Abstract
Parkinson’s disease (PD) is caused by progressive neurodegeneration of dopaminergic (DAergic) neurons with abnormal accumulation of α-synuclein in substantia nigra (SN). Studies have suggested the potential involvement of dopamine, iron, calcium, mitochondria and neuroinflammation in contributing to overwhelmed oxidative stress and neurodegeneration in PD. Function studies on PD-causative mutations of SNCA, PRKN, PINK1, DJ-1, LRRK2, FBXO7 and ATP13A2 further indicate the role of oxidative stress in the pathogenesis of PD. Therefore, it is reasonable that molecules involved in oxidative stress, such as DJ-1, coenzyme Q10, uric acid, 8-hydroxy-2’-deoxyguanosin, homocysteine, retinoic acid/carotenes, vitamin E, glutathione peroxidase, superoxide dismutase, xanthine oxidase and products of lipid peroxidation, could be candidate biomarkers for PD. Applications of antioxidants to modulate oxidative stress could be a strategy in treating PD. Although a number of antioxidants, such as creatine, vitamin E, coenzyme Q10, pioglitazone, melatonin and desferrioxamine, have been tested in clinical trials, none of them have demonstrated conclusive evidence to ameliorate the neurodegeneration in PD patients. Difficulties in clinical studies may be caused by the long-standing progression of neurodegeneration, lack of biomarkers for premotor stage of PD and inadequate drug delivery across blood–brain barrier. Solutions for these challenges will be warranted for future studies with novel antioxidative treatment in PD patients.
1. Introduction
Parkinson’s disease (PD) is a common neurodegenerative disease mainly involved in the progressive loss of dopaminergic (DAergic) neurons with accumulation of α-synuclein in substantia nigra (SN) of ventral midbrain [1]. These neurons secret dopamine (DA) and play a vital role in controlling the ease and balance of movements [1]. With the fall of striatal DA level below 70–80%, the clinical presentations of PD, including bradykinesia, resting tremor, rigidity and postural instability would be developed [2]. In addition, PD also displays olfactory deficits, rapid eye movement sleep disorders, depression, constipation and impairments of cognitive functions, including by affecting cholinergic, serotonergic and noradrenergic systems [1].
The etiologies of PD remain elusive. One of the causative genetic variants for PD, mutations in LRRK2, accounts for a number of autosomal dominantly inherited PD [3]. Mutations in PRKN cause early-onset PD with autosomal recessive inheritance [3]. Other genes, such as SNCA, PINK1, DJ1, ATP13A2, GIGYF2 and HTRA2, have also been identified as the causative genes for familiar and early-onset PD [3]. These genes have been implicated to be involved in the ubiquitin protein degradation pathway, oxidative stress response, cell survival, apoptosis and mitochondrial function [4].
Normal cellular functions and reactions, such as oxidative phosphorylation in mitochondria, generate free reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), superoxide anion (O2-) and nitric oxide. Although ROS are essential molecules for redox signaling and cellular functions, ROS-mediated oxidative damages, such as lipid peroxidation in cell and organelle membranes, protein oxidation by cross-linking, fragmentation and carbonyl group formation, as well as DNA and RNA oxidation, occur within cells [5]. Antioxidative responses up-regulate a number of antioxidants to reduce these radicals. However, as the required endogenous antioxidants are not sufficient in PD, uncontrolled production of ROS may excessively produce non-physiological and toxic ROS levels referred to as oxidative stress.
2. ROS Production in the PD Brain
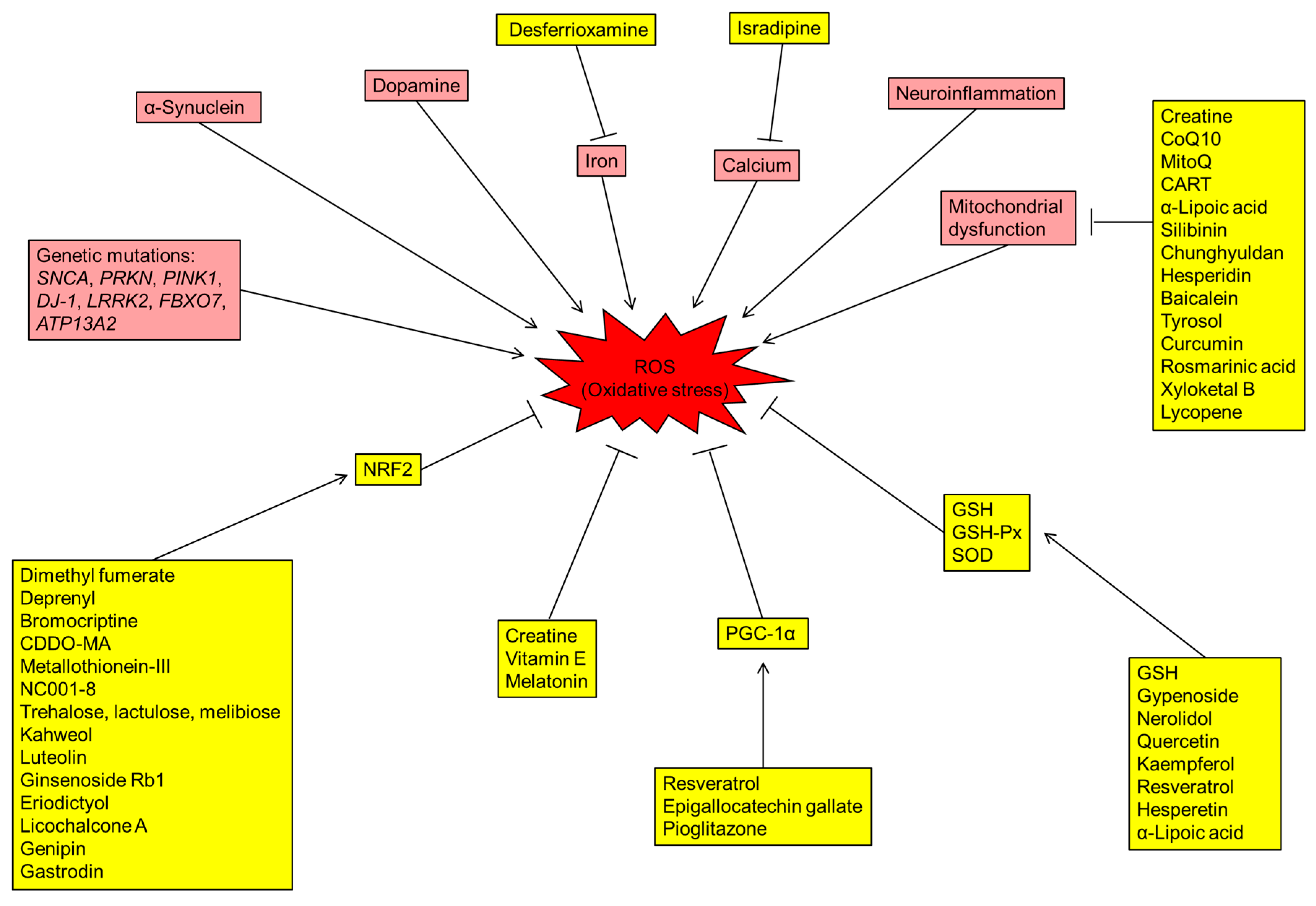
The brain requires plenty of oxygen supply, and a significant amount of oxygen is converted to ROS [6]. The over-production of ROS in the brain increases oxidative stress in PD patients. Lines of evidence suggest that the DA metabolism, high levels of iron and calcium in SN, mitochondrial dysfunction and neuroinflammation contribute to the increased oxidative stress and DAergic neuronal loss in the brains of PD patients (Figure 1).
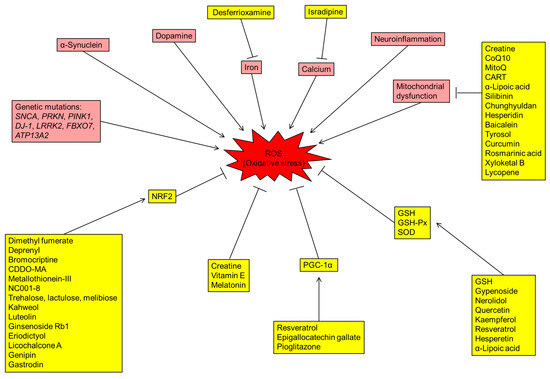
Figure 1.
Schematic diagram representing the mechanisms of reactive oxygen species (ROS) production, antioxidative stress pathways and potential drugs with different targets for Parkinson’s disease (PD). Boxes shaded in red are indicative of genetic and environmental factors involved in the production of ROS. The drugs/proteins with potential in treating PD by reducing oxidative stress are indicated in boxes shaded in yellow. CoQ10: coenzyme Q10; CART: cocaine- and amphetamine-regulated transcript; GSH: glutathione; GSH-Px: glutathione peroxidase; NRF2: nuclear factor erythroid-2-related factor 2; PGC-1α: PPARγ coactivator-1α; ROS: radical oxidative species; SOD: superoxide dismutase.
2.1. Dopamine
Dopamine demonstrates auto-oxidation to form dopamine quinones and free radicals, which could contribute to neurodegeneration in PD [7]. The cyclization of dopamine quinones forms aminochrome, which generates superoxide and down-regulates antioxidative nicotinamide adenine dinucleotide phosphate (NADPH) [8]. The metabolism of DA by monoamine oxidase-B (MAO-B) generates 3,4-dihydroxyphenyl-acetaldehyde, ammonia and H2O2 [9]. H2O2 in DAergic neurons reacts with Fe2+ to form hydroxyl radical [10,11]. Induction of MAO-B in the astrocytes leads to selective loss of DAergic neurons in SN of mice [12].
The transport and storage of DA also increase the production of ROS. The storage of DA requires the transportation through vesicular monoamine transporter 2 (VMAT2) [13]. Overexpression of VMAT2 confers protection against the toxicity generated by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). DAergic neurons with inhibitions of VMAT2 are more vulnerable to oxidative stress [14]. On the other hand, dopamine transporters (DAT) are required in the reuptake of DA [15]. Inhibition of DAT also increases levels of cytosolic DA that is prone to be oxidized [16,17]. Mutations of SNCA and DJ-1 in PD patients are linked to impaired DA reuptake or storage, suggesting the role of DAT in the neuronal susceptibility to oxidative stress [18,19].
2.2. Iron
High levels of iron are reported in SN of PD patients [20]. Iron is an essential metal for tyrosine hydroxylase, which is required for DA synthesis [21]. Iron ions Fe3+ and Fe2+ react with superoxide and H2O2 to generate hydroxyl free radicals that could be toxic to neurons [22]. Exposure of mice to iron generates neuronal loss in SN and DA depletion in striatum, develops parkinsonian phenotypes and becomes more vulnerable to MPTP [23]. Stereotaxic infusion with iron into SN of rats leads to increased levels of iron hydroxyl radicals in striatum [24]. Administrating iron chelator to mice reduces iron levels in the brain and demonstrates neuroprotective effect against iron- or MPTP-induced neurotoxicity [25]. These results suggest that increased levels of iron in SN contribute to oxidative stress and neurodegeneration in PD [24,25].
2.3. Calcium
The regulation of intracellular Ca2+ requires ATP-dependent pumps in mitochondria, which therefore increases ROS generation [26]. In primary cultures of mouse mesencephalic DAergic neurons, oxidative stress can be enhanced by opening of L-type Ca2+ channels, while α-synuclein aggregates potentiate this over-production of ROS in mitochondria [27]. L-type calcium channel Cav1.3 is prevalent in DAergic neurons of SN [28]. This distribution in calcium channel may explain why DAergic neurons in SN are more susceptible to oxidative stress or excitotoxicity. Isradipine, an L-type calcium channel blocker, demonstrates protective effects against α-synuclein, 6-hydroxydopamine (6-OHDA) or MPTP-induced neurotoxicity in DAergic neurons [29,30,31], supporting the association between calcium influx through L-type CA2+ channels and PD pathogenesis.
2.4. Mitochondria Dysfunction
The mitochondria are primary sites of ROS production. In complex I (nicotinamide adenine dinucleotide dehydrogenase) and complex III (cytochrome bc1), the premature electron leakage to oxygen generates O2- in mitochondria [32]. Mitochondrial dysfunction leads to excessive ROS production. On the other hand, ROS are also harmful to the electron transport chain itself [33,34]. The connection between dysfunction of mitochondria and PD is firstly demonstrated by MPTP-induced parkinsonism among drug abusers [35]. In the brain, MPTP is metabolized into 1-methyl-4-phenylpyridinuim (MPP+), which enters into DAergic neurons by DAT. MPP+ selectively inhibits complex I and leads to death of DAergic neurons in SN [35,36]. In PD patients, the complex I activity in SN is also reduced [37,38,39]. Genes encoding mitochondrial proteins are also down-regulated in DAergic neurons from PD patients [40]. The mitochondrial dysfunction in PD involves mitochondrial biogenesis, fusion/fission and mitophagy [41]. Furthermore, a number of genetic mutations, such as LRRK2, PRKN, DJ-1, PINK1, FBXO7 and ATP13A2, in PD patients also provide clues of mitochondrial dysfunction in PD pathogenesis (see below).
2.5. Neuroinflammation
Neuroinflammation, mainly contributed by microglia, is now well recognized as a prominent pathological feature and a potential source of oxidative stress in PD [42]. Microglia are highly motile phagocytes and comprise 10% to 15% of the total cells in the brain [43]. Microglia can be activated and acquire phagocytic ability by α-synuclein [44,45]. Activated microglia are significant sources of oxidative stress [43] and can produce glutamate and proinflammatory factors including tumor necrotizing factor (TNF)-α, interleukin (IL)-1β and IL-6 to promote neurodegeneration [46]. In PD patients, activated microglia in SN along with an increase of pro-inflammatory factors in the brain and cerebrospinal fluid (CSF) are detected [47,48,49]. Microglial activation is observed in cell and animal models induced by MPTP, rotenone, 6-OHDA and lipopolysaccharide (LPS) [50,51,52,53,54]. The dead neurons release oxidized lipids, proteins and DNA, all of which in turn activate microglia, forming a neurotoxic vicious cycle [55]. Since the midbrain contains more microglia, the activation of microglia could be remarkably harmful to DAergic neurons in midbrain [56]. Interestingly, genetic studies find the associations between PD and single nucleotide polymorphisms within human leukocyte antigen (HLA) -DRA, DRB1, DRB5 and DQB1 regions [57,58,59,60,61,62,63,64]. These clinical studies further support the contribution of neuroinflammation to the pathogenesis of PD.
2.6. Oxidative Stress in Other Neurodegenerative Disorders
Similar to PD, aggregations of disease-specific misfolded proteins are main pathological features in Alzheimer’s disease (AD), Huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS). AD is characterized by the presence of senile plaques, composed of β amyloid peptides (Aβ) [65]. The aggregation of Aβ activates neuroinflammation, impairs mitochondrial function and generates ROS [66]. The long polyglutamine (polyQ) tract encoded by expanded CAG trinucleotide repeats in the exon 1 of HUNTINGTIN (HTT) forms intranuclear and intracytoplasmic aggregates in HD [67], while a significant amount of oxidized proteins has been found in these aggregations [68]. Various mutations of TAR DNA binding protein (TDP-43) are found in patients with ALS [69]. These TDP-43 mutant proteins increase oxidative stress, mitochondrial dysfunction and lipid peroxidation [70]. These findings suggest that oxidative stress participates in pathogenesis of neurodegeneration in different neurological diseases.
3. Gene Mutations of PD Patients Involving Oxidative Stress
3.1. α-Synuclein (SNCA)
Mutations of SNCA are discovered in familial PD patients with autosomal dominant inheritance [71]. SNCA encodes α-synuclein, which is abundantly accumulated in Lewy bodies in degenerated DAergic neurons of PD patients [71]. Although the function of α-synuclein remains unclear, several lines of evidence suggest its role in the generation of oxidative stress [72]. Overexpression or misfolding of α-synuclein increases ROS production [73,74] and cell susceptibility to oxidative stress [74,75,76,77]. SNCA-transgenic mice demonstrate increased susceptibility to MPTP and 6-OHDA [78,79,80]. DAergic neurons derived from induced pluripotent stem cells (iPSCs) from a PD patient carrying triplication of the SNCA demonstrate high expression levels of markers of oxidative stress and augmented susceptibility to H2O2, suggesting that the overdose of α-synuclein intrinsically changes the balance of ROS production and antioxidant activities in DAergic neurons [81]. α-Synuclein may interact with phospholipase D2 to disrupt vesicular membrane integrity and recycling, leading to reduced vesicles for DA storage [82,83]. Furthermore, the accumulations of α-synuclein within the inner mitochondrial membrane inhibit activity of complex I, resulting in mitochondrial dysfunction and increased oxidative stress [84,85,86]. As an agonist of toll-like receptor 2, oligomeric α-synuclein demonstrates the potential to activate microglia [87], leading to elevated ROS production followed by the secretion of TNF-α, IL-1β and IL-6 [45,88,89]. The neuron-to-neuron transfer and propagation of α-synuclein triggers more aggregations and cytotoxic cascades [90,91,92]. It is worth noting that increased ROS may contribute to the aggregation of α-synuclein [71], which in turn increases oxidative stress, creating another neurotoxic vicious cycle.
3.2. PARKIN (PRKN)
Loss-of-function mutations of PRKN are found in early-onset PD patients with autosomal recessive inheritance [93]. PRKN encodes an E3 ubiquitin ligase PARKIN, which protects neurons against α-synuclein toxicity and oxidative stress [94]. In accordance with oxidative stress, PARKIN ubiquitinates mitochondrial proteins involving mitochondrial fusion and activates mitophagy to scavenge dipolarized mitochondria [95]. Knockout of PRKN in SNCA-transgenic mice exacerbates α-synuclein-induced mitochondrial dysfunction [96]. DAergic neurons derived from iPSCs carrying PRKN mutations demonstrate a greater amount of ROS [97,98]. On the other hand, oxidative stress down-regulates E3 ligase activity of PARKIN [99]. In PRKN-overexpressing DAergic neurons, treatment with DA decreases E3 ubiquitin ligase activity of PARKIN [99]. These findings indicate that PARKIN indirectly regulates oxidative stress and mitochondrial quality by mitophagy, while oxidative stress diminishes its activity.
3.3. PTEN-Induced Putative Kinase 1 (PINK1)
Mutations in PINK1, encoding a mitochondria-targeted kinase, are discovered in familial PD patients with autosomal recessive inheritance [95]. PINK1 is a key regulator of mitochondrial function by stabilization of cristae and control of mitophagy [95]. PINK1 deficiency leads to shortening, bulging and fragmentation of mitochondria [100,101,102,103,104] and loss of complex I activity [105,106,107]. Knockdown of PINK1 in SH-SY5Y cells diminishes mitochondrial membrane potential, reduces mitochondrial DNA synthesis and ATP production [106]. PINK1 knockout mice demonstrate an increased number of larger mitochondria with impaired function in striatum and increased susceptibility to H2O2 [107]. Neurons derived from iPSCs carrying a PINK1 mutation demonstrate increased susceptibility to MPP+ and H2O2 [108]. PINK1 interacts with PARKIN to regulate mitochondrial function and oxidative stress. Upon oxidative stress, PINK1 is required for the recruitment of PARKIN to mitochondria and initiate mitophagy to scavenge damaged mitochondria [109,110].
3.4. DJ-1
DJ-1 encodes a multifunctional protein participating in antioxidative stress mechanisms and mitochondrial regulation [111]. Mutations in DJ-1 are discovered in early-onset PD patients with autosomal recessive inheritance [112]. Knockdown of DJ-1 potentiates the cell death in oxidative stress [113,114,115]. DJ-1 knockout mice demonstrate increased susceptibility to MPTP and 6-OHDA [116,117], while overexpressing DJ-1 reduces MPTP-induced neuronal loss in SN [116,118,119]. DJ-1 regulates the activities of nuclear factor erythroid-2-related factor 2 (NRF2) [120] and VMAT2 [121]. In low oxidative stress, NRF2 forms a complex with kelch-like ECH-associated protein 1, leading to its degradation by ubiquitination [122]. In accordance with oxidative stress, DJ-1 separates NRF2 form this complex, and the translocation of NRF2 the nucleus activates antioxidative gene expressions, leading to the reduction of ROS [111]. DJ-1 also up-regulates VMAT2 expression and activity and then enhances reuptake of excess DA into synaptic vesicles [121]. Furthermore, DJ-1 could be a potent inhibitor of death-associated protein (DAXX)/apoptosis signal-regulating kinase 1 (ASK1) cell-death pathway [123]. In H2O2-treated SH-SY5Y cells and MPTP-treated mice, DJ-1 sequesters DAXX in the nucleus to prevents its interaction with ASK1, resulting in down-regulation of apoptosis induced by oxidative stress [123,124]. Pathogenic mutations of DJ-1 lose their neuroprotective potentials against oxidative stress [125].
3.5. Leucine-Rich Repeat Kinase 2 (LRRK2)
Mutations in the LRRK2 are the most common genetic causes in PD patients [93,126,127]. LRRK2 contains a resistance to audiogenic seizures (RAS) of complex proteins (ROC) G-domain and a kinase domain. A number of pathogenic mutations of LRRK2 identified in its kinase domain increase kinase activity, causing neuronal apoptosis [126]. Inhibition of LRRK2 kinase activity protects cells against mitochondrial dysfunction [128]. Overexpression of LRRK2 in SH-SY5Y cells generates fragmented mitochondria and increases ROS production and susceptibility to H2O2 [129,130]. DAergic neurons derived from iPSCs carrying LRRK2 mutations are more vulnerable to MPP+, H2O2 and 6-OHDA [108,131]. LRRK2 facilitates the translocation of dynamin-related protein 1 to mitochondria and then induces abnormal fission of mitochondria [129,132]. Mutations in LRRK2 kinase domain also up-regulates phosphorylation of peroxiredoxin 3 (PRDX3), resulting in reduction of peroxidase activity and over-production of ROS. Post-mortem brain analysis from patients carrying LRRK2 G2019S mutation consistently reveals increased phosphorylation of PRDX3 [133].
3.6. FBXO7
Mutations of FBXO7, an adaptor in Skp-Cullin-F-box ubiquitin E3 ligase complex, are found in early-onset familial PD patients with autosomal recessive inheritance [134]. FBXO7 involves many crucial cellular functions, including mitochondria and proteasome [135]. It is also a stress-responsive protein [136]. Stress challenges with H2O2 translocate FBXO7 to mitochondria and form FBXO7 aggregates [136], which are also seen in brains of PD patients [136]. The accumulation of FBXO7 aggregates in mitochondria leads to impaired mitochondria integrity and increased generation of ROS [136]. ROS may further facilitate FBXO7 aggregation [136], resulting in a vicious cycle of FBXO7 aggregation, mitochondria impairment and ROS generation. On the other hand, FBXO7 can interact with PARKIN and promote PARKIN recruitment to mitochondria to modulate mitophagy [137]. FBXO7 mutations induce impairment of mitophagy as well as exaggerate FBXO7 aggregation in mitochondria [137], all of which contribute to FBXO7-associated neurodegeneration in PD.
3.7. ATP13A2
Mutations in ATP13A2 are discovered in sporadic PD patients and Kufor–Rakeb syndrome (KRS), a rare early-onset atypical parkinsonism with pyramidal degeneration and dementia and autosomal recessive inheritance [138]. ATP13A2 is a P-type ATPase, which plays an important role in active transportation of cations across endosomal or lysosomal membranes [139]. The levels of ATP13A2 are reduced in DAergic neurons in SN of PD patients [140]. Lines of evidence also suggest that ATP13A2 attenuates mitochondrial dysfunction. Overexpression of ATP13A2 enhances viability of SH-SY5Y cells exposed to rotenone and MPP+ [141,142]. Fibroblasts of KRS patients carrying ATP13A2 mutations demonstrate enhanced mitochondrial fragmentation, reduced mitochondrial DNA integrity and decreased ATP production [143]. Olfactory neurospheres from patients carrying ATP13A2 mutations are also vulnerable to Zn2+-induced mitochondrial fragmentation and depolarization, as well as demonstrate reduction of ATP synthesis [144]. Knockdown of ATP13A2 in SH-SY5Y cells induces mitochondrial fragmentation and increases ROS production [145].
4. Candidate Biomarkers for Oxidative Stress in Parkinson’s Diseases
Many studies have focused on finding biomarkers that reflect oxidative stress in PD (Table 1). In addition to improve our understanding of PD pathogenesis, these molecular biomarkers may also useful in PD diagnosis and monitoring progression of neurodegeneration in PD patients.

Table 1.
Potential biomarkers involving oxidative stress for Parkinson’s disease.
4.1. DJ-1
Elevated levels of DJ-1 in CSF or plasma are reported in PD patients [146,147,151]. On the other hand, other studies demonstrate contradicting results, which show low levels of DJ-1 in CSF of PD patients [148,149,150]. Serum levels of DJ-1 in PD patients and normal controls are similar [152]. PD patients also demonstrate higher DJ-1 levels in saliva compared to normal controls [153,154]. The levels of 4-hydroxy-2-nonenal (HNE)-modified DJ-1 isoform are significantly altered in whole blood of advanced-stage PD patients [155]. In erythrocytes and urine, levels of oxidized DJ-1 are higher in PD patients compared to normal controls [156,157], suggesting that oxidized DJ-1 could be a biomarker candidate for PD.
4.2. Coenzyme Q10 (CoQ10)
CoQ10 potentiates mitochondrial electron transport chain and reduces ROS production [190]. CoQ10 also scavenges free radicals to protect mitochondrial and lipid membranes against ROS [190]. In PD patients, reduction in plasma levels of total CoQ10 and increase in ratio of oxidized CoQ10/total CoQ10 are reported [158]. The ratio of reduced form/total CoQ10 in platelets is decreased in PD patients [159], suggesting the role of CoQ10 oxidation in PD pathogenesis.
4.3. Uric Acid
Uric acid is a potent antioxidant and may be protective against PD [191]. In cultured SN neurons from mice, uric acid prevents death of DAergic neurons caused by H2O2 or MPP+ [192,193]. Elevations of cerebral uric acid in 6-OHDA-lesioned rodents improve parkinsonian phenotypes [194,195]. Two clinical trials consistently suggest that higher levels of uric acid in serum are closely related to a slower progression of PD [160,161]. High levels of uric acid in CSF are also correlated to a slower rate of clinical progression [160], as well as lower changes of unified PD rating scale (UPDRS) scores [161]. PD patients with cognitive dysfunction also have lower serum levels of uric acid compared to those without cognitive dysfunction [162]. These results suggest uric acid as a protective biomarker for PD.
4.4. 8-Hydroxy-2’-Deoxyguanosine (8-OHdG)
8-OHdG is generated by oxidation of guanine residues and hydroxyl radicals and in DNA [196]. It is a biomarker of DNA damage due to oxidative stress [196]. 8-OHdG concentrations are selectively elevated in SN of PD patients [197]. PD patients consistently demonstrate higher 8-OHdG levels in CSF compared to normal controls [163,164]. Furthermore, the levels of 8-OHdG in CSF are positively correlated with the duration of illness and oxidized CoQ10 in total CoQ10 [164]. PD patients demonstrate increased urinary levels of 8-OHdG [165,166], which is also correlated with the motor score of UPDRS [165].
4.5. Homocysteine
Homocysteine is an intermediate product of transulfuration methylation cycle [198]. Chronic administration of homocysteine in mice causes DAergic neuronal loss in SN by inhibiting mitochondrial activity and increasing oxidative stress [199]. Homocysteine levels in plasma of PD patients are higher compared to AD and normal controls [168]. Increased homocysteine levels are also seen in CSF of patients with PD. High homocysteine levels in plasma are correlated with worse cognition in PD patients [168,200]. It is noteworthy that levodopa therapy may increase plasma homocysteine levels, while supplement with folate or vitamin B12 can reverse levodopa-induced hyperhomocysteinemia [201].
4.6. Retinoic Acid (RA) and Carotenoids
RA and carotenoids demonstrate antioxidative effects in cell and animal models for PD. RA attenuates 6-OHDA and MPP+-mediated neurotoxicity in SH-SY5Y cells [202,203]. Administration with RA agonist prevents interferon (IFN)-γ/LPS-induced DAergic neuronal loss in rat midbrain slice cultures [204]. RA levels in plasma of PD patients are reduced [169]. PD patients also demonstrate reduced levels of α- and β-carotenes and lycopene in serum, which also inversely correlated with motor part of UPDRS scores and Hoehn and Yahr stage [170].
4.7. Vitamin E
Vitamin E, a lipophilic antioxidant, prevents lipids from oxidative stress [205]. Plasma levels of vitamin E are reduced in PD patients [171]. However, other studies show that serum or plasma levels of vitamin E are not different between PD patients and normal controls [172,173,174,175].
4.8. Glutathione Peroxidase (GSH-Px), Superoxide Dismutase (SOD) and Xanthine Oxidase
GSH-Px and SOD are two important antioxidative enzymes [206]. In PD patients, current reports of GSH-Px activities demonstrate wide variability, and could be decreased [176,177] or not altered in erythrocytes [178], or increased in serum [179,180]. A few studies indicate lower SOD activities in erythrocytes of PD patients [177,181], whereas increased [182] or unchanged [179] SOD activities in plasma or serum of PD patients are also reported. The catalyzation of hypoxanthine to xanthine by xanthine oxidase generates O2- and H2O2 [207]. One study shows increased blood xanthine oxidase activities in PD patients compared to normal controls [179].
4.9. Nuclear Factor Erythroid 2-Related Factor 2 (NRF2)
NRF2 pathway is an antioxidative signaling pathway involved in the pathogenic processes of PD. NFR2 inactivation is observed in MPTP- or 6-OHDA-treated SH-SY5Y cells or mice [98,208]. Overexpressing α-synuclein in ventral midbrain of NRF2 knockout mice down-regulates expression levels of NRF2-downstream genes such as nicotinamide adenine dinucleotide phosphate quinone oxidoreductase-1 (NQO1) and heme oxygenase-1 (HO-1), as well as exacerbates neurodegeneration of DAergic neurons [209]. The expressions of NRF2 and NQO1 in DAergic neurons derived from iPSCs carrying a PARKIN mutation are also down-regulated [97]. Recently, a small clinical study demonstrates that levels of NRF2 in peripheral leukocytes are elevated in PD patients [183]. Larger studies with correlation between NRF2 pathway and clinical severity in PD are warranted to confirm the potential of NRF2 as a biomarker for PD.
4.10. Lipid Peroxidation Products
Lipid peroxidation disturbs membrane organization and functional impairment of proteins and DNA [210]. A number of studies demonstrate altered levels of products of lipid peroxidation, such as isoprostanes [211,212], HNE [213] and malondialdehyde (MDA) [214,215,216], in brain tissues of neurodegenerative patients. The level of HNE in CSF is elevated in PD patients [184]. High levels of MDA have been identified in plasma of PD patients [171,182,185,186], although other studies demonstrate similar serum levels of MDA levels between PD patients and normal controls [179,187]. Plasma levels of F2-isoprostanes are also elevated in PD patients [166], whereas another study demonstrates that plasma levels of F2-isoprostanes in PD patients are similar to those in normal controls [188]. PD patients also demonstrate high levels of oxidized low-density lipoproteins (LDL) compared to normal controls [189].
5. Potentials of Antioxidants in Treating Parkinson’s Diseases
Current treatments for PD are prescribing levodopa with aromatic 1-amino acid decarboxylase inhibitors, dopamine agonists (eg. pramipexole, ropinirole), catechol-O-methyltransferase inhibitors (e.g., entacapone), MAO-B inhibitors (e.g., selegiline, rasagiline) and anti-cholinergic medications. Although these treatments are helpful to relieve symptoms and maintain patients’ quality of life, none of them can halt or even slow neurodegenerative processes. Considering the important role of oxidative stress in the pathogenesis of PD, antioxidants could be reasonable therapeutic strategies to modify PD progression (Table 2).
5.1. Creatine
Creatine is crucial to maintain energy levels particularly in the brain and cardiac and skeletal muscles [217]. It also improves mitochondrial function and serves as an antioxidant directly [218]. In MPTP-treated mice, creatine administration reduces DAergic neuronal degeneration and DA depletion [219]. A number of clinical trials have been reported to assess the potentials of creatine in treating PD patients (Table 2). A randomized, placebo-controlled study shows that creatine (2–4 g/day) has no effect on UPDRS scores but may improve moods in PD patients [220]. A larger randomized, double-blind, placebo-controlled trial on 200 PD patients shows significant reduction of UPDRS scores by creatinine (10 g/day) [221]. Another small double-blind study on 20 PD patients demonstrates that creatine (5 g/day) can improve muscle strength by resistance training [222]. However, a phase III clinical trial conducted by the National Institute of Neurological Disorders and Stroke on 1741 PD patients shows negative results by taking creatine 10 g/day for at least 5 years [223]. A potential explanation for the lack of clinical benefits in creatine and other antioxidants could be that approximately 50% of DAergic neurons and 80% of striatal DA levels have already been lost when clinical features manifest [224]. The fates of the surviving neurons may have been determined and are very hard to be altered by antioxidants.
5.2. Vitamin E
One population-based study shows inverse association between consumption of vitamin E and incidence of PD [225]. However, this association cannot be recapitulated by other similar studies [226,227]. In an open-labeled trial, the combination of vitamin C and E may slow disease progression in early-stage PD patients [228], while the use of levodopa could be delayed by long-term treatment with vitamin E [228]. However, another study show that vitamin E cannot slow neurodegeneration, decrease cognitive decline and mortality in PD patients (Table 2) [229]. A long-standing study further suggests that vitamin E has no effects on mortality in early-stage PD patients within 8.2 years of observation [230].
5.3. CoQ10
Platelet levels of CoQ10 are lower in PD patients [38]. In a small study on 15 PD patients, treatment with CoQ10 (400~800 mg/day) was well-tolerated and increased plasma levels of CoQ10 in a dose-dependent manner, whereas it demonstrates no change in UPDRS scores [231]. A randomized, double-blind, placebo-controlled clinical trial on 80 early-stage PD patients shows a significant reduction in UPDRS scores dependent on doses by administration of CoQ10 (300~1200 mg/day) [232]. This potential benefit is recapitulated by other small double-blind or open-labeled trials (Table 2) [233,234]. However, large clinical studies cannot reproduce the findings of above studies. Two larger randomized, double-blind, placebo-controlled trials do not show any benefit from higher dosages of CoQ10 (1200~2400 mg/day) [235,236]. Nanoparticular CoQ10 at 300 mg/d (equivalent to CoQ10 1200 mg/day) also displays no benefit in another randomized, double-blind, placebo-controlled trial on 131 PD patients [237]. On the other hand, administration with reduced form of CoQ10 (ubiquinol-10, 300 mg/day) in a randomized, double-blind, placebo-controlled trial on 64 PD patients in Japan shows improvement of UPDRS [238].
The limited potential to penetrate BBB may reduce the concentration of CoQ10 in CNS [239,240]. Therefore a mitochondria-targeted CoQ10 analog MitoQ is designed to enhance penetrance to BBB [241]. MitoQ is modified from CoQ10 by covalent binding of lipophilic triphenylphosphonium cation and the antioxidant moiety of CoQ10 [242]. With lipophilic property and its positive charge, MitoQ is efficiently cross BBB and accumulates within mitochondria [241,242]. However, in a randomized, double-blind, placebo-controlled trial on 128 patients, MitoQ (40 or 80 mg/day) cannot reduce the progression of PD [240].
5.4. PPARγ Coactivator-1α (PGC-1α) Agonist
PGC-1α is a pivotal regulator of mitochondrial biogenesis [243]. It also up-regulates antioxidative proteins such as GSH-Px, catalase, and SOD [244]. Genome-wide analysis revealed that many PGC-1α-driven gene expressions are down-regulated in PD patients [245]. Neurons in PGC-1α knockout mice demonstrate increased susceptibility to MPTP [246], while DAergic neurons in PGC-1α-transgenic mice are resistant to MPTP-induced neurotoxicity [247]. In rodents, administration of resveratrol, a PGC-1α activator, up-regulates genes involving mitochondrial biogenesis [248,249,250] and demonstrates neuroprotection against MPTP- [247,251,252,253] and 6-OHDA-induced neurotoxicity [254,255,256]. Epigallocatechin gallate, an important component of green, up-regulates PGC-1α to improve mitochondrial function and DA neuronal survival in MPP+-treated PC12 cells [257]. Another PGC-1α agonist, pioglitazone, demonstrates protective potentials against DAergic neuronal loss in an MPTP-treated rat [258]. It also mitigates the reduction of DA in striatum of rotenone-treated rats [259]. However, a population-based study does not find the relationship between pioglitazone use and PD incidence among diabetic patients [260]. A randomized, double-blind, placebo-controlled trial of pioglitazone (15 or 45 mg/day) on 210 PD patients could not show a difference of UPDRS scores between treatment and placebo groups (Table 2) [261]. Of note, pioglitazone is a substrate of CYP2C8 [262], and administration of CYP2C8 inducer, such as rifampin, phenobarbital and phenytoin, could reduce its blood level and neuroprotective effects.
5.5. Glutathione (GSH), GSH-Px and SOD
GSH reduces the production of ROS and provides protection from oxidative stress [206]. Decreased levels of GSH are discovered in SN of PD patients [263,264,265,266]. Therefore, restoring the level of GSH may be a strategy to prevent damages of oxidative stress in DA neurons. PD patients with intravenous infusion of GSH (1200 mg/d) demonstrates 42% decline in disability compared to vehicle-treated controls [267]. However, another study shows no significant PD improvement by intravenous administration of GSH (700 mg/d) (Table 2) [268]. A larger randomized, double-blind, placebo-controlled trial on 45 PD patients shows intranasal GSH administration (300 or 600 mg/d) displays PD improvement similar to placebo [269].
Natural antioxidants are enriched in herb medicines and may have neuroprotective effects in PD by enhancing activities of GSH-Px or SOD. For example, gypenosides, extracted from Gynostemma pentaphyllum, may mitigate MPTP-induced reduction of GSH and SOD activities in mouse SN [270]. Nerolidol, found in essential oils from plants, up-regulates levels of SOD and GSH in a rotenone-treated PD mouse model [271]. Quercetin, abundant in fruits and vegetables, red wine and olive oil, restores GSH levels in striatum of 6-OHDA-treated rats [272,273]. Kaempferol, a flavonol present in tea, apple, grapefruit and broccoli, up-regulates SOD and GSH-Px activities in SN of MPTP-treated mice [274]. Resveratrol [256] and hesperetin [275] up-regulate of levels of GSH as well as activities of GSH-Px and SOD in SN of 6-OHDA-treated rats. Clinical studies will be warranted to confirm the application of these natural antioxidants in treating PD.
5.6. NRF2 Enhancer
The critical role of the NRF2 pathway in oxidative stress points to its potential as a target for treating PD. In cell or animal models for PD, several compounds demonstrate neuroprotective effects by up-regulating the NRF2 pathway. Dimethyl fumarate, a potent NRF2 enhancer in treating multiple sclerosis, demonstrates neuroprotection against MPTP- and α-synuclein-induced neurotoxicity in mice by activating NRF2 and HO-1 [276,277]. Deprenyl, a selective MAO-B inhibitor, up-regulates NRF2 and NQO1 to protect PC12 and SH-SY5Y cells against MPP+-induced toxicity [278,279]. Bromocriptine, a dopamine agonist, up-regulates NRF2 and NQO1 to protect PC12 cells from oxidative damages by H2O2 [280]. Administration of synthetic NRF2 activator CDDO-MA reduces MPTP-induced DAergic degeneration, ROS production and α-synuclein accumulation in SN of mice [281]. Treatment with metallothionein-III in 6-OHDA-treated SH-SY5Y cells up-regulates NRF2 and HO-1 expression, as well as reduces ROS production and cell apoptosis [282]. Indole derivative NC001-8 also protects DAergic neurons derived from SH-SY5Y or iPSCs carrying PRKN mutation against MPP+ and H2O2-induced neurotoxicity by up-regulating NRF2 and NQO1 [98]. By up-regulating autophagy and NRF2 pathway, disaccharides, including trehalose, lactulose and melibiose, demonstrate neuroprotective effects against α-synuclein-induced neurotoxicity [283]. Several natural compounds, such as kahweol [284], luteolin [285], ginsenoside Rb1 [286], eriodictyol [287], licochalcone A [288], genipin [97] and gastrodin [289] also demonstrate neuroprotective effects by up-regulating NRF2 pathway in different PD models. Clinical trials targeting NRF2 pathway in the future may offer a possible strategy to modify neurodegeneration in PD.
5.7. Melatonin
Because of its amphiphilicity, melatonin passes across BBB and possesses antioxidant properties in CNS [290]. Melatonin reduces oxidative stress and prevents neuronal degeneration in the nigrostriatal pathway of MPTP-treated mice [291,292,293,294]. The neuroprotective potential of melatonin is consistently demonstrated in 6-OHDA [295], paraquat [296,297] and rotenone-treated mice [298]. However, in a small clinical trial on 18 PD patients, 4-week administration of melatonin (3 mg/day) to PD patients has no benefit on motor performance, although this treatment may improve subjective quality of sleep (Table 2) [299]. The reduced bioavailability and first-pass effect for oral administration may reduce the level of melatonin in the targeted brain region [300].
5.8. Iron Chelator
The accumulation of iron in SN and its close association with production of oxidative stress suggest the application of iron chelators in treating PD. In MPTP-treated mice, desferrioxamine, a common iron chelator, inhibits iron accumulation as well as normalizes the levels of hydroxyl radical and lipid peroxidation [25]. Intraventricular injection of desferrioxamine increases DA levels in striatum of 6-OHDA-treated mice [301,302]. In a placebo-controlled, double-blind trial on 40 PD patients, treatment with desferrioxamine (30 mg/kg/day) for 12 months demonstrates lower magnetic-resonance-imaging-detected iron levels in SN compared to those with treatment for 6 months [303]. Patients with 12-month treatment also display better UPDRS scores compared to those with 6-month treatment (Table 2) [303].

Table 2.
Clinical trials of antioxidants in treating Parkinson’s diseases.
Table 2.
Clinical trials of antioxidants in treating Parkinson’s diseases.
| Antioxidant | Number of Patients (Treatment/Placebo) | Follow-up | Dosage | Route | Effect | References |
|---|---|---|---|---|---|---|
| Creatine | ||||||
| 60 (40/20) | 2 years | 4 g/day | Oral | No | [220] | |
| 134 (67/67) | 1 year | 10 g/day | Oral | Beneficial | [221] | |
| 20 (10/10) | 12 weeks | 5 g/day | Oral | Beneficial | [222] | |
| 1741 (874/867) | 5 years | 10 g/day | Oral | No | [223] | |
| Vitamine E | ||||||
| 400 (202/199) | 2 years | 2000 IU/day | Oral | No | [229] | |
| Coenzyme Q10 (CoQ10) | ||||||
| 28 (14/14) | 4 weeks | 360 mg/day | Oral | Beneficial | [234] | |
| 80 (64/16) | 16 months | 300~1200 mg/day | Oral | Beneficial * | [232] | |
| 142 (71/71) | 1 year | 2400 mg/day | Oral | No | [235] | |
| 600 (397/203) | 16 months | 1200~2400 mg/day | Oral | No | [236] | |
| Ubiquinol-10 | ||||||
| 64 (36/28) | 96 weeks | 300 mg/day | Oral | No | [238] | |
| Nanoparticular CoQ10 | ||||||
| 131 (64/67) | 3 months | 300 mg/day | Oral | No | [237] | |
| MitoQ | ||||||
| 130 (89/41) | 1 year | 40~80 mg/day | Oral | No | [240] | |
| Pioglitazone | ||||||
| 210 (139/71) | 44 weeks | 15~45 mg/day | Oral | No | [261] | |
| Glutathione | ||||||
| 20 (10/10) | 12 weeks | 1400 mg t.i.w. for 4 weeks | Intravenous | No | [268] | |
| 43 (28/15) | 3 months | 300~600 mg/d | Intranasal | No | [269] | |
| Melatonin | ||||||
| 18 (8/10) | 4 weeks | 3 mg/d | Oral | No | [299] | |
| Desferrioxamine | ||||||
| 40 (21 with 12-month treatment/19 with 6-month treatment) | 12 months | 30 mg/kg/day | Oral | Beneficial | [303] | |
* In the group with coenzyme Q10 1200 mg/day.
5.9. Mitochondria-Targeted Antioxidant
The association of mitochondrial dysfunction and production of ROS represent mitochondria as a potential target for treating PD. Cocaine- and amphetamine-regulated transcript (CART) demonstrate their potential to be localized to mitochondria and protect SH-SY5Y cells and rat cortical and hippocampal neurons against H2O2-induced oxidative stress [304]. α-Lipoic acid protects mitochondria by up-regulating GSH levels, maintaining mitochondrial membrane potentials and inhibiting ROS production in different cell models for PD [305,306,307]. Silibinin, a major constituent of milk thistle seeds, maintains function and integrity of mitochondria and inhibits apoptosis in MPP+-treated rats [308]. The herbal medicine chunghyuldan inhibits ROS production and apoptosis, as well as maintains mitochondrial membrane potential, in 6-OHDA-treated PC12 cells [309]. Hesperidin, a flavanone rich in citrus, maintains mitochondrial membrane potential and inhibits ROS production and apoptosis in rotenone-treated SK-N-SH cells [310]. The flavonoid baicalein maintains mitochondrial integrity and ATP production, as well as reduces apoptosis, in 6-OHDA-treated SH-SY5Y and rotenone-treated PC12 cells [311,312,313]. In catecholaminergic neurons, tyrosol in olive oil protects cells against MPP+-induced toxicity and apoptosis by improving ATP production and maintaining mitochondria membrane potential [314]. Curcumin from the spice turmeric reduces ROS production and keeps mitochondrial integrity and function, as well as inhibits apoptosis, in PC12 cells overexpressing α-synuclein [315]. In MES23.5 DAergic cells, rosmarinic acid in Perilla frutescens exerts neuroprotection against 6-OHDA-induced toxicity by decreasing ROS production and maintaining mitochondrial membrane potential [316]. The marine compound xyloketal B attenuates MPP+-induced ROS production and reduction of mitochondria membrane potential in PC12 cells and Caenorhabditis elegans [317]. Moreover, the carotenoid lycopene reduces ROS production and mitochondrial damages, as well as improves ATP production, in MPP+-treated SH-SY5Y cells and rotenone-treated rats [318,319]. More clinical studies are essential to validate the neuroprotective effects of these compounds.
6. Conclusion Remarks
The pathogenesis of PD remains elusive. Lines of evidence suggest accumulation of α-synuclein, DA, iron and calcium depositions, mitochondrial dysfunction and neuroinflammation generate overwhelming oxidative stress in SN of PD patients. Deciphering the functional properties of mutations in SNCA, PARKIN, PINK1, LRRK2, DJ-1, FBXO7 and ATP13A2 further indicates that mitochondrial dysfunction and oxidative stress play crucial roles in PD neurodegeneration. Various biomarkers of oxidative stress, such as DJ-1, CoQ10, uric acid, 8OHdG, homocysteine, retinoic acid, vitamin E and products of lipid peroxidation are aiming to improve the early diagnosis of PD, predict its progression and monitor the therapeutic efficacy. Although numerous studies in cell and animal models support the potential of antioxidants in treating PD, many of these results cannot be reproduced in clinical trials. Novel drug delivery approaches by chemical modifications [320,321], liposomes [322,323], nanoparticles [324,325] and nanoemulsions [326,327] would be helpful to efficiently deliver antioxidants to SN and other affected brain regions of PD patients. It is also important to develop useful biomarkers as surrogate endpoints for clinical trials and to identify very early PD patients, particularly in the pre-motor stage. Carrying out robust clinical trials in large populations with application of objective biomarkers and assessment of confounding factors carefully will be essential to consolidate the therapeutic potentials of antioxidants for PD.
Author Contributions
Writing—original draft preparation, K.-H.C. and C.-M.C.; writing—review and editing, K.-H.C. and C.-M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by CMRPG3H147 and CMRPG3J127 from Chang Gung Memorial Hospital, Taoyuan, Taiwan.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Lozano, A.M. Parkinson’s disease. First of two parts. N. Engl. J. Med. 1998, 339, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Blauwendraat, C.; Nalls, M.A.; Singleton, A.B. The genetic architecture of Parkinson’s disease. Lancet Neurol. 2020, 19, 170–178. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Dunbar, G.L. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef]
- Thanan, R.; Oikawa, S.; Hiraku, Y.; Ohnishi, S.; Ma, N.; Pinlaor, S.; Yongvanit, P.; Kawanishi, S.; Murata, M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2014, 16, 193–217. [Google Scholar] [CrossRef]
- Valera-Alberni, M.; Canto, C. Mitochondrial stress management: A dynamic journey. Cell Stress 2018, 2, 253–274. [Google Scholar] [CrossRef]
- Monzani, E.; Nicolis, S.; Dell’Acqua, S.; Capucciati, A.; Bacchella, C.; Zucca, F.A.; Mosharov, E.V.; Sulzer, D.; Zecca, L.; Casella, L. Dopamine, Oxidative stress and protein-quinone modifications in Parkinson’s and other neurodegenerative diseases. Angew. Chem. Int. Ed. Engl. 2019, 58, 6512–6527. [Google Scholar] [CrossRef]
- Segura-Aguilar, J.; Paris, I. Mechanisms of dopamine oxidation and Parkinson’s disease. In Handbook of Neurotoxicity; Kostrzewa, R.M., Ed.; Springer New York: New York, NY, USA, 2014; pp. 865–883. [Google Scholar] [CrossRef]
- Graves, S.M.; Xie, Z.; Stout, K.A.; Zampese, E.; Burbulla, L.F.; Shih, J.C.; Kondapalli, J.; Patriarchi, T.; Tian, L.; Brichta, L.; et al. Dopamine metabolism by a monoamine oxidase mitochondrial shuttle activates the electron transport chain. Nat. Neurosci. 2020, 23, 15–20. [Google Scholar] [CrossRef]
- Kumar, M.J.; Andersen, J.K. Perspectives on MAO-B in aging and neurological disease: Where do we go from here? Mol. Neurobiol. 2004, 30, 77–89. [Google Scholar] [CrossRef]
- Nagatsu, T.; Sawada, M. Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson’s disease: Possible implications of glial cells. J. Neural. Transm. Suppl. 2006. [Google Scholar] [CrossRef]
- Mallajosyula, J.K.; Kaur, D.; Chinta, S.J.; Rajagopalan, S.; Rane, A.; Nicholls, D.G.; Di Monte, D.A.; Macarthur, H.; Andersen, J.K. MAO-B elevation in mouse brain astrocytes results in Parkinson’s pathology. PLoS ONE 2008, 3, e1616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, R.; Wang, G. Impact of dopamine oxidation on dopaminergic neurodegeneration. ACS Chem. Neurosci. 2019, 10, 945–953. [Google Scholar] [CrossRef]
- Uhl, G.R.; Li, S.; Takahashi, N.; Itokawa, K.; Lin, Z.; Hazama, M.; Sora, I. The VMAT2 gene in mice and humans: Amphetamine responses, locomotion, cardiac arrhythmias, aging, and vulnerability to dopaminergic toxins. FASEB J. 2000, 14, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- McHugh, P.C.; Buckley, D.A. The structure and function of the dopamine transporter and its role in CNS diseases. Vitam. Horm. 2015, 98, 339–369. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.G. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol. 1978, 14, 633–643. [Google Scholar]
- Hastings, T.G. Enzymatic oxidation of dopamine: The role of prostaglandin H synthase. J. Neurochem. 1995, 64, 919–924. [Google Scholar] [CrossRef]
- Rcom-H’cheo-Gauthier, A.; Goodwin, J.; Pountney, D.L. Interactions between calcium and alpha-synuclein in neurodegeneration. Biomolecules 2014, 4, 795–811. [Google Scholar] [CrossRef]
- Luk, B.; Mohammed, M.; Liu, F.; Lee, F.J. A physical interaction between the dopamine transporter and DJ-1 facilitates increased dopamine reuptake. PLoS ONE 2015, 10, e0136641. [Google Scholar] [CrossRef]
- Mochizuki, H.; Choong, C.J.; Baba, K. Parkinson’s disease and iron. J. Neural. Transm. 2020, 127, 181–187. [Google Scholar] [CrossRef]
- Nagatsu, T.; Nagatsu, I. Tyrosine hydroxylase (TH), its cofactor tetrahydrobiopterin (BH4), other catecholamine-related enzymes, and their human genes in relation to the drug and gene therapies of Parkinson’s disease (PD): Historical overview and future prospects. J. Neural. Transm. 2016, 123, 1255–1278. [Google Scholar] [CrossRef]
- Bresgen, N.; Eckl, P.M. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules 2015, 5, 808–847. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Peng, J.; Chinta, S.J.; Rajagopalan, S.; Di Monte, D.A.; Cherny, R.A.; Andersen, J.K. Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiol. Aging 2007, 28, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Sziraki, I.; Mohanakumar, K.P.; Rauhala, P.; Kim, H.G.; Yeh, K.J.; Chiueh, C.C. Manganese: A transition metal protects nigrostriatal neurons from oxidative stress in the iron-induced animal model of parkinsonism. Neuroscience 1998, 85, 1101–1111. [Google Scholar] [CrossRef]
- Lan, J.; Jiang, D.H. Desferrioxamine and vitamin E protect against iron and MPTP-induced neurodegeneration in mice. J. Neural. Transm. 1997, 104, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C. Calcium signaling and reactive oxygen species in mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef]
- Dryanovski, D.I.; Guzman, J.N.; Xie, Z.; Galteri, D.J.; Volpicelli-Daley, L.A.; Lee, V.M.; Miller, R.J.; Schumacker, P.T.; Surmeier, D.J. Calcium entry and alpha-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J. Neurosci. 2013, 33, 10154–10164. [Google Scholar] [CrossRef]
- Hurley, M.J.; Brandon, B.; Gentleman, S.M.; Dexter, D.T. Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins. Brain 2013, 136, 2077–2097. [Google Scholar] [CrossRef]
- Ilijic, E.; Guzman, J.N.; Surmeier, D.J. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol. Dis. 2011, 43, 364–371. [Google Scholar] [CrossRef]
- Lautenschlager, J.; Stephens, A.D.; Fusco, G.; Strohl, F.; Curry, N.; Zacharopoulou, M.; Michel, C.H.; Laine, R.; Nespovitaya, N.; Fantham, M.; et al. C-terminal calcium binding of alpha-synuclein modulates synaptic vesicle interaction. Nat. Commun. 2018, 9, 712. [Google Scholar] [CrossRef]
- Wang, Q.M.; Xu, Y.Y.; Liu, S.; Ma, Z.G. Isradipine attenuates MPTP-induced dopamine neuron degeneration by inhibiting up-regulation of L-type calcium channels and iron accumulation in the substantia nigra of mice. Oncotarget 2017, 8, 47284–47295. [Google Scholar] [CrossRef]
- Kausar, S.; Wang, F.; Cui, H. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef]
- Hang, L.; Thundyil, J.; Lim, K.L. Mitochondrial dysfunction and Parkinson disease: A Parkin-AMPK alliance in neuroprotection. Ann. N. Y. Acad. Sci. 2015, 1350, 37–47. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Przedborski, S. Targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 365–375. [Google Scholar] [CrossRef]
- Schapira, A.H.; Cooper, J.M.; Dexter, D.; Jenner, P.; Clark, J.B.; Marsden, C.D. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1989, 1, 1269. [Google Scholar] [CrossRef]
- Shults, C.W.; Haas, R.H.; Passov, D.; Beal, M.F. Coenzyme Q10 levels correlate with the activities of complexes I and II/III in mitochondria from parkinsonian and nonparkinsonian subjects. Ann. Neurol. 1997, 42, 261–264. [Google Scholar] [CrossRef]
- Keeney, P.M.; Xie, J.; Capaldi, R.A.; Bennett, J.P., Jr. Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J. Neurosci. 2006, 26, 5256–5264. [Google Scholar] [CrossRef]
- Elstner, M.; Morris, C.M.; Heim, K.; Bender, A.; Mehta, D.; Jaros, E.; Klopstock, T.; Meitinger, T.; Turnbull, D.M.; Prokisch, H. Expression analysis of dopaminergic neurons in Parkinson’s disease and aging links transcriptional dysregulation of energy metabolism to cell death. Acta Neuropathol. 2011, 122, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Davis, R.L.; Sue, C.M. Mitochondrial dysfunction in Parkinson’s disease: New mechanistic insights and therapeutic perspectives. Curr. Neurol. Neurosci. Rep. 2018, 18, 21. [Google Scholar] [CrossRef]
- Hassanzadeh, K.; Rahimmi, A. Oxidative stress and neuroinflammation in the story of Parkinson’s disease: Could targeting these pathways write a good ending? J. Cell. Physiol. 2018, 234, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Pei, Z.; Miller, D.S.; Wu, X.; Block, M.L.; Wilson, B.; Zhang, W.; Zhou, Y.; Hong, J.S.; et al. Aggregated alpha-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J. 2005, 19, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Maguire-Zeiss, K.A.; Giuliano, R.; Prifti, L.; Venkatesh, K.; Federoff, H.J. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol. Aging 2008, 29, 1690–1701. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Harada, M.; Narabayashi, H.; Inagaki, H.; Minami, M.; Nagatsu, T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci. Lett. 1996, 211, 13–16. [Google Scholar] [CrossRef]
- Hunot, S.; Dugas, N.; Faucheux, B.; Hartmann, A.; Tardieu, M.; Debre, P.; Agid, Y.; Dugas, B.; Hirsch, E.C. FcepsilonRII/CD23 is expressed in Parkinson’s disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-alpha in glial cells. J. Neurosci. 1999, 19, 3440–3447. [Google Scholar] [CrossRef]
- Gerhard, A.; Pavese, N.; Hotton, G.; Turkheimer, F.; Es, M.; Hammers, A.; Eggert, K.; Oertel, W.; Banati, R.B.; Brooks, D.J. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 2006, 21, 404–412. [Google Scholar] [CrossRef]
- McNaught, K.S.; Mytilineou, C.; Jnobaptiste, R.; Yabut, J.; Shashidharan, P.; Jennert, P.; Olanow, C.W. Impairment of the ubiquitin-proteasome system causes dopaminergic cell death and inclusion body formation in ventral mesencephalic cultures. J. Neurochem. 2002, 81, 301–306. [Google Scholar] [CrossRef]
- McNaught, K.S.; Jenner, P. Altered glial function causes neuronal death and increases neuronal susceptibility to 1-methyl-4-phenylpyridinium- and 6-hydroxydopamine-induced toxicity in astrocytic/ventral mesencephalic co-cultures. J. Neurochem. 1999, 73, 2469–2476. [Google Scholar] [CrossRef]
- Iravani, M.M.; Sadeghian, M.; Leung, C.C.; Jenner, P.; Rose, S. Lipopolysaccharide-induced nigral inflammation leads to increased IL-1beta tissue content and expression of astrocytic glial cell line-derived neurotrophic factor. Neurosci. Lett. 2012, 510, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Iravani, M.M.; Leung, C.C.; Sadeghian, M.; Haddon, C.O.; Rose, S.; Jenner, P. The acute and the long-term effects of nigral lipopolysaccharide administration on dopaminergic dysfunction and glial cell activation. Eur. J. Neurosci. 2005, 22, 317–330. [Google Scholar] [CrossRef]
- Herrera, A.J.; Castano, A.; Venero, J.L.; Cano, J.; Machado, A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol. Dis. 2000, 7, 429–447. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef]
- Qian, L.; Flood, P.M.; Hong, J.S. Neuroinflammation is a key player in Parkinson’s disease and a prime target for therapy. J. Neural. Transm. 2010, 117, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Tamouza, R.; Delord, M.; Krishnamoorthy, R.; Tzourio, C.; Mulot, C.; Nacfer, M.; Lambert, J.C.; Beaune, P.; Laurent-Puig, P.; et al. Association between Parkinson’s disease and the HLA-DRB1 locus. Mov. Disord. 2012, 27, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Wu, Y.R.; Chen, Y.C.; Fung, H.C.; Chen, C.M. Association of genetic variants within HLA-DR region with Parkinson’s disease in Taiwan. Neurobiol. Aging 2020, 87, 140 e113–140 e118. [Google Scholar] [CrossRef]
- Hamza, T.H.; Zabetian, C.P.; Tenesa, A.; Laederach, A.; Montimurro, J.; Yearout, D.; Kay, D.M.; Doheny, K.F.; Paschall, J.; Pugh, E.; et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat. Genet. 2010, 42, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Hill-Burns, E.M.; Factor, S.A.; Zabetian, C.P.; Thomson, G.; Payami, H. Evidence for more than one Parkinson’s disease-associated variant within the HLA region. PLoS ONE 2011, 6, e27109. [Google Scholar] [CrossRef] [PubMed]
- International Parkinson Disease Genomics Consortium; Nalls, M.A.; Plagnol, V.; Hernandez, D.G.; Sharma, M.; Sheerin, U.M.; Saad, M.; Simon-Sanchez, J.; Schulte, C.; Lesage, S.; et al. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet 2011, 377, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Pankratz, N.; Beecham, G.W.; DeStefano, A.L.; Dawson, T.M.; Doheny, K.F.; Factor, S.A.; Hamza, T.H.; Hung, A.Y.; Hyman, B.T.; Ivinson, A.J.; et al. Meta-analysis of Parkinson’s disease: Identification of a novel locus, RIT2. Ann. Neurol. 2012, 71, 370–384. [Google Scholar] [CrossRef]
- Simon-Sanchez, J.; van Hilten, J.J.; van de Warrenburg, B.; Post, B.; Berendse, H.W.; Arepalli, S.; Hernandez, D.G.; de Bie, R.M.; Velseboer, D.; Scheffer, H.; et al. Genome-wide association study confirms extant PD risk loci among the Dutch. Eur. J. Hum. Genet. 2011, 19, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Wissemann, W.T.; Hill-Burns, E.M.; Zabetian, C.P.; Factor, S.A.; Patsopoulos, N.; Hoglund, B.; Holcomb, C.; Donahue, R.J.; Thomson, G.; Erlich, H.; et al. Association of Parkinson disease with structural and regulatory variants in the HLA region. Am. J. Hum. Genet. 2013, 93, 984–993. [Google Scholar] [CrossRef]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Tonnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Nance, M.A. Genetics of Huntington disease. Handb. Clin. Neurol. 2017, 144, 3–14. [Google Scholar] [CrossRef]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A mini review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Mathis, S.; Goizet, C.; Soulages, A.; Vallat, J.M.; Masson, G.L. Genetics of amyotrophic lateral sclerosis: A review. J. Neurol. Sci. 2019, 399, 217–226. [Google Scholar] [CrossRef]
- Prasad, A.; Bharathi, V.; Sivalingam, V.; Girdhar, A.; Patel, B.K. Molecular mechanisms of TDP-43 misfolding and pathology in amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2019, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.J.; Pervaiz, N.; Abbasi, A.A. The Parkinson disease gene SNCA: Evolutionary and structural insights with pathological implication. Sci. Rep. 2016, 6, 24475. [Google Scholar] [CrossRef]
- Vekrellis, K.; Xilouri, M.; Emmanouilidou, E.; Rideout, H.J.; Stefanis, L. Pathological roles of alpha-synuclein in neurological disorders. Lancet Neurol. 2011, 10, 1015–1025. [Google Scholar] [CrossRef]
- Winklhofer, K.F.; Haass, C. Mitochondrial dysfunction in Parkinson’s disease. Biochim. Biophys. Acta 2010, 1802, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Junn, E.; Mouradian, M.M. Human alpha-synuclein over-expression increases intracellular reactive oxygen species levels and susceptibility to dopamine. Neurosci. Lett. 2002, 320, 146–150. [Google Scholar] [CrossRef]
- Ko, L.; Mehta, N.D.; Farrer, M.; Easson, C.; Hussey, J.; Yen, S.; Hardy, J.; Yen, S.H. Sensitization of neuronal cells to oxidative stress with mutated human alpha-synuclein. J. Neurochem. 2000, 75, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Hyun, D.; Halliwell, B.; Jenner, P. Effect of the overexpression of wild-type or mutant alpha-synuclein on cell susceptibility to insult. J. Neurochem. 2001, 76, 998–1009. [Google Scholar] [CrossRef]
- Perfeito, R.; Ribeiro, M.; Rego, A.C. Alpha-synuclein-induced oxidative stress correlates with altered superoxide dismutase and glutathione synthesis in human neuroblastoma SH-SY5Y cells. Arch. Toxicol. 2017, 91, 1245–1259. [Google Scholar] [CrossRef]
- Song, D.D.; Shults, C.W.; Sisk, A.; Rockenstein, E.; Masliah, E. Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp. Neurol. 2004, 186, 158–172. [Google Scholar] [CrossRef]
- Piltonen, M.; Savolainen, M.; Patrikainen, S.; Baekelandt, V.; Myohanen, T.T.; Mannisto, P.T. Comparison of motor performance, brain biochemistry and histology of two A30P alpha-synuclein transgenic mouse strains. Neuroscience 2013, 231, 157–168. [Google Scholar] [CrossRef]
- Nieto, M.; Gil-Bea, F.J.; Dalfo, E.; Cuadrado, M.; Cabodevilla, F.; Sanchez, B.; Catena, S.; Sesma, T.; Ribe, E.; Ferrer, I.; et al. Increased sensitivity to MPTP in human alpha-synuclein A30P transgenic mice. Neurobiol. Aging 2006, 27, 848–856. [Google Scholar] [CrossRef]
- Byers, B.; Cord, B.; Nguyen, H.N.; Schule, B.; Fenno, L.; Lee, P.C.; Deisseroth, K.; Langston, J.W.; Pera, R.R.; Palmer, T.D. SNCA triplication Parkinson’s patient’s iPSC-derived DA neurons accumulate alpha-synuclein and are susceptible to oxidative stress. PLoS ONE 2011, 6, e26159. [Google Scholar] [CrossRef]
- Lotharius, J.; Brundin, P. Pathogenesis of Parkinson’s disease: Dopamine, vesicles and alpha-synuclein. Nat. Rev. Neurosci. 2002, 3, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.H.; Rhim, H.; Kim, S.Y.; Sung, Y.M.; Lee, M.Y.; Choi, J.Y.; Wolozin, B.; Chang, J.S.; Lee, Y.H.; Kwon, T.K.; et al. alpha-Synuclein interacts with phospholipase D isozymes and inhibits pervanadate-induced phospholipase D activation in human embryonic kidney-293 cells. J. Biol. Chem. 2002, 277, 12334–12342. [Google Scholar] [CrossRef]
- Chinta, S.J.; Mallajosyula, J.K.; Rane, A.; Andersen, J.K. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci. Lett. 2010, 486, 235–239. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, C.; Yin, J.; Li, X.; Cheng, F.; Li, Y.; Yang, H.; Ueda, K.; Chan, P.; Yu, S. alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity. Neurosci. Lett. 2009, 454, 187–192. [Google Scholar] [CrossRef]
- Devi, L.; Raghavendran, V.; Prabhu, B.M.; Avadhani, N.G.; Anandatheerthavarada, H.K. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J. Biol. Chem. 2008, 283, 9089–9100. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ho, D.H.; Suk, J.E.; You, S.; Michael, S.; Kang, J.; Joong Lee, S.; Masliah, E.; Hwang, D.; Lee, H.J.; et al. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 2013, 4, 1562. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.P.; Chartrand, K.; Reynolds, A.; Vitvitsky, V.; Banerjee, R.; Gendelman, H.E. Ion channel blockade attenuates aggregated alpha synuclein induction of microglial reactive oxygen species: Relevance for the pathogenesis of Parkinson’s disease. J. Neurochem. 2007, 100, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Klegeris, A.; Pelech, S.; Giasson, B.I.; Maguire, J.; Zhang, H.; McGeer, E.G.; McGeer, P.L. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol. Aging 2008, 29, 739–752. [Google Scholar] [CrossRef]
- Braidy, N.; Gai, W.P.; Xu, Y.H.; Sachdev, P.; Guillemin, G.J.; Jiang, X.M.; Ballard, J.W.; Horan, M.P.; Fang, Z.M.; Chong, B.H.; et al. Alpha-synuclein transmission and mitochondrial toxicity in primary human foetal enteric neurons in vitro. Neurotox. Res. 2014, 25, 170–182. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.M.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J. Exp. Med. 2012, 209, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Bandres-Ciga, S.; Diez-Fairen, M.; Kim, J.J.; Singleton, A.B. Genetics of Parkinson’s disease: An introspection of its journey towards precision medicine. Neurobiol. Dis. 2020, 137, 104782. [Google Scholar] [CrossRef]
- Barodia, S.K.; Creed, R.B.; Goldberg, M.S. Parkin and PINK1 functions in oxidative stress and neurodegeneration. Brain Res. Bull. 2017, 133, 51–59. [Google Scholar] [CrossRef]
- Ge, P.; Dawson, V.L.; Dawson, T.M. PINK1 and Parkin mitochondrial quality control: A source of regional vulnerability in Parkinson’s disease. Mol. Neurodegener. 2020, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Stichel, C.C.; Zhu, X.R.; Bader, V.; Linnartz, B.; Schmidt, S.; Lubbert, H. Mono- and double-mutant mouse models of Parkinson’s disease display severe mitochondrial damage. Hum. Mol. Genet. 2007, 16, 2377–2393. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Lee-Chen, G.J.; Wu, Y.R.; Chen, Y.J.; Lin, J.L.; Li, M.; Chen, I.C.; Lo, Y.S.; Wu, H.C.; Chen, C.M. Impairment of proteasome and anti-oxidative pathways in the induced pluripotent stem cell model for sporadic Parkinson’s disease. Parkinsonism Relat. Disord. 2016, 24, 81–88. [Google Scholar] [CrossRef]
- Wei, P.C.; Lee-Chen, G.J.; Chen, C.M.; Wu, Y.R.; Chen, Y.J.; Lin, J.L.; Lo, Y.S.; Yao, C.F.; Chang, K.H. Neuroprotection of indole-derivative compound NC001-8 by the regulation of the NRF2 pathway in Parkinson’s disease cell models. Oxid. Med. Cell. Longev. 2019, 2019, 5074367. [Google Scholar] [CrossRef]
- LaVoie, M.J.; Ostaszewski, B.L.; Weihofen, A.; Schlossmacher, M.G.; Selkoe, D.J. Dopamine covalently modifies and functionally inactivates parkin. Nat. Med. 2005, 11, 1214–1221. [Google Scholar] [CrossRef]
- Sandebring, A.; Thomas, K.J.; Beilina, A.; van der Brug, M.; Cleland, M.M.; Ahmad, R.; Miller, D.W.; Zambrano, I.; Cowburn, R.F.; Behbahani, H.; et al. Mitochondrial alterations in PINK1 deficient cells are influenced by calcineurin-dependent dephosphorylation of dynamin-related protein 1. PLoS ONE 2009, 4, e5701. [Google Scholar] [CrossRef]
- Lutz, A.K.; Exner, N.; Fett, M.E.; Schlehe, J.S.; Kloos, K.; Lammermann, K.; Brunner, B.; Kurz-Drexler, A.; Vogel, F.; Reichert, A.S.; et al. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J. Biol. Chem. 2009, 284, 22938–22951. [Google Scholar] [CrossRef]
- Cui, M.; Tang, X.; Christian, W.V.; Yoon, Y.; Tieu, K. Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the mitochondrial division inhibitor mdivi-1. J. Biol. Chem. 2010, 285, 11740–11752. [Google Scholar] [CrossRef]
- Dagda, R.K.; Cherra, S.J., 3rd; Kulich, S.M.; Tandon, A.; Park, D.; Chu, C.T. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J. Biol. Chem. 2009, 284, 13843–13855. [Google Scholar] [CrossRef]
- Wood-Kaczmar, A.; Gandhi, S.; Yao, Z.; Abramov, A.Y.; Miljan, E.A.; Keen, G.; Stanyer, L.; Hargreaves, I.; Klupsch, K.; Deas, E.; et al. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE 2008, 3, e2455. [Google Scholar] [CrossRef]
- Morais, V.A.; Verstreken, P.; Roethig, A.; Smet, J.; Snellinx, A.; Vanbrabant, M.; Haddad, D.; Frezza, C.; Mandemakers, W.; Vogt-Weisenhorn, D.; et al. Parkinson’s disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol. Med. 2009, 1, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Gegg, M.E.; Cooper, J.M.; Schapira, A.H.; Taanman, J.W. Silencing of PINK1 expression affects mitochondrial DNA and oxidative phosphorylation in dopaminergic cells. PLoS ONE 2009, 4, e4756. [Google Scholar] [CrossRef] [PubMed]
- Gautier, C.A.; Kitada, T.; Shen, J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc. Natl. Acad. Sci. USA 2008, 105, 11364–11369. [Google Scholar] [CrossRef] [PubMed]
- Cooper, O.; Seo, H.; Andrabi, S.; Guardia-Laguarta, C.; Graziotto, J.; Sundberg, M.; McLean, J.R.; Carrillo-Reid, L.; Xie, Z.; Osborn, T.; et al. Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease. Sci. Transl. Med. 2012, 4, 141ra190. [Google Scholar] [CrossRef]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef]
- Ziviani, E.; Tao, R.N.; Whitworth, A.J. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc. Natl. Acad. Sci. USA 2010, 107, 5018–5023. [Google Scholar] [CrossRef]
- Dolgacheva, L.P.; Berezhnov, A.V.; Fedotova, E.I.; Zinchenko, V.P.; Abramov, A.Y. Role of DJ-1 in the mechanism of pathogenesis of Parkinson’s disease. J. Bioenerg. Biomembr. 2019, 51, 175–188. [Google Scholar] [CrossRef]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Mullett, S.J.; Hinkle, D.A. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol. Dis. 2009, 33, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Mullett, S.J.; Di Maio, R.; Greenamyre, J.T.; Hinkle, D.A. DJ-1 expression modulates astrocyte-mediated protection against neuronal oxidative stress. J. Mol. Neurosci. 2013, 49, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Taira, T.; Saito, Y.; Niki, T.; Iguchi-Ariga, S.M.; Takahashi, K.; Ariga, H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004, 5, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Paterna, J.C.; Leng, A.; Weber, E.; Feldon, J.; Bueler, H. DJ-1 and Parkin modulate dopamine-dependent behavior and inhibit MPTP-induced nigral dopamine neuron loss in mice. Mol. Ther. 2007, 15, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Inden, M.; Taira, T.; Kitamura, Y.; Yanagida, T.; Tsuchiya, D.; Takata, K.; Yanagisawa, D.; Nishimura, K.; Taniguchi, T.; Kiso, Y.; et al. PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson’s disease rat model. Neurobiol. Dis. 2006, 24, 144–158. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, Y.J.; Hwang, I.Y.; Youdim, M.B.; Park, K.S.; Oh, Y.J. Nuclear translocation of DJ-1 during oxidative stress-induced neuronal cell death. Free Radic. Biol. Med. 2012, 53, 936–950. [Google Scholar] [CrossRef]
- Kim, R.H.; Smith, P.D.; Aleyasin, H.; Hayley, S.; Mount, M.P.; Pownall, S.; Wakeham, A.; You-Ten, A.J.; Kalia, S.K.; Horne, P.; et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc. Natl. Acad. Sci. USA 2005, 102, 5215–5220. [Google Scholar] [CrossRef]
- Clements, C.M.; McNally, R.S.; Conti, B.J.; Mak, T.W.; Ting, J.P. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. USA 2006, 103, 15091–15096. [Google Scholar] [CrossRef]
- Ishikawa, S.; Tanaka, Y.; Takahashi-Niki, K.; Niki, T.; Ariga, H.; Iguchi-Ariga, S.M. Stimulation of vesicular monoamine transporter 2 activity by DJ-1 in SH-SY5Y cells. Biochem. Biophys. Res. Commun. 2012, 421, 813–818. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Kazantsev, A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018, 285, 3576–3590. [Google Scholar] [CrossRef]
- Junn, E.; Taniguchi, H.; Jeong, B.S.; Zhao, X.; Ichijo, H.; Mouradian, M.M. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc. Natl. Acad. Sci. USA 2005, 102, 9691–9696. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, S.; Diwakar, L.; Saeed, U.; Agarwal, V.; Ramakrishnan, S.; Iyengar, S.; Ravindranath, V. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson’s disease: Protection by alpha-lipoic acid. FASEB J. 2007, 21, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Waak, J.; Weber, S.S.; Gorner, K.; Schall, C.; Ichijo, H.; Stehle, T.; Kahle, P.J. Oxidizable residues mediating protein stability and cytoprotective interaction of DJ-1 with apoptosis signal-regulating kinase 1. J. Biol. Chem. 2009, 284, 14245–14257. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Vila, M.; Klein, C.; Rascol, O. LRRK2 in Parkinson disease: Challenges of clinical trials. Nat. Rev. Neurol. 2020, 16, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Rui, Q.; Ni, H.; Li, D.; Gao, R.; Chen, G. The Role of LRRK2 in neurodegeneration of Parkinson disease. Curr. Neuropharmacol. 2018, 16, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, C.; Crosio, C.; Vitale, C.; Sanna, G.; Carri, M.T.; Barone, P. Apoptotic mechanisms in mutant LRRK2-mediated cell death. Hum. Mol. Genet. 2007, 16, 1319–1326. [Google Scholar] [CrossRef]
- Wang, X.; Yan, M.H.; Fujioka, H.; Liu, J.; Wilson-Delfosse, A.; Chen, S.G.; Perry, G.; Casadesus, G.; Zhu, X. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum. Mol. Genet. 2012, 21, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.Y.; Park, J.M.; Kim, C.H.; Han, B.S.; Kim, K.S.; Seol, W. LRRK2 enhances oxidative stress-induced neurotoxicity via its kinase activity. Exp. Cell. Res. 2010, 316, 649–656. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Byers, B.; Cord, B.; Shcheglovitov, A.; Byrne, J.; Gujar, P.; Kee, K.; Schule, B.; Dolmetsch, R.E.; Langston, W.; et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 2011, 8, 267–280. [Google Scholar] [CrossRef]
- Niu, J.; Yu, M.; Wang, C.; Xu, Z. Leucine-rich repeat kinase 2 disturbs mitochondrial dynamics via Dynamin-like protein. J. Neurochem. 2012, 122, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Angeles, D.C.; Gan, B.H.; Onstead, L.; Zhao, Y.; Lim, K.L.; Dachsel, J.; Melrose, H.; Farrer, M.; Wszolek, Z.K.; Dickson, D.W.; et al. Mutations in LRRK2 increase phosphorylation of peroxiredoxin 3 exacerbating oxidative stress-induced neuronal death. Hum. Mutat. 2011, 32, 1390–1397. [Google Scholar] [CrossRef]
- Conedera, S.; Apaydin, H.; Li, Y.; Yoshino, H.; Ikeda, A.; Matsushima, T.; Funayama, M.; Nishioka, K.; Hattori, N. FBXO7 mutations in Parkinson’s disease and multiple system atrophy. Neurobiol. Aging 2016, 40, 192.e1–192.e5. [Google Scholar] [CrossRef]
- Joseph, S.; Schulz, J.B.; Stegmuller, J. Mechanistic contributions of FBXO7 to Parkinson disease. J. Neurochem. 2018, 144, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Xie, S.P.; Sathiyamoorthy, S.; Saw, W.T.; Sing, T.Y.; Ng, S.H.; Chua, H.P.; Tang, A.M.; Shaffra, F.; Li, Z.; et al. F-box protein 7 mutations promote protein aggregation in mitochondria and inhibit mitophagy. Hum. Mol. Genet. 2015, 24, 6314–6330. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Sathiyamoorthy, S.; Angeles, D.C.; Tan, E.K. Linking F-box protein 7 and parkin to neuronal degeneration in Parkinson’s disease (PD). Mol. Brain 2016, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Blair, N.F.; Sue, C.M. The role of ATP13A2 in Parkinson’s disease: Clinical phenotypes and molecular mechanisms. Mov. Disord. 2015, 30, 770–779. [Google Scholar] [CrossRef]
- de Tezanos Pinto, F.; Adamo, H.P. The strategic function of the P5-ATPase ATP13A2 in toxic waste disposal. Neurochem. Int. 2018, 112, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Dehay, B.; Ramirez, A.; Martinez-Vicente, M.; Perier, C.; Canron, M.H.; Doudnikoff, E.; Vital, A.; Vila, M.; Klein, C.; Bezard, E. Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc. Natl. Acad. Sci. USA 2012, 109, 9611–9616. [Google Scholar] [CrossRef]
- Holemans, T.; Sorensen, D.M.; van Veen, S.; Martin, S.; Hermans, D.; Kemmer, G.C.; Van den Haute, C.; Baekelandt, V.; Gunther Pomorski, T.; Agostinis, P.; et al. A lipid switch unlocks Parkinson’s disease-associated ATP13A2. Proc. Natl. Acad. Sci. USA 2015, 112, 9040–9045. [Google Scholar] [CrossRef]
- Covy, J.P.; Waxman, E.A.; Giasson, B.I. Characterization of cellular protective effects of ATP13A2/PARK9 expression and alterations resulting from pathogenic mutants. J. Neurosci. Res. 2012, 90, 2306–2316. [Google Scholar] [CrossRef]
- Grunewald, A.; Arns, B.; Seibler, P.; Rakovic, A.; Munchau, A.; Ramirez, A.; Sue, C.M.; Klein, C. ATP13A2 mutations impair mitochondrial function in fibroblasts from patients with Kufor-Rakeb syndrome. Neurobiol. Aging 2012, 33, 1843.e1–1843.e7. [Google Scholar] [CrossRef]
- Park, J.S.; Koentjoro, B.; Veivers, D.; Mackay-Sim, A.; Sue, C.M. Parkinson’s disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction. Hum. Mol. Genet. 2014, 23, 2802–2815. [Google Scholar] [CrossRef] [PubMed]
- Gusdon, A.M.; Zhu, J.; Van Houten, B.; Chu, C.T. ATP13A2 regulates mitochondrial bioenergetics through macroautophagy. Neurobiol. Dis. 2012, 45, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.K.; Eeftens, J.M.; Aerts, M.B.; Esselink, R.A.; Bloem, B.R.; Kuiperij, H.B.; Verbeek, M.M. CSF levels of DJ-1 and tau distinguish MSA patients from PD patients and controls. Parkinsonism Relat. Disord. 2014, 20, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Waragai, M.; Wei, J.; Fujita, M.; Nakai, M.; Ho, G.J.; Masliah, E.; Akatsu, H.; Yamada, T.; Hashimoto, M. Increased level of DJ-1 in the cerebrospinal fluids of sporadic Parkinson’s disease. Biochem. Biophys. Res. Commun. 2006, 345, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Shi, M.; Chung, K.A.; Quinn, J.F.; Peskind, E.R.; Galasko, D.; Jankovic, J.; Zabetian, C.P.; Leverenz, J.B.; Baird, G.; et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain 2010, 133, 713–726. [Google Scholar] [CrossRef]
- Shi, M.; Bradner, J.; Hancock, A.M.; Chung, K.A.; Quinn, J.F.; Peskind, E.R.; Galasko, D.; Jankovic, J.; Zabetian, C.P.; Kim, H.M.; et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann. Neurol. 2011, 69, 570–580. [Google Scholar] [CrossRef]
- Waragai, M.; Sekiyama, K.; Sekigawa, A.; Takamatsu, Y.; Fujita, M.; Hashimoto, M. alpha-Synuclein and DJ-1 as potential biological fluid biomarkers for Parkinson’s Disease. Int. J. Mol. Sci. 2010, 11, 4257–4266. [Google Scholar] [CrossRef]
- Waragai, M.; Nakai, M.; Wei, J.; Fujita, M.; Mizuno, H.; Ho, G.; Masliah, E.; Akatsu, H.; Yokochi, F.; Hashimoto, M. Plasma levels of DJ-1 as a possible marker for progression of sporadic Parkinson’s disease. Neurosci. Lett. 2007, 425, 18–22. [Google Scholar] [CrossRef]
- Maita, C.; Tsuji, S.; Yabe, I.; Hamada, S.; Ogata, A.; Maita, H.; Iguchi-Ariga, S.M.; Sasaki, H.; Ariga, H. Secretion of DJ-1 into the serum of patients with Parkinson’s disease. Neurosci. Lett. 2008, 431, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Devic, I.; Hwang, H.; Edgar, J.S.; Izutsu, K.; Presland, R.; Pan, C.; Goodlett, D.R.; Wang, Y.; Armaly, J.; Tumas, V.; et al. Salivary alpha-synuclein and DJ-1: Potential biomarkers for Parkinson’s disease. Brain 2011, 134, e178. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.M.; Noyce, A.J.; Warner, T.T.; Giovannoni, G.; Proctor, G.B. Elevated salivary protein in Parkinson’s disease and salivary DJ-1 as a potential marker of disease severity. Parkinsonism Relat. Disord. 2015, 21, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Cook, T.J.; Zabetian, C.P.; Leverenz, J.B.; Peskind, E.R.; Hu, S.C.; Cain, K.C.; Pan, C.; Edgar, J.S.; Goodlett, D.R.; et al. DJ-1 isoforms in whole blood as potential biomarkers of Parkinson disease. Sci. Rep. 2012, 2, 954. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Hamakubo, T.; Yoshida, Y.; Ogawa, Y.; Hara, Y.; Fujimura, H.; Imai, Y.; Iwanari, H.; Mochizuki, Y.; Shichiri, M.; et al. Preparation and application of monoclonal antibodies against oxidized DJ-1. Significant elevation of oxidized DJ-1 in erythrocytes of early-stage Parkinson disease patients. Neurosci. Lett. 2009, 465, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Jeong, S.; Lee, S.I.; Seol, W.; Seo, H.; Son, I.; Ho, D.H. Oxidized DJ-1 levels in urine samples as a putative biomarker for Parkinson’s disease. Parkinsons Dis. 2018, 2018, 1241757. [Google Scholar] [CrossRef]
- Sohmiya, M.; Tanaka, M.; Tak, N.W.; Yanagisawa, M.; Tanino, Y.; Suzuki, Y.; Okamoto, K.; Yamamoto, Y. Redox status of plasma coenzyme Q10 indicates elevated systemic oxidative stress in Parkinson’s disease. J. Neurol. Sci. 2004, 223, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Gotz, M.E.; Gerstner, A.; Harth, R.; Dirr, A.; Janetzky, B.; Kuhn, W.; Riederer, P.; Gerlach, M. Altered redox state of platelet coenzyme Q10 in Parkinson’s disease. J. Neural. Transm. 2000, 107, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; LeWitt, P.A.; Xu, K.; Eberly, S.; Watts, A.; Matson, W.R.; Marras, C.; Kieburtz, K.; Rudolph, A.; Bogdanov, M.B.; et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch. Neurol. 2009, 66, 1460–1468. [Google Scholar] [CrossRef]
- Schwarzschild, M.A.; Schwid, S.R.; Marek, K.; Watts, A.; Lang, A.E.; Oakes, D.; Shoulson, I.; Ascherio, A.; Parkinson Study Group, P.I.; Hyson, C.; et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch. Neurol. 2008, 65, 716–723. [Google Scholar] [CrossRef]
- Wang, X.J.; Luo, W.F.; Wang, L.J.; Mao, C.J.; Wang, L.; Liu, C.F. Study on uric acid and the related factors associated with cognition in the patients with Parkinson’s disease. Zhonghua Yi Xue Za Zhi 2009, 89, 1633–1635. [Google Scholar] [CrossRef]
- Gmitterova, K.; Heinemann, U.; Gawinecka, J.; Varges, D.; Ciesielczyk, B.; Valkovic, P.; Benetin, J.; Zerr, I. 8-OHdG in cerebrospinal fluid as a marker of oxidative stress in various neurodegenerative diseases. Neurodegener. Dis. 2009, 6, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Isobe, C.; Abe, T.; Terayama, Y. Levels of reduced and oxidized coenzyme Q-10 and 8-hydroxy-2’-deoxyguanosine in the cerebrospinal fluid of patients with living Parkinson’s disease demonstrate that mitochondrial oxidative damage and/or oxidative DNA damage contributes to the neurodegenerative process. Neurosci. Lett. 2010, 469, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Nakamura, T.; Watanabe, H.; Uchida, K.; Hama, T.; Hara, T.; Niimi, Y.; Ito, M.; Ohno, K.; Sobue, G. Urinary 8-hydroxydeoxyguanosine correlate with hallucinations rather than motor symptoms in Parkinson’s disease. Parkinsonism Relat. Disord. 2011, 17, 46–49. [Google Scholar] [CrossRef]
- Seet, R.C.S.; Lee, C.-Y.J.; Lim, E.C.H.; Tan, J.J.H.; Quek, A.M.L.; Chong, W.-L.; Looi, W.-F.; Huang, S.-H.; Wang, H.; Chan, Y.-H.; et al. Oxidative damage in Parkinson disease: Measurement using accurate biomarkers. Free Radic. Biol. Med. 2010, 48, 560–566. [Google Scholar] [CrossRef]
- Isobe, C.; Murata, T.; Sato, C.; Terayama, Y. Increase of total homocysteine concentration in cerebrospinal fluid in patients with Alzheimer’s disease and Parkinson’s disease. Life Sci. 2005, 77, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, M.C.; Gurol, M.E.; Raju, S.; Diaz-Arrastia, R.; Locascio, J.J.; Tennis, M.; Hyman, B.T.; Growdon, J.H.; Greenberg, S.M.; Bottiglieri, T. Association of homocysteine with plasma amyloid beta protein in aging and neurodegenerative disease. Neurology 2005, 65, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Foy, C.J.; Passmore, A.P.; Vahidassr, M.D.; Young, I.S.; Lawson, J.T. Plasma chain-breaking antioxidants in Alzheimer’s disease, vascular dementia and Parkinson’s disease. QJM 1999, 92, 39–45. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, J.; Shim, E.; Chung, E.J.; Jang, S.H.; Koh, S.B. Association of serum carotenoid, retinol, and tocopherol concentrations with the progression of Parkinson’s Disease. Nutr. Res. Pract. 2017, 11, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Liu, J.L.; Wu, Y.R.; Chen, Y.C.; Cheng, H.S.; Cheng, M.L.; Chiu, D.T. Increased oxidative damage in peripheral blood correlates with severity of Parkinson’s disease. Neurobiol. Dis. 2009, 33, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Battisti, C.; Formichi, P.; Dotti, M.T. Plasma levels of vitamin E in Parkinson’s disease. J. Neural. Transm. Suppl. 1995, 45, 267–270. [Google Scholar] [PubMed]
- Nicoletti, G.; Crescibene, L.; Scornaienchi, M.; Bastone, L.; Bagala, A.; Napoli, I.D.; Caracciolo, M.; Quattrone, A. Plasma levels of vitamin E in Parkinson’s disease. Arch. Gerontol. Geriatr. 2001, 33, 7–12. [Google Scholar] [CrossRef]
- Ahlskog, J.E.; Uitti, R.J.; Low, P.A.; Tyce, G.M.; Nickander, K.K.; Petersen, R.C.; Kokmen, E. No evidence for systemic oxidant stress in Parkinson’s or Alzheimer’s disease. Mov. Disord. 1995, 10, 566–573. [Google Scholar] [CrossRef]
- Fernandez-Calle, P.; Molina, J.A.; Jimenez-Jimenez, F.J.; Vazquez, A.; Pondal, M.; Garcia-Ruiz, P.J.; Urra, D.G.; Domingo, J.; Codoceo, R. Serum levels of alpha-tocopherol (vitamin E) in Parkinson’s disease. Neurology 1992, 42, 1064–1066. [Google Scholar] [CrossRef]
- Abraham, S.; Soundararajan, C.C.; Vivekanandhan, S.; Behari, M. Erythrocyte antioxidant enzymes in Parkinson’s disease. Indian J. Med. Res. 2005, 121, 111–115. [Google Scholar]
- Kilinç, A.; Yalçin, A.S.; Yalçin, D.; Taga, Y.; Emerk, K. Increased erythrocyte susceptibility to lipid peroxidation in human Parkinson’s disease. Neurosci. Lett. 1988, 87, 307–310. [Google Scholar] [CrossRef]
- Poirier, J.; Barbeau, A. Erythrocyte antioxidant activity in human patients with Parkinson’s disease. Neurosci. Lett. 1987, 75, 345–348. [Google Scholar] [CrossRef]
- Gokce Cokal, B.; Yurtdas, M.; Keskin Guler, S.; Gunes, H.N.; Atac Ucar, C.; Aytac, B.; Durak, Z.E.; Yoldas, T.K.; Durak, I.; Cubukcu, H.C. Serum glutathione peroxidase, xanthine oxidase, and superoxide dismutase activities and malondialdehyde levels in patients with Parkinson’s disease. Neurol. Sci. 2017, 38, 425–431. [Google Scholar] [CrossRef]
- Kalra, J.; Rajput, A.H.; Mantha, S.V.; Prasad, K. Serum antioxidant enzyme activity in Parkinson’s disease. Mol. Cell. Biochem. 1992, 110, 165–168. [Google Scholar] [CrossRef]
- Baillet, A.; Chanteperdrix, V.; Trocmé, C.; Casez, P.; Garrel, C.; Besson, G. The role of oxidative stress in amyotrophic lateral sclerosis and Parkinson’s disease. Neurochem. Res. 2010, 35, 1530–1537. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, P.; Kumar, B.; Prabhakar, S.; Gill, K.D. Plasma lipid peroxidation and antioxidant status of Parkinson’s disease patients in the Indian population. Parkinsonism Relat. Disord. 2008, 14, 52–57. [Google Scholar] [CrossRef]
- Petrillo, S.; Schirinzi, T.; Di Lazzaro, G.; D’Amico, J.; Colona, V.L.; Bertini, E.; Pierantozzi, M.; Mari, L.; Mercuri, N.B.; Piemonte, F.; et al. Systemic activation of Nrf2 pathway in Parkinson’s disease. Mov. Disord. 2020, 35, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Selley, M.L. (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson’s disease. Free Radic. Biol. Med. 1998, 25, 169–174. [Google Scholar] [CrossRef]
- de Farias, C.C.; Maes, M.; Bonifácio, K.L.; Bortolasci, C.C.; de Souza Nogueira, A.; Brinholi, F.F.; Matsumoto, A.K.; do Nascimento, M.A.; de Melo, L.B.; Nixdorf, S.L.; et al. Highly specific changes in antioxidant levels and lipid peroxidation in Parkinson’s disease and its progression: Disease and staging biomarkers and new drug targets. Neurosci. Lett. 2016, 617, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, J.; Bandyopadhyay, S.K.; Banerjee, T.K.; Mukherjee, S.C.; Chakraborty, D.P.; Ray, B.C.; Rao, V.R. Plasma levels of lipid peroxides in patients with Parkinson’s disease. Eur. Rev. Med. Pharmacol. Sci. 2009, 13, 129–132. [Google Scholar]
- Molina, J.A.; Jimenez-Jimenez, F.J.; Fernandez-Calle, P.; Lalinde, L.; Tenias, J.M.; Pondal, M.; Vazquez, A.; Codoceo, R. Serum lipid peroxides in patients with Parkinson’s disease. Neurosci. Lett. 1992, 136, 137–140. [Google Scholar] [CrossRef]
- Irizarry, M.C.; Yao, Y.; Hyman, B.T.; Growdon, J.H.; Pratico, D. Plasma F2A isoprostane levels in Alzheimer’s and Parkinson’s disease. Neurodegener. Dis. 2007, 4, 403–405. [Google Scholar] [CrossRef]
- Andican, G.; Konukoglu, D.; Bozluolcay, M.; Bayulkem, K.; Firtiina, S.; Burcak, G. Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson’s disease. Acta Neurol. Belg. 2012, 112, 155–159. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Xu, H.; Luo, X.; Yu, J.; Liu, J.; Chang, R.C. Neuroprotection of Coenzyme Q10 in Neurodegenerative Diseases. Curr. Top. Med. Chem. 2016, 16, 858–866. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J. Adv. Res. 2017, 8, 487–493. [Google Scholar] [CrossRef]
- Cipriani, S.; Desjardins, C.A.; Burdett, T.C.; Xu, Y.; Xu, K.; Schwarzschild, M.A. Urate and its transgenic depletion modulate neuronal vulnerability in a cellular model of Parkinson’s disease. PLoS ONE 2012, 7, e37331. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, S.; Desjardins, C.A.; Burdett, T.C.; Xu, Y.; Xu, K.; Schwarzschild, M.A. Protection of dopaminergic cells by urate requires its accumulation in astrocytes. J. Neurochem. 2012, 123, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Burdett, T.C.; Desjardins, C.A.; Logan, R.; Cipriani, S.; Xu, Y.; Schwarzschild, M.A. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 300–305. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Q.L.; Zhang, N.; Hua, W.Y.; Huang, Y.X.; Di, P.W.; Huang, T.; Xu, X.S.; Liu, C.F.; Hu, L.F.; et al. Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson’s disease: Linking to Akt/GSK3beta signaling pathway. J. Neurochem. 2012, 123, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2’ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Alam, Z.I.; Jenner, A.; Daniel, S.E.; Lees, A.J.; Cairns, N.; Marsden, C.D.; Jenner, P.; Halliwell, B. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997, 69, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.S. Homocysteine metabolism, atherosclerosis, and diseases of aging. Compr. Physiol. 2015, 6, 471–505. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Paul, R.; Giri, A.; Borah, A. Chronic exposure of homocysteine in mice contributes to dopamine loss by enhancing oxidative stress in nigrostriatum and produces behavioral phenotypes of Parkinson’s disease. Biochem. Biophys. Rep. 2016, 6, 47–53. [Google Scholar] [CrossRef]
- Zoccolella, S.; Lamberti, P.; Iliceto, G.; Dell’Aquila, C.; Diroma, C.; Fraddosio, A.; Lamberti, S.V.; Armenise, E.; Defazio, G.; de Mari, M.; et al. Elevated plasma homocysteine levels in L-dopa-treated Parkinson’s disease patients with dyskinesias. Clin. Chem. Lab. Med. 2006, 44, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Espay, A.J.; Zadikoff, C.; Suchowersky, O.; Martin, W.R.; Lafontaine, A.L.; Ranawaya, R.; Camicioli, R.; Lang, A.E. Vitamins and entacapone in levodopa-induced hyperhomocysteinemia: A randomized controlled study. Neurology 2006, 66, 1941–1943. [Google Scholar] [CrossRef] [PubMed]
- Tieu, K.; Zuo, D.M.; Yu, P.H. Differential effects of staurosporine and retinoic acid on the vulnerability of the SH-SY5Y neuroblastoma cells: Involvement of bcl-2 and p53 proteins. J. Neurosci. Res. 1999, 58, 426–435. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Lau, W.K.; Yu, M.S.; Lai, C.S.; Yeung, S.C.; So, K.F.; Chang, R.C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Katsuki, H.; Kurimoto, E.; Takemori, S.; Kurauchi, Y.; Hisatsune, A.; Isohama, Y.; Izumi, Y.; Kume, T.; Shudo, K.; Akaike, A. Retinoic acid receptor stimulation protects midbrain dopaminergic neurons from inflammatory degeneration via BDNF-mediated signaling. J. Neurochem. 2009, 110, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory redox interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2019, 54, 287–293. [Google Scholar] [CrossRef]
- Kostić, D.A.; Dimitrijević, D.S.; Stojanović, G.S.; Palić, I.R.; Đorđević, A.S.; Ickovski, J.D. Xanthine oxidase: Isolation, assays of activity, and inhibition. J. Chem. 2015, 2015, 294858. [Google Scholar] [CrossRef]
- Jakel, R.J.; Townsend, J.A.; Kraft, A.D.; Johnson, J.A. Nrf2-mediated protection against 6-hydroxydopamine. Brain Res. 2007, 1144, 192–201. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Ulusoy, A.; Innamorato, N.G.; Sahin, G.; Rabano, A.; Kirik, D.; Cuadrado, A. alpha-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson’s disease. Hum. Mol. Genet. 2012, 21, 3173–3192. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Nourooz-Zadeh, J.; Liu, E.H.; Yhlen, B.; Anggard, E.E.; Halliwell, B. F4-isoprostanes as specific marker of docosahexaenoic acid peroxidation in Alzheimer’s disease. J. Neurochem. 1999, 72, 734–740. [Google Scholar] [CrossRef]
- Pratico, D.; V, M.Y.L.; Trojanowski, J.Q.; Rokach, J.; Fitzgerald, G.A. Increased F2-isoprostanes in Alzheimer’s disease: Evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998, 12, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Yoritaka, A.; Hattori, N.; Uchida, K.; Tanaka, M.; Stadtman, E.R.; Mizuno, Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl. Acad. Sci. USA 1996, 93, 2696–2701. [Google Scholar] [CrossRef]
- Dexter, D.T.; Carter, C.J.; Wells, F.R.; Javoy-Agid, F.; Agid, Y.; Lees, A.; Jenner, P.; Marsden, C.D. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J. Neurochem. 1989, 52, 381–389. [Google Scholar] [CrossRef]
- Browne, S.E.; Ferrante, R.J.; Beal, M.F. Oxidative Stress in Huntington’s Disease. Brain Pathol. 1999, 9, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Stoy, N.; Mackay, G.M.; Forrest, C.M.; Christofides, J.; Egerton, M.; Stone, T.W.; Darlington, L.G. Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J. Neurochem. 2005, 93, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Rae, C.D.; Broer, S. Creatine as a booster for human brain function. How might it work? Neurochem. Int. 2015, 89, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.T.; Ferrante, R.J.; Klivenyi, P.; Yang, L.; Klein, A.M.; Mueller, G.; Kaddurah-Daouk, R.; Beal, M.F. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp. Neurol. 1999, 157, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.; Koch, W.; Elstner, M.; Schombacher, Y.; Bender, J.; Moeschl, M.; Gekeler, F.; Muller-Myhsok, B.; Gasser, T.; Tatsch, K.; et al. Creatine supplementation in Parkinson disease: A placebo-controlled randomized pilot trial. Neurology 2006, 67, 1262–1264. [Google Scholar] [CrossRef]
- NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology 2006, 66, 664–671. [Google Scholar] [CrossRef]
- Hass, C.J.; Collins, M.A.; Juncos, J.L. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: A randomized trial. Neurorehabil. Neural. Repair 2007, 21, 107–115. [Google Scholar] [CrossRef]
- Kieburtz, K.; Tilley, B.C.; Elm, J.J.; Babcock, D.; Hauser, R.; Ross, G.W.; Augustine, A.H.; Augustine, E.U.; Aminoff, M.J.; Bodis-Wollner, I.G.; et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: A randomized clinical trial. JAMA 2015, 313, 584–593. [Google Scholar] [CrossRef]
- Iarkov, A.; Barreto, G.E.; Grizzell, J.A.; Echeverria, V. Strategies for the treatment of Parkinson’s disease: Beyond dopamine. Front. Aging Neurosci. 2020, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- de Rijk, M.C.; Breteler, M.M.; den Breeijen, J.H.; Launer, L.J.; Grobbee, D.E.; van der Meche, F.G.; Hofman, A. Dietary antioxidants and Parkinson disease. The Rotterdam Study. Arch. Neurol. 1997, 54, 762–765. [Google Scholar] [CrossRef]
- Logroscino, G.; Marder, K.; Cote, L.; Tang, M.X.; Shea, S.; Mayeux, R. Dietary lipids and antioxidants in Parkinson’s disease: A population-based, case-control study. Ann. Neurol. 1996, 39, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Hernán, M.A.; Chen, H.; Spiegelman, D.; Willett, W.C.; Ascherio, A. Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology 2002, 59, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S. A pilot trial of high-dose alpha-tocopherol and ascorbate in early Parkinson’s disease. Ann. Neurol. 1992, 32, S128–S132. [Google Scholar] [CrossRef]
- Parkinson Study, G. Effects of tocopherol and deprenyl on the progression of disability in early Parkinson’s disease. N. Engl. J. Med. 1993, 328, 176–183. [Google Scholar] [CrossRef]
- Parkinson Study Group. Mortality in DATATOP: A multicenter trial in early Parkinson’s disease. Ann. Neurol. 1998, 43, 318–325. [Google Scholar] [CrossRef]
- Shults, C.W.; Beal, M.F.; Fontaine, D.; Nakano, K.; Haas, R.H. Absorption, tolerability, and effects on mitochondrial activity of oral coenzyme Q10 in parkinsonian patients. Neurology 1998, 50, 793–795. [Google Scholar] [CrossRef]
- Shults, C.W.; Oakes, D.; Kieburtz, K.; Beal, M.F.; Haas, R.; Plumb, S.; Juncos, J.L.; Nutt, J.; Shoulson, I.; Carter, J.; et al. Effects of coenzyme Q10 in early Parkinson disease: Evidence of slowing of the functional decline. Arch. Neurol. 2002, 59, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Horstink, M.W.; van Engelen, B.G. The effect of coenzyme Q10 therapy in Parkinson disease could be symptomatic. Arch. Neurol. 2003, 60, 1170–1172. [Google Scholar] [CrossRef]
- Muller, T.; Buttner, T.; Gholipour, A.F.; Kuhn, W. Coenzyme Q10 supplementation provides mild symptomatic benefit in patients with Parkinson’s disease. Neurosci. Lett. 2003, 341, 201–204. [Google Scholar] [CrossRef]
- The NINDS NET-PD Investigators. A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Neurology 2007, 68, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Parkinson Study Group QE3 Investigators; Beal, M.F.; Oakes, D.; Shoulson, I.; Henchcliffe, C.; Galpern, W.R.; Haas, R.; Juncos, J.L.; Nutt, J.G.; Voss, T.S.; et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: No evidence of benefit. JAMA Neurol. 2014, 71, 543–552. [Google Scholar] [CrossRef]
- Storch, A.; Jost, W.H.; Vieregge, P.; Spiegel, J.; Greulich, W.; Durner, J.; Muller, T.; Kupsch, A.; Henningsen, H.; Oertel, W.H.; et al. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Arch. Neurol. 2007, 64, 938–944. [Google Scholar] [CrossRef]
- Yoritaka, A.; Kawajiri, S.; Yamamoto, Y.; Nakahara, T.; Ando, M.; Hashimoto, K.; Nagase, M.; Saito, Y.; Hattori, N. Randomized, double-blind, placebo-controlled pilot trial of reduced coenzyme Q10 for Parkinson’s disease. Parkinsonism Relat. Disord. 2015, 21, 911–916. [Google Scholar] [CrossRef]
- Smith, R.A.; Murphy, M.P. Mitochondria-targeted antioxidants as therapies. Discov. Med. 2011, 11, 106–114. [Google Scholar]
- Snow, B.J.; Rolfe, F.L.; Lockhart, M.M.; Frampton, C.M.; O’Sullivan, J.D.; Fung, V.; Smith, R.A.; Murphy, M.P.; Taylor, K.M.; Protect Study, G. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov. Disord. 2010, 25, 1670–1674. [Google Scholar] [CrossRef]
- Murphy, M.P.; Smith, R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef]
- Kelso, G.F.; Porteous, C.M.; Coulter, C.V.; Hughes, G.; Porteous, W.K.; Ledgerwood, E.C.; Smith, R.A.; Murphy, M.P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J. Biol. Chem. 2001, 276, 4588–4596. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of mitochondrial biogenesis as a way for active longevity: Interaction between the Nrf2 and PGC-1alpha signaling pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Rius-Perez, S.; Torres-Cuevas, I.; Millan, I.; Ortega, A.L.; Perez, S. PGC-1alpha, inflammation, and oxidative Stress: An integrative view in metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Liao, Z.; Locascio, J.J.; Lesniak, K.A.; Roderick, S.S.; Watt, M.L.; Eklund, A.C.; Zhang-James, Y.; Kim, P.D.; Hauser, M.A.; et al. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson’s disease. Sci. Transl. Med. 2010, 2, 52ra73. [Google Scholar] [CrossRef]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jager, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef]
- Mudo, G.; Makela, J.; Di Liberto, V.; Tselykh, T.V.; Olivieri, M.; Piepponen, P.; Eriksson, O.; Malkia, A.; Bonomo, A.; Kairisalo, M.; et al. Transgenic expression and activation of PGC-1alpha protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell. Mol. Life. Sci. 2012, 69, 1153–1165. [Google Scholar] [CrossRef]
- Rocha-Gonzalez, H.I.; Ambriz-Tututi, M.; Granados-Soto, V. Resveratrol: A natural compound with pharmacological potential in neurodegenerative diseases. CNS Neurosci. Ther. 2008, 14, 234–247. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Lofrumento, D.D.; Nicolardi, G.; Cianciulli, A.; De Nuccio, F.; La Pesa, V.; Carofiglio, V.; Dragone, T.; Calvello, R.; Panaro, M.A. Neuroprotective effects of resveratrol in an MPTP mouse model of Parkinson’s-like disease: Possible role of SOCS-1 in reducing pro-inflammatory responses. Innate Immun. 2014, 20, 249–260. [Google Scholar] [CrossRef]
- Blanchet, J.; Longpre, F.; Bureau, G.; Morissette, M.; DiPaolo, T.; Bronchti, G.; Martinoli, M.G. Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.T.; Ko, M.C.; Chen, B.Y.; Huang, J.C.; Hsieh, C.W.; Lee, M.C.; Chiou, R.Y.; Wung, B.S.; Peng, C.H.; Yang, Y.L. Neuroprotective effects of resveratrol on MPTP-induced neuron loss mediated by free radical scavenging. J. Agric. Food Chem. 2008, 56, 6910–6913. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Wu, Q.; Lu, Y.F.; Gong, Q.H.; Shi, J.S. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur. J. Pharmacol. 2008, 600, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Fu, Q.; Ma, R.; Xiang, J. Protective effect of resveratrol derived from Polygonum cuspidatum and its liposomal form on nigral cells in parkinsonian rats. J. Neurol. Sci. 2011, 304, 29–34. [Google Scholar] [CrossRef]
- Khan, M.M.; Ahmad, A.; Ishrat, T.; Khan, M.B.; Hoda, M.N.; Khuwaja, G.; Raza, S.S.; Khan, A.; Javed, H.; Vaibhav, K.; et al. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson’s disease. Brain Res. 2010, 1328, 139–151. [Google Scholar] [CrossRef]
- Ye, Q.; Ye, L.; Xu, X.; Huang, B.; Zhang, X.; Zhu, Y.; Chen, X. Epigallocatechin-3-gallate suppresses 1-methyl-4-phenyl-pyridine-induced oxidative stress in PC12 cells via the SIRT1/PGC-1alpha signaling pathway. BMC Complement. Altern. Med. 2012, 12, 82. [Google Scholar] [CrossRef]
- Barbiero, J.K.; Santiago, R.M.; Persike, D.S.; da Silva Fernandes, M.J.; Tonin, F.S.; da Cunha, C.; Lucio Boschen, S.; Lima, M.M.; Vital, M.A. Neuroprotective effects of peroxisome proliferator-activated receptor alpha and gamma agonists in model of parkinsonism induced by intranigral 1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine. Behav. Brain Res. 2014, 274, 390–399. [Google Scholar] [CrossRef]
- Ulusoy, G.K.; Celik, T.; Kayir, H.; Gursoy, M.; Isik, A.T.; Uzbay, T.I. Effects of pioglitazone and retinoic acid in a rotenone model of Parkinson’s disease. Brain Res. Bull. 2011, 85, 380–384. [Google Scholar] [CrossRef]
- Wu, H.F.; Kao, L.T.; Shih, J.H.; Kao, H.H.; Chou, Y.C.; Li, I.H.; Kao, S. Pioglitazone use and Parkinson’s disease: A retrospective cohort study in Taiwan. BMJ Open 2018, 8, e023302. [Google Scholar] [CrossRef]
- NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators. Pioglitazone in early Parkinson’s disease: A phase 2, multicentre, double-blind, randomised trial. Lancet Neurol. 2015, 14, 795–803. [Google Scholar] [CrossRef]
- Tan, M.L.; Yoshida, K.; Zhao, P.; Zhang, L.; Nolin, T.D.; Piquette-Miller, M.; Galetin, A.; Huang, S.M. Effect of chronic kidney disease on nonrenal elimination pathways: A systematic assessment of CYP1A2, CYP2C8, CYP2C9, CYP2C19, and OATP. Clin. Pharmacol. Ther. 2018, 103, 854–867. [Google Scholar] [CrossRef]
- Jenner, P. Altered mitochondrial function, iron metabolism and glutathione levels in Parkinson’s disease. Acta Neurol. Scand. Suppl. 1993, 146, 6–13. [Google Scholar]
- Sian, J.; Dexter, D.T.; Lees, A.J.; Daniel, S.; Agid, Y.; Javoy-Agid, F.; Jenner, P.; Marsden, C.D. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994, 36, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Sastre, M.; Bohl, J.R.; de Vos, R.A.; Del Tredici, K. Parkinson’s disease: Lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 2007, 113, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Dexter, D.T.; Sian, J.; Schapira, A.H.; Marsden, C.D. Oxidative stress as a cause of nigral cell death in Parkinson’s disease and incidental Lewy body disease. The Royal Kings and Queens Parkinson’s Disease Research Group. Ann. Neurol. 1992, 32, S82–S87. [Google Scholar] [CrossRef] [PubMed]
- Sechi, G.; Deledda, M.G.; Bua, G.; Satta, W.M.; Deiana, G.A.; Pes, G.M.; Rosati, G. Reduced intravenous glutathione in the treatment of early Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 1996, 20, 1159–1170. [Google Scholar] [CrossRef]
- Hauser, R.A.; Lyons, K.E.; McClain, T.; Carter, S.; Perlmutter, D. Randomized, double-blind, pilot evaluation of intravenous glutathione in Parkinson’s disease. Mov. Disord. 2009, 24, 979–983. [Google Scholar] [CrossRef]
- Mischley, L.K.; Lau, R.C.; Shankland, E.G.; Wilbur, T.K.; Padowski, J.M. Phase IIb study of intranasal glutathione in Parkinson’s disease. J. Parkinsons Dis. 2017, 7, 289–299. [Google Scholar] [CrossRef]
- Wang, P.; Niu, L.; Gao, L.; Li, W.X.; Jia, D.; Wang, X.L.; Gao, G.D. Neuroprotective effect of gypenosides against oxidative injury in the substantia nigra of a mouse model of Parkinson’s disease. J. Int. Med. Res. 2010, 38, 1084–1092. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Abul Khair, S.B.; Ojha, S.; Haque, M.E. Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neurosci. 2016, 17, 58. [Google Scholar] [CrossRef]
- Haleagrahara, N.; Siew, C.J.; Mitra, N.K.; Kumari, M. Neuroprotective effect of bioflavonoid quercetin in 6-hydroxydopamine-induced oxidative stress biomarkers in the rat striatum. Neurosci. Lett. 2011, 500, 139–143. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Li, S.; Pu, X.P. Neuroprotective effect of kaempferol against a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Biol. Pharm. Bull. 2011, 34, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Kiasalari, Z.; Khalili, M.; Baluchnejadmojarad, T.; Roghani, M. Protective effect of oral hesperetin against unilateral striatal 6-hydroxydopamine damage in the rat. Neurochem. Res. 2016, 41, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; Garcia-Yague, A.J.; Scannevin, R.H.; Casarejos, M.J.; Kugler, S.; Rabano, A.; Cuadrado, A. Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in Parkinson’s disease. Antioxid. Redox Signal. 2016, 25, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Casili, G.; Biundo, F.; Crupi, R.; Cordaro, M.; Cuzzocrea, S.; Esposito, E. The neuroprotective effect of dimethyl fumarate in an MPTP-mouse, odel of Parkinson’s disease: Involvement of reactive oxygen species/nuclear factor-kappaB/nuclear transcription factor related to NF-E2. Antioxid. Redox Signal. 2017, 27, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Lv, F.; Xu, W.; Zhang, L.; Jing, P.; Cao, X. Deprenyl prevents MPP(+)-induced oxidative damage in PC12 cells by the upregulation of Nrf2-mediated NQO1 expression through the activation of PI3K/Akt and Erk. Toxicology 2011, 290, 286–294. [Google Scholar] [CrossRef]
- Nakaso, K.; Nakamura, C.; Sato, H.; Imamura, K.; Takeshima, T.; Nakashima, K. Novel cytoprotective mechanism of anti-parkinsonian drug deprenyl: PI3K and Nrf2-derived induction of antioxidative proteins. Biochem. Biophys. Res. Commun. 2006, 339, 915–922. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, K.M.; Kim, S.W.; Hwang, O.; Choi, H.J. Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: Novel cytoprotective mechanism against oxidative damage. Pharmacol. Res. 2008, 57, 325–331. [Google Scholar] [CrossRef]
- Yang, L.; Calingasan, N.Y.; Thomas, B.; Chaturvedi, R.K.; Kiaei, M.; Wille, E.J.; Liby, K.T.; Williams, C.; Royce, D.; Risingsong, R.; et al. Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS ONE 2009, 4, e5757. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Kim, H.G.; Han, E.H.; Jeong, H.G. Metallothionein-III protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a PI3K and ERK/Nrf2-dependent manner. Toxicol. Appl. Pharmacol. 2008, 231, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Lin, C.H.; Wu, Y.R.; Yen, C.Y.; Huang, Y.T.; Lin, J.L.; Lin, C.Y.; Chen, W.L.; Chao, C.Y.; Lee-Chen, G.J.; et al. Lactulose and melibiose inhibit alpha-synuclein aggregation and up-regulate autophagy to reduce neuronal vulnerability. Cells 2020, 9, 1230. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Jeong, H.G. The coffee diterpene kahweol induces heme oxygenase-1 via the PI3K and p38/Nrf2 pathway to protect human dopaminergic neurons from 6-hydroxydopamine-derived oxidative stress. FEBS Lett. 2008, 582, 2655–2662. [Google Scholar] [CrossRef]
- Wruck, C.J.; Claussen, M.; Fuhrmann, G.; Romer, L.; Schulz, A.; Pufe, T.; Waetzig, V.; Peipp, M.; Herdegen, T.; Gotz, M.E. Luteolin protects rat PC12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. J. Neural. Transm. Suppl. 2007. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Jeong, H.G. Ginsenoside Rb1 protects against 6-hydroxydopamine-induced oxidative stress by increasing heme oxygenase-1 expression through an estrogen receptor-related PI3K/Akt/Nrf2-dependent pathway in human dopaminergic cells. Toxicol. Appl. Pharmacol. 2010, 242, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Jing, X.; Ren, D.; Wei, X.; Zhang, X. Eriodictyol protects against H(2)O(2)-induced neuron-like PC12 cell death through activation of Nrf2/ARE signaling pathway. Neurochem. Int. 2012, 61, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, J.; Ju, C.; Yang, D.; Chen, G.; Xu, S.; Zeng, Y.; Yan, X.; Wang, W.; Liu, D.; et al. Licochalcone A prevents the loss of dopaminergic neurons by inhibiting microglial activation in lipopolysaccharide (LPS)-induced Parkinson’s disease models. Int. J. Mol. Sci. 2017, 18, 2043. [Google Scholar] [CrossRef]
- Jiang, G.; Hu, Y.; Liu, L.; Cai, J.; Peng, C.; Li, Q. Gastrodin protects against MPP(+)-induced oxidative stress by up regulates heme oxygenase-1 expression through p38 MAPK/Nrf2 pathway in human dopaminergic cells. Neurochem. Int. 2014, 75, 79–88. [Google Scholar] [CrossRef]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Coto-Montes, A.; Monti, M.G.; Ortiz, G.G.; Reiter, R.J. Melatonin is protective against MPTP-induced striatal and hippocampal lesions. Life Sci. 1996, 60, PL23–PL29. [Google Scholar] [CrossRef]
- Liu, C.L.; Huang, Y.S.; Hosokawa, M.; Miyashita, K.; Hu, M.L. Inhibition of proliferation of a hepatoma cell line by fucoxanthin in relation to cell cycle arrest and enhanced gap junctional intercellular communication. Chem. Biol. Interact. 2009, 182, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Mohanakumar, K.P. Melatonin protects against oxidative stress caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the mouse nigrostriatum. J. Pineal Res. 2004, 36, 25–32. [Google Scholar] [CrossRef]
- Antolin, I.; Mayo, J.C.; Sainz, R.M.; del Brio Mde, L.; Herrera, F.; Martin, V.; Rodriguez, C. Protective effect of melatonin in a chronic experimental model of Parkinson’s disease. Brain Res. 2002, 943, 163–173. [Google Scholar] [CrossRef]
- Dabbeni-Sala, F.; Di Santo, S.; Franceschini, D.; Skaper, S.D.; Giusti, P. Melatonin protects against 6-OHDA-induced neurotoxicity in rats: A role for mitochondrial complex I activity. FASEB J. 2001, 15, 164–170. [Google Scholar] [CrossRef]
- Singhal, N.K.; Srivastava, G.; Patel, D.K.; Jain, S.K.; Singh, M.P. Melatonin or silymarin reduces maneb- and paraquat-induced Parkinson’s disease phenotype in the mouse. J. Pineal Res. 2011, 50, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, D.; Reiter, R.J.; Attia, A.M.; Hara, M.; Burgos, A.; Nistico, G. Potent protective effect of melatonin on in vivo paraquat-induced oxidative damage in rats. Life Sci. 1994, 56, 83–89. [Google Scholar] [CrossRef]
- Saravanan, K.S.; Sindhu, K.M.; Mohanakumar, K.P. Melatonin protects against rotenone-induced oxidative stress in a hemiparkinsonian rat model. J. Pineal Res. 2007, 42, 247–253. [Google Scholar] [CrossRef]
- Medeiros, C.A.; Carvalhedo de Bruin, P.F.; Lopes, L.A.; Magalhaes, M.C.; de Lourdes Seabra, M.; de Bruin, V.M. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson’s disease. A randomized, double blind, placebo-controlled study. J. Neurol. 2007, 254, 459–464. [Google Scholar] [CrossRef]
- Lane, E.A.; Moss, H.B. Pharmacokinetics of melatonin in man: First pass hepatic metabolism. J. Clin. Endocrinol. Metab. 1985, 61, 1214–1216. [Google Scholar] [CrossRef]
- Ben-Shachar, D.; Eshel, G.; Finberg, J.P.; Youdim, M.B. The iron chelator desferrioxamine (Desferal) retards 6-hydroxydopamine-induced degeneration of nigrostriatal dopamine neurons. J. Neurochem. 1991, 56, 1441–1444. [Google Scholar] [CrossRef]
- Ben-Shachar, D.; Eshel, G.; Riederer, P.; Youdim, M.B. Role of iron and iron chelation in dopaminergic-induced neurodegeneration: Implication for Parkinson’s disease. Ann. Neurol. 1992, 32 Suppl, S105–S110. [Google Scholar] [CrossRef]
- Devos, D.; Moreau, C.; Devedjian, J.C.; Kluza, J.; Petrault, M.; Laloux, C.; Jonneaux, A.; Ryckewaert, G.; Garcon, G.; Rouaix, N.; et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid. Redox Signal. 2014, 21, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Meshul, C.K.; Thuillier, P.; Goldberg, N.R.; Reddy, P.H. CART peptide is a potential endogenous antioxidant and preferentially localized in mitochondria. PLoS ONE 2012, 7, e29343. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Li, G.R.; Lu, Y.; Liu, Z.Q.; Chang, M.; Yao, M.; Cheng, W.; Hu, L.S. alpha-lipoic acid protects dopaminergic neurons against MPP+-induced apoptosis by attenuating reactive oxygen species formation. Int. J. Mol. Med. 2013, 32, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jia, H.; Liu, J.; Ao, N.; Yan, B.; Shen, W.; Wang, X.; Li, X.; Luo, C.; Liu, J. Combined R-alpha-lipoic acid and acetyl-L-carnitine exerts efficient preventative effects in a cellular model of Parkinson’s disease. J. Cell. Mol. Med. 2010, 14, 215–225. [Google Scholar] [CrossRef]
- Abdin, A.A.; Sarhan, N.I. Intervention of mitochondrial dysfunction-oxidative stress-dependent apoptosis as a possible neuroprotective mechanism of alpha-lipoic acid against rotenone-induced parkinsonism and L-dopa toxicity. Neurosci. Res. 2011, 71, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Geed, M.; Garabadu, D.; Ahmad, A.; Krishnamurthy, S. Silibinin pretreatment attenuates biochemical and behavioral changes induced by intrastriatal MPP+ injection in rats. Pharmacol. Biochem. Behav. 2014, 117, 92–103. [Google Scholar] [CrossRef]
- Kim, H.G.; Ju, M.S.; Kim, D.H.; Hong, J.; Cho, S.H.; Cho, K.H.; Park, W.; Lee, E.H.; Kim, S.Y.; Oh, M.S. Protective effects of Chunghyuldan against ROS-mediated neuronal cell death in models of Parkinson’s disease. Basic Clin. Pharmacol. Toxicol. 2010, 107, 958–964. [Google Scholar] [CrossRef]
- Tamilselvam, K.; Braidy, N.; Manivasagam, T.; Essa, M.M.; Prasad, N.R.; Karthikeyan, S.; Thenmozhi, A.J.; Selvaraju, S.; Guillemin, G.J. Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 102741. [Google Scholar] [CrossRef]
- Lee, H.J.; Noh, Y.H.; Lee, D.Y.; Kim, Y.S.; Kim, K.Y.; Chung, Y.H.; Lee, W.B.; Kim, S.S. Baicalein attenuates 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. Eur. J. Cell. Biol. 2005, 84, 897–905. [Google Scholar] [CrossRef]
- Li, X.X.; He, G.R.; Mu, X.; Xu, B.; Tian, S.; Yu, X.; Meng, F.R.; Xuan, Z.H.; Du, G.H. Protective effects of baicalein against rotenone-induced neurotoxicity in PC12 cells and isolated rat brain mitochondria. Eur. J. Pharmacol. 2012, 674, 227–233. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yu, H.T.; Pu, X.P.; Du, G.H. Baicalein prevents 6-hydroxydopamine-induced mitochondrial dysfunction in SH-SY5Y cells via inhibition of mitochondrial oxidation and up-regulation of DJ-1 protein expression. Molecules 2013, 18, 14726–14738. [Google Scholar] [CrossRef] [PubMed]
- Dewapriya, P.; Himaya, S.W.; Li, Y.X.; Kim, S.K. Tyrosol exerts a protective effect against dopaminergic neuronal cell death in in vitro model of Parkinson’s disease. Food Chem. 2013, 141, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, Y.; Li, X.; Ross, C.A.; Smith, W.W. Curcumin protects against A53T alpha-synuclein-induced toxicity in a PC12 inducible cell model for Parkinsonism. Pharmacol. Res. 2011, 63, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Jiang, H.; Li, R.; Wang, J.; Song, N.; Xu, H.M.; Xie, J.X. Rosmarinic acid inhibits 6-OHDA-induced neurotoxicity by anti-oxidation in MES23.5 cells. J. Mol. Neurosci. 2009, 39, 220–225. [Google Scholar] [CrossRef]
- Lu, X.L.; Yao, X.L.; Liu, Z.; Zhang, H.; Li, W.; Li, Z.; Wang, G.L.; Pang, J.; Lin, Y.; Xu, Z.; et al. Protective effects of xyloketal B against MPP+-induced neurotoxicity in Caenorhabditis elegans and PC12 cells. Brain Res. 2010, 1332, 110–119. [Google Scholar] [CrossRef]
- Kaur, H.; Chauhan, S.; Sandhir, R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson’s disease. Neurochem. Res. 2011, 36, 1435–1443. [Google Scholar] [CrossRef]
- Yi, F.; He, X.; Wang, D. Lycopene protects against MPP(+)-induced cytotoxicity by maintaining mitochondrial function in SH-SY5Y cells. Neurochem. Res. 2013, 38, 1747–1757. [Google Scholar] [CrossRef]
- Armstrong, J.S.; Whiteman, M.; Rose, P.; Jones, D.P. The coenzyme Q10 analog decylubiquinone inhibits the redox-activated mitochondrial permeability transition: Role of mitochondrial respiratory complex III. J. Biol. Chem. 2003, 278, 49079–49084. [Google Scholar] [CrossRef]
- Youk, H.J.; Lee, E.; Choi, M.K.; Lee, Y.J.; Jun, H.C.; Kim, S.H.; Lee, C.H.; Lim, S.J. Enhanced anticancer efficacy of α-tocopheryl succinate by conjugation with polyethylene glycol. J. Control. Release 2005, 107, 43–52. [Google Scholar] [CrossRef]
- Minko, T.; Stefanov, A.; Pozharov, V. Selected Contribution: Lung hypoxia: Antioxidant and antiapoptotic effects of liposomal α-tocopherol. J. Appl. Physiol. 2002, 93, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.G.; Liang, J.H.; Cai, Y.J. Preparation of vitamin E liposomes by the thin film method and study on its leakage rate. Adv. Mater. Res. 2011, 236-238, 2207–2210. [Google Scholar] [CrossRef]
- Abla, M.J.; Banga, A.K. Formulation of tocopherol nanocarriers and in vitro delivery into human skin. Int. J. Cosmet Sci. 2014, 36, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, N.; Lopez-Garcia, M.; Ambrose, D.; Lee, J.; Annelin, C.; Peterson, T. Development and evaluation of resveratrol, Vitamin E, and epigallocatechin gallate loaded lipid nanoparticles for skin care applications. Eur. J. Pharm. Biopharm. 2017, 117, 286–291. [Google Scholar] [CrossRef]
- Morais Diane, J.M.; Burgess, J. Vitamin E nanoemulsions characterization and analysis. Int. J. Pharm. 2014, 465, 455–463. [Google Scholar] [CrossRef]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Formation and stabilization of nanoemulsion-based vitamin E delivery systems using natural biopolymers: Whey protein isolate and gum arabic. Food Chem. 2015, 188, 256–263. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).