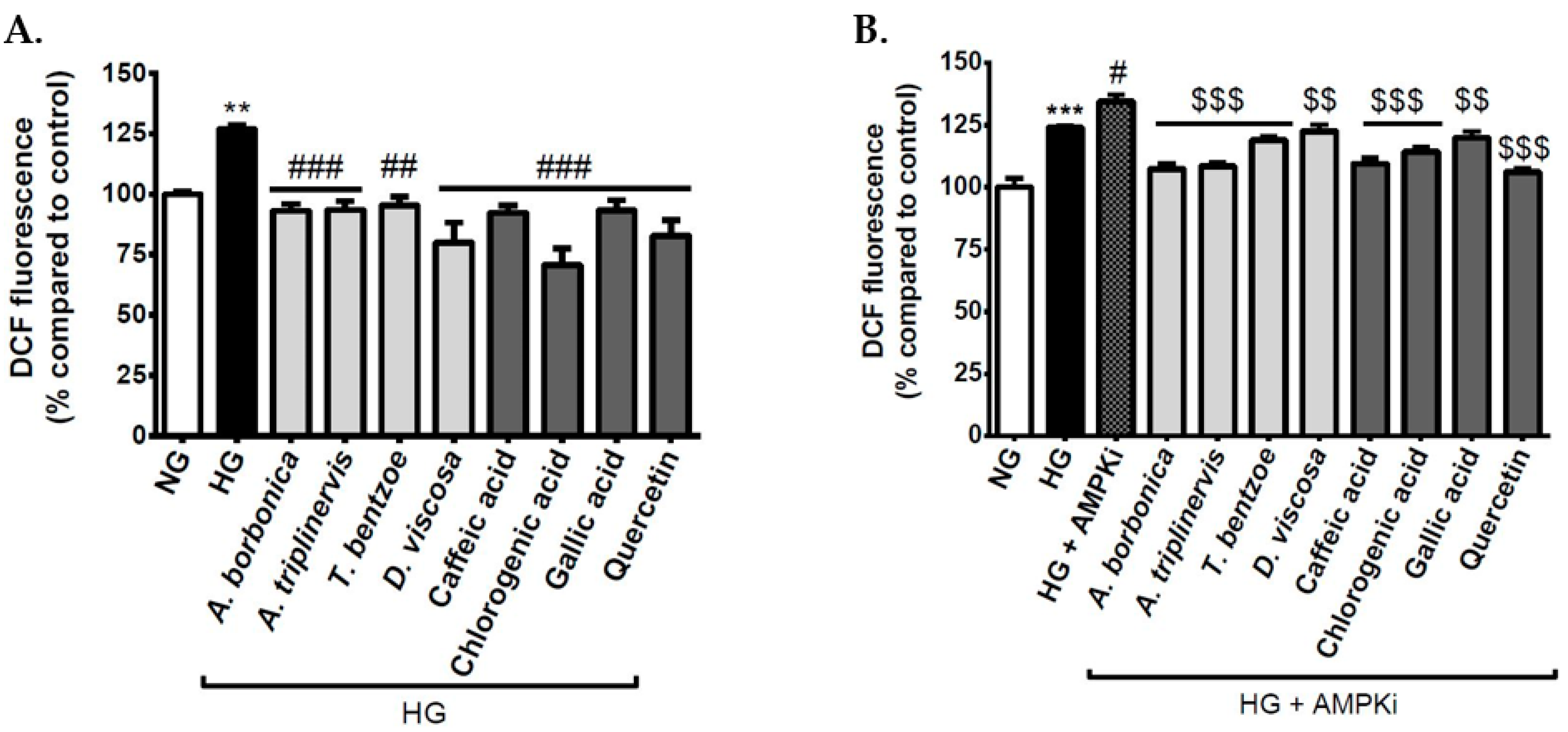
3.4. Effect of Signaling Pathway Inhibitors and Polyphenols on Intracellular ROS Levels on Cerebral Endothelial Cells in Hyperglycemic Condition
3.5. Effect of Polyphenols on SOD Activity on Cerebral Endothelial Cells in Hyperglycemic Condition
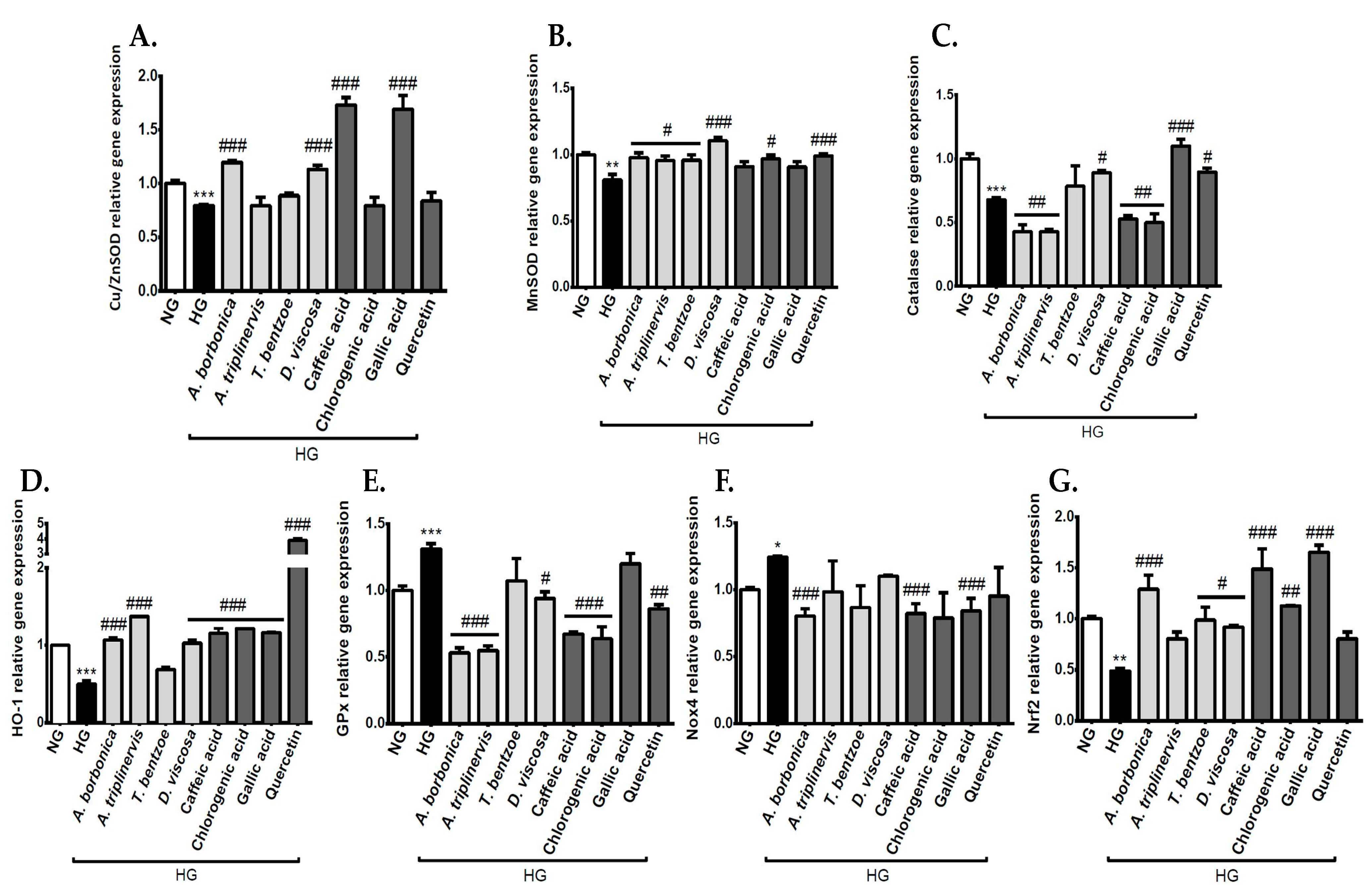
3.6. Effect of Polyphenols on the Expression of Genes Encoding Redox Markers on Endothelial Cells in Hyperglycemic Condition
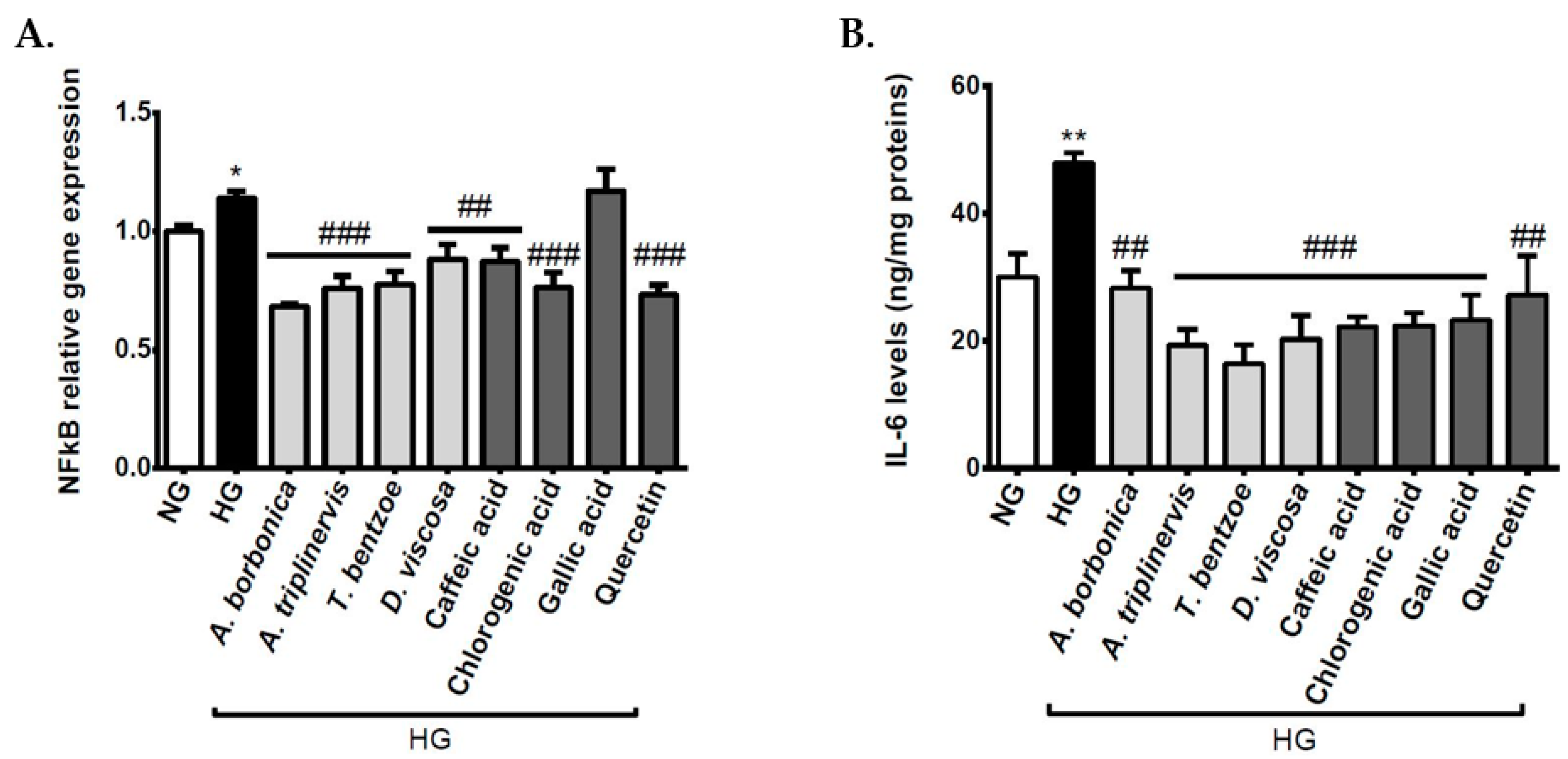
3.7. Effect of Polyphenols on Inflammatory Markers on Cerebral Endothelial Cells in Hyperglycemic Condition
3.8. Effect of Polyphenols on Vasoactive Markers on Cerebral Endothelial Cells in Hyperglycemic Condition
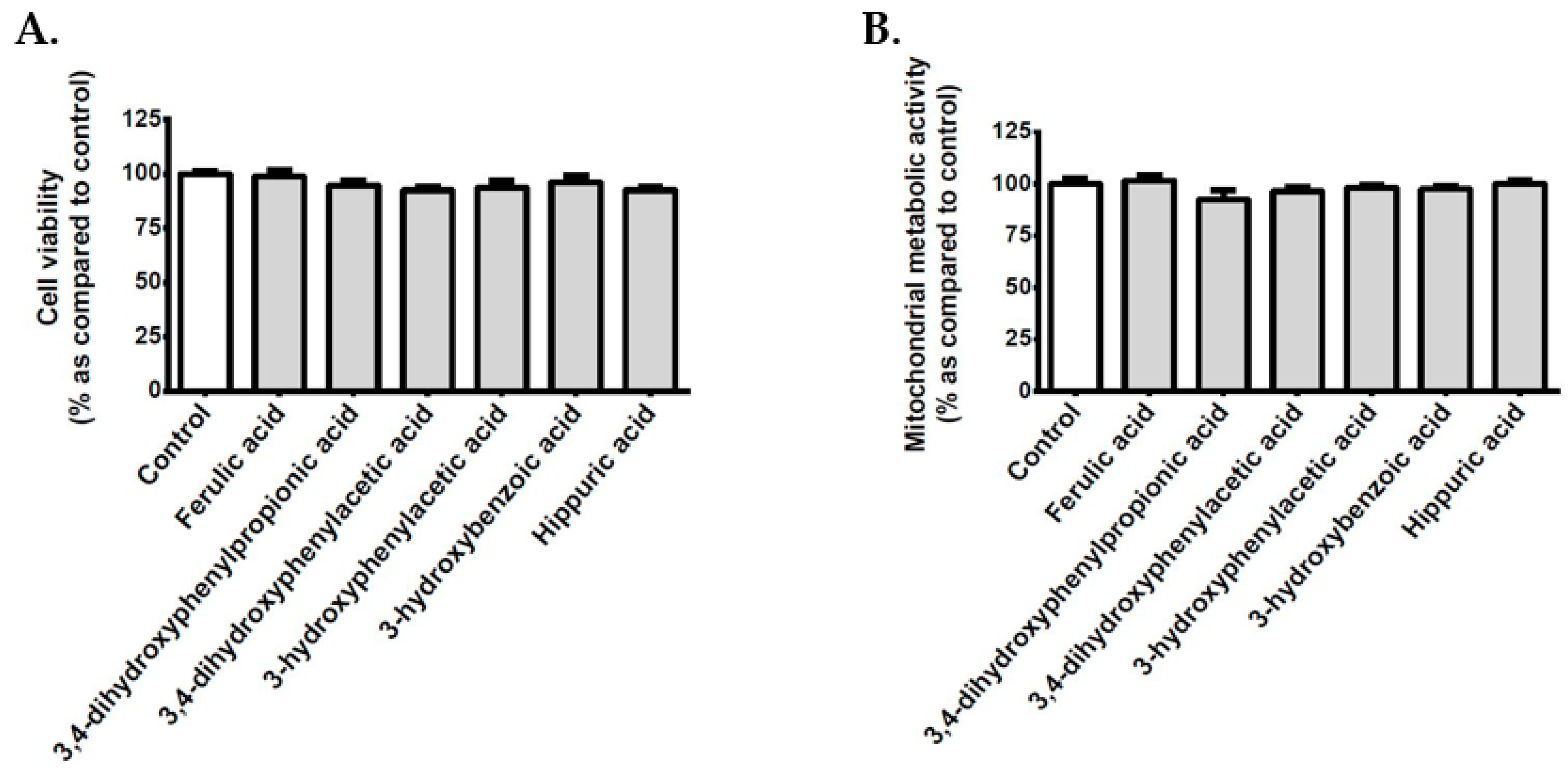
3.9. Effect of Polyphenol Related Circulating Metabolites on Cerebral Endothelial Cell Viability, Redox, Inflammatory and Vasoactive Markers in Hyperglycemic Condition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2009, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Persp. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Kolster, M.; Wilhelmi, M.; Schrimpf, C.; Hilfiker, A.; Haverich, A.; Aper, T. Outgrowing endothelial and smooth muscle cells for tissue engineering approaches. J. Tissue Eng. 2017, 8, 2041731417698852. [Google Scholar] [CrossRef]
- Bellien, J.; Iacob, M.; Monteil, C.; Remy-Jouet, I.; Roche, C.; Duflot, T.; Vendeville, C.; Gutierrez, L.; Thuillez, C.; Richard, V.; et al. Physiological role of endothelin-1 in flow-mediated vasodilatation in humans and impact of cardiovascular risk factors. J. Hypertens. 2017, 35, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Friedman, A. Overview and introduction: The blood-brain barrier in health and disease. Epilepsia 2012, 53, 1–6. [Google Scholar] [CrossRef]
- Sena, C.M.; Pereira, A.M.; Seiça, R. Endothelial dysfunction: A major mediator of diabetic vascular disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2013, 1832, 2216–2231. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Shi, H. Hyperglycemia as a Risk Factor of Ischemic Stroke. J. Drug Metab. Toxicol. 2014, 4, 153. [Google Scholar]
- Sajja, R.K.; Prasad, S.; Cucullo, L. Impact of altered glycaemia on blood-brain barrier endothelium: An in vitro study using the hCMEC/D3 cell line. Fluids Barriers CNS 2014, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Simpson, I.A.; Appel, N.M.; Hokari, M.; Oki, J.; Holman, G.D.; Maher, F.; Koehler-Stec, E.M.; Vannucci, S.J.; Smith, Q.R. Blood-brain barrier glucose transporter: Effects of hypo- and hyperglycemia revisited. J. Neurochem. 1999, 72, 238–247. [Google Scholar] [CrossRef]
- Guzik, T.J.; Mussa, S.; Gastaldi, D.; Sadowski, J.; Ratnatunga, C.; Pillai, R.; Channon, K.M. Mechanisms of Increased Vascular Superoxide Production in Human Diabetes Mellitus. Role of NAD(P)H Oxidase and Endothelial Nitric Oxide Synthase. Circulation 2002, 105, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Hink, U.; Li, H.; Mollnau, H.; Oelze, M.; Matheis, E.; Hartmann, M.; Skatchkov, M.; Thaiss, F.; Stahl, R.A.K.; Warnholtz, A.; et al. Mechanisms Underlying Endothelial Dysfunction in Diabetes Mellitus. Circ. Res. 2001, 88, e14–e22. [Google Scholar] [CrossRef]
- Mates, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Kassab, A.; Piwowar, A. Cell oxidant stress delivery and cell dysfunction onset in type 2 diabetes. Biochimie 2012, 94, 1837–1848. [Google Scholar] [CrossRef]
- Takayama, T.; Wada, A.; Tsutamoto, T.; Ohnishi, M.; Fujii, M.; Isono, T.; Horie, M. Contribution of Vascular NAD(P)H Oxidase to Endothelial Dysfunction in Heart Failure and the Therapeutic Effects of HMG-CoA Reductase Inhibitor. Circ. J. 2004, 68, 1067–1075. [Google Scholar] [CrossRef][Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2011, 107, 1058–1070. [Google Scholar] [CrossRef]
- Wingler, K.; Hermans, J.J.; Schiffers, P.; Moens, A.; Paul, M.; Schmidt, H.H. NOX1, 2, 4, 5: Counting out oxidative stress. Br. J. Pharmacol. 2011, 164, 866–883. [Google Scholar] [CrossRef]
- Guzik, T.J.; West, N.E.J.; Black, E.; McDonald, D.; Ratnatunga, C.; Pillai, R.; Channon, K.M. Vascular Superoxide Production by NAD(P)H Oxidase: Association With Endothelial Dysfunction and Clinical Risk Factors. Circ. Res. 2000, 86, e85–e90. [Google Scholar] [CrossRef]
- Sharma, A.; Rizky, L.; Stefanovic, N.; Tate, M.; Ritchie, R.H.; Ward, K.W.; de Haan, J.B. The nuclear factor (erythroid-derived 2)-like 2 (Nrf2) activator dh404 protects against diabetes-induced endothelial dysfunction. Cardiovasc. Diabetol. 2017, 16, 33. [Google Scholar] [CrossRef]
- Cui, W.; Min, X.; Xu, X.; Du, B.; Luo, P. Role of Nuclear Factor Erythroid 2-Related Factor 2 in Diabetic Nephropathy. J. Diabetes Res. 2017, 2017, 3797802. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Liang, J. Activation of the Nrf2 defense pathway contributes to neuroprotective effects of phloretin on oxidative stress injury after cerebral ischemia/reperfusion in rats. J. Neurol. Sci. 2015, 351, 88–92. [Google Scholar] [CrossRef]
- Thorwald, M.A.; Godoy-Lugo, J.A.; Rodriguez, G.J.; Rodriguez, M.A.; Jamal, M.; Kinoshita, H.; Nakano, D.; Nishiyama, A.; Forman, H.J.; Ortiz, R.M. Nrf2-related gene expression is impaired during a glucose challenge in type II diabetic rat hearts. Free Radic. Biol. Med. 2019, 130, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Somade, O.T.; Ajayi, B.O.; Tajudeen, N.O.; Atunlute, E.M.; James, A.S.; Kehinde, S.A. Camphor elicits up-regulation of hepatic and pulmonary pro-inflammatory cytokines and chemokines via activation of NF-kB in rats. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2019, 26, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef]
- Eid, A.A.; Lee, D.Y.; Roman, L.J.; Khazim, K.; Gorin, Y. Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent endothelial nitric oxide synthase uncoupling and matrix protein expression. Mol. Cell. Biol. 2013, 33, 3439–3460. [Google Scholar] [CrossRef]
- Jang, H.J.; Ridgeway, S.D.; Kim, J.A. Effects of the green tea polyphenol epigallocatechin-3-gallate on high-fat diet-induced insulin resistance and endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1444–E1451. [Google Scholar] [CrossRef]
- Mellor, D.D.; Madden, L.A.; Smith, K.A.; Kilpatrick, E.S.; Atkin, S.L. High-polyphenol chocolate reduces endothelial dysfunction and oxidative stress during acute transient hyperglycaemia in Type 2 diabetes: A pilot randomized controlled trial. Diabet. Med. 2012, 30, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Septembre-Malaterre, A.; Stanislas, G.; Douraguia, E.; Gonthier, M.P. Evaluation of nutritional and antioxidant properties of the tropical fruits banana, litchi, mango, papaya, passion fruit and pineapple cultivated in Reunion French Island. Food Chem. 2016, 212, 225–233. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef]
- Yu, P.; Wang, L.; Tang, F.; Zeng, L.; Zhou, L.; Song, X.; Jia, W.; Chen, J.; Yang, Q. Resveratrol Pretreatment Decreases Ischemic Injury and Improves Neurological Function Via Sonic Hedgehog Signaling After Stroke in Rats. Mol. Neurobiol. 2017, 54, 212–226. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Smadja, J.M.; Marodon, C. Le Grand Livre des Plantes Médicinales de l’île de La Réunion Inscrites à la Pharmacopée Française; Éditions Orphie: Saint Denis, France, 2016; p. 231. [Google Scholar]
- Poullain, C.; Girard-Valenciennes, E.; Smadja, J. Plants from reunion island: Evaluation of their free radical scavenging and antioxidant activities. J. Ethnopharmacol. 2004, 95, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, R. Tisaneurs et Plantes Médicinales Indigènes à la Réunion; Éditions Orphie: Saint Denis, France, 2016; p. 435. [Google Scholar]
- Septembre-Malaterre, A.; Le Sage, F.; Hatia, S.; Catan, A.; Janci, L.; Gonthier, M.P. Curcuma longa polyphenols improve insulin-mediated lipid accumulation and attenuate proinflammatory response of 3T3-L1 adipose cells during oxidative stress through regulation of key adipokines and antioxidant enzymes. Biofactors 2016, 42, 418–430. [Google Scholar] [CrossRef]
- Le Sage, F.; Meilhac, O.; Gonthier, M.P. Anti-inflammatory and antioxidant effects of polyphenols extracted from Antirhea borbonica medicinal plant on adipocytes exposed to Porphyromonas gingivalis and Escherichia coli lipopolysaccharides. Pharmacol. Res. 2017, 119, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Marimoutou, M.; Le Sage, F.; Smadja, J.; Lefebvre d’Hellencourt, C.; Gonthier, M.P.; Robert-Da Silva, C. Antioxidant polyphenol-rich extracts from the medicinal plants Antirhea borbonica, Doratoxylon apetalum and Gouania mauritiana protect 3T3-L1 preadipocytes against H2O2, TNFalpha and LPS inflammatory mediators by regulating the expression of superoxide dismutase and NF-kappaB genes. J. Inflamm. (London) 2015, 12, 10. [Google Scholar]
- Arcambal, A.; Taïlé, J.; Couret, D.; Planesse, C.; Veeren, B.; Diotel, N.; Gauvin-Bialecki, A.; Meilhac, O.; Gonthier, M.P. Protective Effects of Antioxidant Polyphenols Against Hyperglycemia-Mediated Alterations in Cerebral Endothelial Cells and a Mouse Stroke Model. Mol. Nutr. Food Res. 2020, e1900779. [Google Scholar] [CrossRef]
- Hatia, S.; Septembre-Malaterre, A.; Le Sage, F.; Badiou-Beneteau, A.; Baret, P.; Payet, B.; Lefebvre d’Hellencourt, C.; Gonthier, M.P. Evaluation of antioxidant properties of major dietary polyphenols and their protective effect on 3T3-L1 preadipocytes and red blood cells exposed to oxidative stress. Free Radic. Res. 2014, 48, 387–401. [Google Scholar] [CrossRef]
- Arcambal, A.; Taïlé, J.; Rondeau, P.; Viranaïcken, W.; Meilhac, O.; Gonthier, M.P. Hyperglycemia modulates redox, inflammatory and vasoactive markers through specific signaling pathways in cerebral endothelial cells: Insights on insulin protective action. Free Radic. Biol. Med. 2019, 130, 59–70. [Google Scholar] [CrossRef]
- Bainor, A.; Chang, L.; McQuade, T.J.; Webb, B.; Gestwicki, J.E. Bicinchoninic acid (BCA) assay in low volume. Anal. Biochem. 2010, 410, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.S.; Wahid, S.; Mustafa, S.; Al-Saleh, E.; Dhaunsi, G.S.; Al-Mulla, F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur. J. Pharmacol. 2005, 511, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Ruskovska, T.; Massaro, M.; Carluccio, M.A.; Arola-Arnal, A. Systematic bioinformatic analysis of nutrigenomic data of flavanols in cell models of cardiometabolic disease. Food Funct. 2020, 11, 5040–5064. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef]
- Chen, F.; Qian, L.H.; Deng, B.; Liu, Z.M.; Zhao, Y.; Le, Y.Y. Resveratrol protects vascular endothelial cells from high glucose-induced apoptosis through inhibition of NADPH oxidase activation-driven oxidative stress. CNS Neurosci. Ther. 2013, 19, 675–681. [Google Scholar] [CrossRef]
- Samoisy, A.K.; Mahomoodally, M.F. Ethnopharmacological analysis of medicinal plants used against non-communicable diseases in Rodrigues Island, Indian Ocean. J. Ethnopharmacol. 2015, 173, 20–38. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, D.H.; Kim, J.S.; Lee, J.Y.; Park, H.G.; Kim, Y.J.; Oh, Y.K.; Jung, H.C.; Kim, S.I. 5,7-dihydroxy-3,4,6-trimethoxyflavone inhibits the inflammatory effects induced by Bacteroides fragilis enterotoxin via dissociating the complex of heat shock protein 90 and I kappaB alpha and I kappaB kinase-gamma in intestinal epithelial cell culture. Clin. Exp. Immunol. 2009, 155, 541–551. [Google Scholar] [CrossRef]
- Gauvin-Bialecki, A.; Marodon, C. Essential oil of Ayapana triplinervis from Reunion Island: A good natural source of thymohydroquinone dimethyl ether. Biochem. Syst. Ecol. 2008, 36, 853–858. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Li, P.; Zhang, Q.; Kim, H.J.; Gee, S.J.; Hammock, B.D. Phage-displayed peptide that mimics aflatoxins and its application in immunoassay. J. Agric. Food Chem. 2013, 61, 2426–2433. [Google Scholar] [CrossRef]
- Kang, B.; Kim, C.Y.; Hwang, J.; Jo, K.; Kim, S.; Suh, H.J.; Choi, H.S. Punicalagin, a Pomegranate-Derived Ellagitannin, Suppresses Obesity and Obesity-Induced Inflammatory Responses Via the Nrf2/Keap1 Signaling Pathway. Mol. Nutr. Food Res. 2019, 63, e1900574. [Google Scholar] [CrossRef]
- Deng, S.; West, B.J.; Jensen, C.J. A quantitative comparison of phytochemical components in global noni fruits and their commercial products. Food Chem. 2010, 122, 267–270. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Sendra, E.; Sayas-Barberá, E.; Pérez-Álvarez, J.A. Antioxidant properties of pomegranate (Punica granatum L.) bagasses obtained as co-product in the juice extraction. Food Res. Int. 2011, 44, 1217–1223. [Google Scholar] [CrossRef]
- Rolo, A.P.; Palmeira, C.M. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol Appl. Pharmacol. 2006, 212, 167–178. [Google Scholar] [CrossRef]
- Chan, P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2001, 21, 2–14. [Google Scholar] [CrossRef]
- Ashafaq, M.; Raza, S.S.; Khan, M.M.; Ahmad, A.; Javed, H.; Ahmad, M.E.; Tabassum, R.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. Catechin hydrate ameliorates redox imbalance and limits inflammatory response in focal cerebral ischemia. Neurochem. Res. 2012, 37, 1747–1760. [Google Scholar] [CrossRef]
- Morris, G.P.; Wright, A.L.; Tan, R.P.; Gladbach, A.; Ittner, L.M.; Vissel, B. A Comparative Study of Variables Influencing Ischemic Injury in the Longa and Koizumi Methods of Intraluminal Filament Middle Cerebral Artery Occlusion in Mice. PLoS ONE 2016, 11, e0148503. [Google Scholar] [CrossRef]
- Ceriello, A.; dello Russo, P.; Amstad, P.; Cerutti, P. High glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stress. Diabetes 1996, 45, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Pan, C.S.; Mao, X.W.; Liu, Y.Y.; Yan, L.; Zhou, C.M.; Fan, J.Y.; Zhang, S.Y.; Han, J.Y. Role of NADPH oxidase in total salvianolic acid injection attenuating ischemia-reperfusion impaired cerebral microcirculation and neurons: Implication of AMPK/Akt/PKC. Microcirculation 2014, 21, 615–627. [Google Scholar] [CrossRef]
- Shen, Y.; Croft, K.D.; Hodgson, J.M.; Kyle, R.; Lee, I.L.E.; Wang, Y.; Stocker, R.; Ward, N.C. Quercetin and its metabolites improve vessel function by inducing eNOS activity via phosphorylation of AMPK. Biochem. Pharmacol. 2012, 84, 1036–1044. [Google Scholar] [CrossRef]
- Jiang, R.; Hodgson, J.M.; Mas, E.; Croft, K.D.; Ward, N.C. Chlorogenic acid improves ex vivo vessel function and protects endothelial cells against HOCl-induced oxidative damage, via increased production of nitric oxide and induction of Hmox-1. J. Nutr. Biochem. 2016, 27, 53–60. [Google Scholar] [CrossRef]
- Rigoulet, M.; Yoboue, E.D.; Devin, A. Mitochondrial ROS generation and its regulation: Mechanisms involved in H(2)O(2) signaling. Antioxid. Redox Signal. 2011, 14, 459–468. [Google Scholar] [CrossRef]
- Baret, P.; Septembre-Malaterre, A.; Rigoulet, M.; Lefebvre d’Hellencourt, C.; Priault, M.; Gonthier, M.P.; Devin, A. Dietary polyphenols preconditioning protects 3T3-L1 preadipocytes from mitochondrial alterations induced by oxidative stress. Int. J. Biochem. Cell Biol. 2013, 45, 167–174. [Google Scholar] [CrossRef]
- Carvalho, C.; Moreira, P.I. Oxidative Stress: A Major Player in Cerebrovascular Alterations Associated to Neurodegenerative Events. Front. Physiol. 2018, 9, 806. [Google Scholar] [CrossRef]
- Le Sage, F.; Meilhac, O.; Gonthier, M.P. Porphyromonas gingivalis lipopolysaccharide induces pro-inflammatory adipokine secretion and oxidative stress by regulating Toll-like receptor-mediated signaling pathways and redox enzymes in adipocytes. Mol. Cell. Endocrinol. 2017, 446, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.R.; Lu, X.; Sutliff, R.L.; Hart, C.M. Rosiglitazone attenuates NF-κB-mediated Nox4 upregulation in hyperglycemia-activated endothelial cells. Am. J. Physiol. Cell Physiol. 2012, 303, C213–C223. [Google Scholar] [CrossRef]
- Kleinschnitz, C.; Grund, H.; Wingler, K.; Armitage, M.E.; Jones, E.; Mittal, M.; Barit, D.; Schwarz, T.; Geis, C.; Kraft, P.; et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010, 8, e1000479. [Google Scholar] [CrossRef] [PubMed]
- Weidig, P.; McMaster, D.; Bayraktutan, U. High glucose mediates pro-oxidant and antioxidant enzyme activities in coronary endothelial cells. Diabetes Obes. Metab. 2004, 6, 432–441. [Google Scholar] [CrossRef]
- Mates, J.M.; Perez-Gomez, C.; Nunez de Castro, I. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Liu, P.; Yang, X.; Hei, C.; Meli, Y.; Niu, J.; Sun, T.; Li, P.A. Rapamycin Reduced Ischemic Brain Damage in Diabetic Animals Is Associated with Suppressions of mTOR and ERK1/2 Signaling. Int. J. Biol. Sci. 2016, 12, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Gao, Y.; Li, L.; Tang, C.; Wen, G.; Zhou, Y.; Zhou, M.; Mao, L.; Fan, Y. Protective Effects of Quercetin on Mitochondrial Biogenesis in Experimental Traumatic Brain Injury via the Nrf2 Signaling Pathway. PLoS ONE 2016, 11, e0164237. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wang, H.D.; Hu, Z.G.; Wang, Q.F.; Yin, H.X. Activation of Nrf2-ARE pathway in brain after traumatic brain injury. Neurosci. Lett. 2008, 431, 150–154. [Google Scholar] [CrossRef]
- Arredondo, F.; Echeverry, C.; Abin-Carriquiry, J.A.; Blasina, F.; Antúnez, K.; Jones, D.P.; Go, Y.M.; Liang, Y.L.; Dajas, F. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic. Biol. Med. 2010, 49, 738–747. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Z.; Lu, H.; Xu, Z.; Tong, R.; Shi, J.; Jia, G. Recent Advances of Natural Polyphenols Activators for Keap1-Nrf2 Signaling Pathway. Chem. Biodivers. 2019, 16, e1900400. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Barber, A.J.; Spagnuolo, C.; Russo, G.L.; Daglia, M.; Nabavi, S.M.; Sobarzo-Sanchez, E. Nrf2 as molecular target for polyphenols: A novel therapeutic strategy in diabetic retinopathy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 293–312. [Google Scholar] [CrossRef]
- Priestley, J.R.; Kautenburg, K.E.; Casati, M.C.; Endres, B.T.; Geurts, A.M.; Lombard, J.H. The NRF2 knockout rat: A new animal model to study endothelial dysfunction, oxidant stress, and microvascular rarefaction. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H478–H487. [Google Scholar] [CrossRef]
- Ito, H.; Gonthier, M.P.; Manach, C.; Morand, C.; Mennen, L.; Remesy, C.; Scalbert, A. Polyphenol levels in human urine after intake of six different polyphenol-rich beverages. Br. J. Nutr. 2005, 94, 500–509. [Google Scholar] [CrossRef]
- Gonthier, M.P.; Verny, M.A.; Besson, C.; Remesy, C.; Scalbert, A. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003, 133, 1853–1859. [Google Scholar] [CrossRef]
- Gonthier, M.P.; Donovan, J.L.; Texier, O.; Felgines, C.; Remesy, C.; Scalbert, A. Metabolism of dietary procyanidins in rats. Free Radic. Biol. Med. 2003, 35, 837–844. [Google Scholar] [CrossRef]
- Rios, L.Y.; Gonthier, M.P.; Remesy, C.; Mila, I.; Lapierre, C.; Lazarus, S.A.; Williamson, G.; Scalbert, A. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am. J. Clin. Nutr. 2003, 77, 912–918. [Google Scholar] [CrossRef]
- Spencer, J.P.; Abd-el-Mohsen, M.M.; Rice-Evans, C. Cellular uptake and metabolism of flavonoids and their metabolites: Implications for their bioactivity. Arch. Biochem. Biophys. 2004, 423, 148–161. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Toro-Funes, N.; Cifuentes-Gomez, T.; Cortese-Krott, M.; Heiss, C.; Spencer, J.P. Uptake and metabolism of (-)-epicatechin in endothelial cells. Arch. Biochem. Biophys. 2014, 559, 17–23. [Google Scholar] [CrossRef]
- Kitagawa, S. Inhibitory effects of polyphenols on p-glycoprotein-mediated transport. Biol. Pharmaceut. Bull. 2006, 29, 1–6. [Google Scholar] [CrossRef]
- Bansal, T.; Jaggi, M.; Khar, R.K.; Talegaonkar, S. Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J. Pharm. Pharmaceut. Sci. 2009, 12, 46–78. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef]
- Pinheiro Fernandes, F.D.; Fontenele Menezes, A.P.; de Sousa Neves, J.C.; Fonteles, A.A.; da Silva, A.T.; de Araújo Rodrigues, P.; Santos do Carmo, M.R.; de Souza, C.M.; de Andrade, G.M. Caffeic acid protects mice from memory deficits induced by focal cerebral ischemia. Behav. Pharmacol. 2014, 25, 637–647. [Google Scholar] [CrossRef]
- Delveaux, J.; Turpin, C.; Veeren, B.; Diotel, N.; Bravo, S.B.; Begue, F.; Álvarez, E.; Meilhac, O.; Bourdon, E.; Rondeau, P. Antirhea borbonica Aqueous Extract Protects Albumin and Erythrocytes from Glycoxidative Damages. Antioxidants 2020, 9, 415. [Google Scholar]


| Mouse Gene | Sequence |
|---|---|
| Catalase | Forward CCT-CCT-CGT-TCA-GGA-TGT-GGT-T Reverse CGA-GGG-TCA-CGA-ACT-GTG-TCA-G |
| Cu/Zn Superoxide dismutase (Cu/ZnSOD) | Forward GCA-GGG-AAC-CAT-CCA-CTT Reverse TAC-AAC-CTC-TGG-ACC-CGT |
| endothelial Nitric oxide synthase (eNOS) | Forward CTG-TGG-TCT-GGT-GCT-GGT-C Reverse TGG-GGC-AAC-TTG-AAG-AGT-GTG |
| Endothelin-1 (ET-1) | Forward CCC-TTT-GCA-GAA-TGG-ATT-AT Reverse CTG-TAG-TCA-ATG-TGC-TCG-GT |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Forward TTC-ACC-ACC-ATG-GAG-AAG-GC Reverse GGC-ATG-GAC-TGT-GGT-CAT-GA |
| Glutathione peroxidase (GPx) | Forward TGC-TCA-TTG-AGA-ATG-TCG-CGT-CTC Reverse AGG-CAT-TCC-GCA-GGA-AGG-TAA-AGA |
| Heme oxygenase-1 (HO-1) | Forward GGT-CAT-GGC-TTC-CTT-GTA-C Reverse AGT-GAG-GCC-CAT-ACC-AGA-AG |
| MnSOD | Forward ATG-TTG-TGT-CGG-GCG-GCG Reverse AGG-TAG-TAA-GCG-TGC-TCC-CAC-ACG |
| Nuclear factor kappa B (NFκB) | Forward GTG-ATG-GGC-CTT-CAC-ACA-CA Reverse CAT-TTG-AAC-ACT-GCT-TTG-ACT |
| NADPH oxidase 4 (Nox4) | Forward GAT-CAC-AGA-AGG-TCC-CTA-GCA-G Reverse GTT-GAG-GGC-ATT-CAC-CAA-GT |
| Nuclear factor erythroid 2-related factor 2 (Nrf2) | Forward TTG-GCA-GAG-ACA-TTC-CCA-T Reverse GCT-GCC-ACC-GTC-ACT-GGG |
| Medicinal Plant | Retention Time (min) | Molecular Weight (Da) | Parent Ions | Fragment Ions | Identified Compound |
|---|---|---|---|---|---|
| A. borbonica | 2.5 | 354 | 353 | 191 | Chlorogenic acid isomer |
| 3.5 | 354 | 353 | 191 | Chlorogenic acid | |
| 3.7 | 354 | 353 | 191, 173 | Chlorogenic acid isomer | |
| 5.7 | 610 | 609 | 301 | Quercetin-hexoside-ramnoside | |
| 6.0 | 594 | 593 | 447, 285 | Kaempferol-hexoside-ramnoside | |
| 6.2 | 594 | 593 | 455, 285 | Kaempferol-hexoside-ramnoside | |
| 6.4 | 516 | 515 | 353, 191 | Dicaffeoylquinic acid | |
| 6.6 | 516 | 515 | 353, 191 | Dicaffeoylquinic acid | |
| 6.9 | 516 | 515 | 353, 191 | Dicaffeoylquinic acid | |
| A. triplinervis | 3.2 | 154 | 153 | 109 | Protocatechuic acid |
| 4.4 | 180 | 179 | / | Caffeic acid | |
| 5.0 | 356 610 | 355 609 | 193 301, 179 | Feruoyl-hexoside Quercetin-hexoside-ramnoside | |
| 5.2 | 464 | 463 | 301 | Quercetin-hexoside | |
| 5.5 | 550 | 549 | 505, 463, 301 | Quercetin-hexoside-malonate | |
| 6.1 | 696 | 695 | 533, 515, 371, 209 | Tricaffeoyl-glucarate | |
| 6.3 | 194 | 193 | / | Isoferulic acid | |
| 6.8 | 190 | 191 * | 163, 133 * | Ayapin | |
| 7.4 | 176 | 177 * | 145, 133, 121 * | Ayapanin (Herniarin) | |
| T. bentzoe | 1.2 | 482 | 481 | 301 | Hexahydroxydiphenoyl (HHDP)-hexoside |
| 1.4 | 482 | 481 | 301 | HHDP-hexoside | |
| 2.3 | 170 | 169 | 125 | Gallic acid | |
| 2.7 | 782 | 781 | 721, 601, 299, 271 | Punicalin | |
| 3.0 | 934 | 933 | / | Galloyl-punicalin | |
| 3.2 | 934 | 933 | 915, 781, 721, 601 | Galloyl-punicalin | |
| 3.4 | 470 | 469 | 425 | Valoneic acid dilactone | |
| 3.5 | 1084 | 1083 | 781, 601 | Punicalagin | |
| 3.8 | 1084 | 1083 | 781, 601 | Punicalagin | |
| 3.9 | 186 | 1085 | 783, 601, 451 | Digalloyl-gallagyl-hexoside | |
| 4.0 | 634 | 633 | 463, 301 | HHDP-galloyl-hexoside | |
| 4.1 | 1086 | 1085 | 933, 721, 601 | Digalloyl-punicalin | |
| 4.3 | 786 | 785 | 633, 483 | HHDP-digalloyl-hexoside | |
| 4.9 | 938 | 937 | 785, 767, 741, 465, 301 | Tri-galloyl-HHDP-glucose | |
| 5.2 | 302 | 301 | / | Ellagic acid | |
| 5.3 | 940 | 939 | 787, 769, 617 | Pentagalloyl-hexoside | |
| D. viscosa | 3.8 | 1152 | 1151 | 981, 863, 711 | Procyanidin tetramer type A |
| 4.2 | 578 | 557 | 451, 425 | Procyanidin dimer B2 | |
| 4.6 | 864 | 863 | 711, 693, 559 | Procyanidin trimer type A | |
| 5.4 | 624 | 623 | 315 | Isorhamnetin-hexoside-rhamnoside | |
| 9.0 | 344 | 343 | 329, 301, 283 | 5,7-Dihydroxy-3,6,4′-trimethoxyflavone (Santin) |
| Total Polyphenol Contents (g GAE/100 g) | DPPH Reduced (%) | |
|---|---|---|
| Plants | ||
| A. borbonica | 3.44 ± 0.11 b,c | 89.50 ± 1.24 e |
| A. triplinervis | 0.58 ± 0.02 a,c,d | 67.99 ± 1.58 f |
| T. bentzoe | 8.14 ± 0.27 a,b,d | 92.43 ± 0.42 |
| D. viscosa | 3.22 ± 0.12 b,c | 89.95 ± 1.41 |
| Standard antioxidants | ||
| Vitamin C | - | 93.78 ± 0.65 |
| Caffeic acid | - | 92.58 ± 0.63 |
| Chlorogenic acid | - | 89.36 ± 0.75 e |
| Gallic acid | - | 94.04 ± 0.06 |
| Quercetin | - | 92.56 ± 0.54 |
| Cell Conditions | Intracellular ROS Levels (% of Control) | ||
|---|---|---|---|
| Vehicle | NFκB Inhibition | AMPK Inhibition | |
| Normoglycemic condition | 100 ± 2.12 | 80.38 ± 2.92 * | 137.89 ± 3.19 *** |
| Hyperglycemic condition | 127.75 ± 2.66 *** | 80.28 ± 3.21 ### | 142.16 ± 2.88 ## |
| Cell Conditions | Total SOD Activity (U/g Protein) | Cu/ZnSOD Activity (U/g Protein) | MnSOD Activity (U/g Protein) |
|---|---|---|---|
| NG | 9.45 ± 0.41 | 2.28 ± 0.13 | 6.09 ± 0.13 |
| HG | 13.28 ± 0.76 *** | 10.77 ± 0.54 *** | 4.68 ± 0.54 |
| HG + A. borbonica | 10.85 ± 0.36 # | 6.27 ± 0.25 ## | 5.10 ± 0.54 |
| HG + A. triplinervis | 9.09 ± 0.37 ### | 4.05 ± 0.93 ### | 9.01 ± 1.24 ## |
| HG + T. bentzoe | 9.69 ± 0.31 ### | 7.14 ± 0.09 # | 4.84 ± 1.37 |
| HG + D. viscosa | 13.08 ± 0.14 | 7.41 ± 0.77 # | 5.08 ± 0.77 |
| HG + Caffeic acid | 8.73 ± 0.37 ### | 3.80 ± 0.36 ### | 6.42 ± 0.71 |
| HG + Chlorogenic acid | 7.47 ± 0.43 ### | 3.24 ± 0.04 ### | 8.30 ± 0.04 # |
| HG + Gallic acid | 11.16 ± 0.40 # | 1.22 ± 0.64 ### | 11.33 ± 0.64 ### |
| HG + Quercetin | 8.90 ± 0.73 ### | 3.65 ± 1.50 ### | 10.42 ± 1.86 # |
| Cell Conditions | ROS Levels (%) | NFκB/SEAP Activity (%) | IL-6 Secretion (ng/mg Proteins) | NO Levels (%) |
|---|---|---|---|---|
| NG | 100.00 ± 2.46 | 100.00 ± 1.11 | 30.01 ± 3.64 | 100.00 ± 2.54 |
| HG | 118.75 ± 1.89 *** | 133.69 ± 10.46 ** | 47.95 ± 1.61 ** | 70.51 ± 0.94 *** |
| HG + Ferulic acid | 84.33 ± 4.51 ### | 104.40 ± 0.23 ## | 31.96 ± 2.09 # | 80.08 ± 1.13 ## |
| HG + 3,4-Dihydroxyphenylpropionic acid | 104.16 ± 3.95 # | 100.29 ± 3.41 ## | 26.62 ± 8.30 # | 83.42 ± 1.66 ### |
| HG + 3,4-Dihydroxyphenylacetic acid | 84.17 ± 4.14 ### | 102.03 ± 1.10 ## | 23.50 ± 7.90 # | 80.62 ± 0.30 ### |
| HG + 3-Hydroxyphenylacetic acid | 109.38 ± 1.16 | 104.31 ± 2.46 ## | 19.34 ± 1.80 ### | 83.65 ± 1.36 ### |
| HG + 3-Hydroxybenzoic acid | 107.21 ± 0.74 | 102.89 ± 0.58 ## | 28.13 ± 7.20 # | 79.77 ± 0.93 # |
| HG + Hippuric acid | 113.72 ± 0.94 | 95.78 ± 3.18 ## | 26.69 ± 3.00 ## | 91.53 ± 3.36 ### |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taïlé, J.; Arcambal, A.; Clerc, P.; Gauvin-Bialecki, A.; Gonthier, M.-P. Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition. Antioxidants 2020, 9, 573. https://doi.org/10.3390/antiox9070573
Taïlé J, Arcambal A, Clerc P, Gauvin-Bialecki A, Gonthier M-P. Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition. Antioxidants. 2020; 9(7):573. https://doi.org/10.3390/antiox9070573
Chicago/Turabian StyleTaïlé, Janice, Angélique Arcambal, Patricia Clerc, Anne Gauvin-Bialecki, and Marie-Paule Gonthier. 2020. "Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition" Antioxidants 9, no. 7: 573. https://doi.org/10.3390/antiox9070573
APA StyleTaïlé, J., Arcambal, A., Clerc, P., Gauvin-Bialecki, A., & Gonthier, M.-P. (2020). Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition. Antioxidants, 9(7), 573. https://doi.org/10.3390/antiox9070573