Antioxidant Activity in Supramolecular Carotenoid Complexes Favored by Nonpolar Environment and Disfavored by Hydrogen Bonding
Abstract
1. Introduction
2. Oxidation Potentials of Carotenoids as Functions of Conjugation Length and Electron Donor/Acceptor Substituents
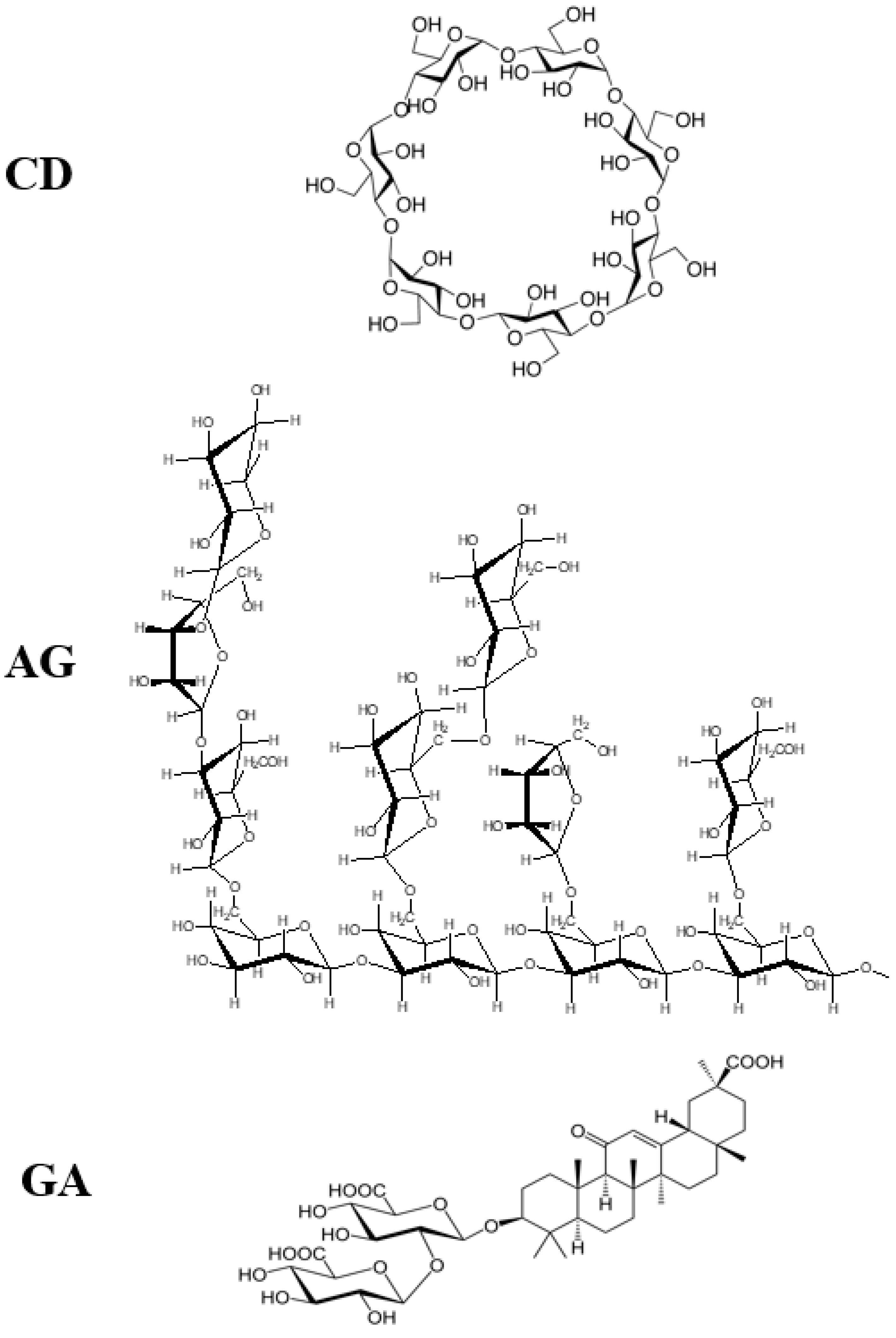
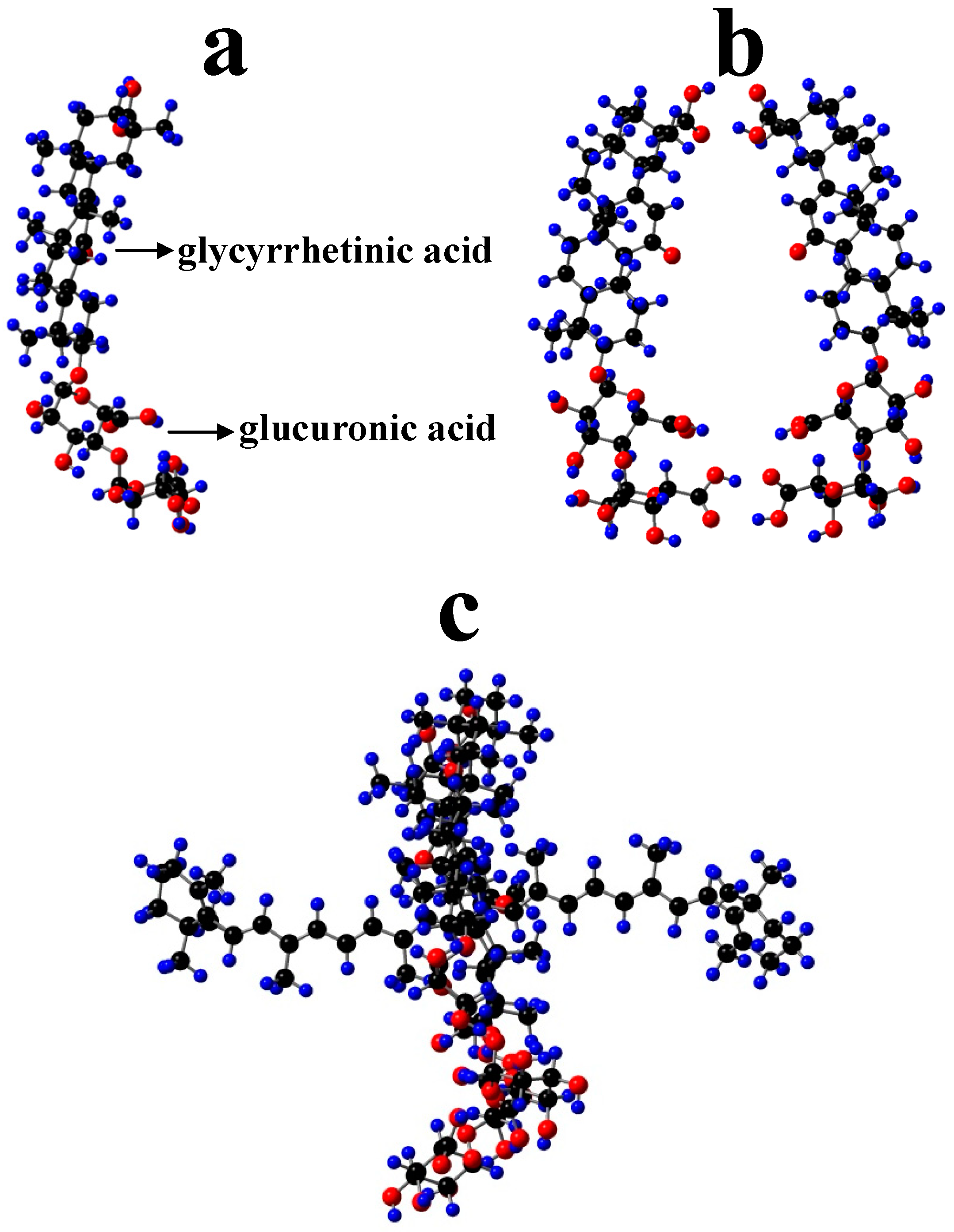
3. Oxidation Potentials of Carotenoids in Polar and Nonpolar Environments
4. Carotenoids Imbedded in Mesoporous Molecular Sieves MCM-41
5. Carotenoids Anchored on TiO2
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parker, R.S. Carotenoids in human blood and tissues. J. Nutr. 1989, 119, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Helzlsouer, K.J.; Alberg, A.J.; Norkus, E.P.; Morris, J.S.; Hoffman, S.C.; Comstock, G.W. Prospective study of serum micronutrients and ovarian cancer. J. Natl. Cancer Inst. 1996, 88, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, K.; Skibsted, L.H. Carotenoid scavenging of radicals. Z. Lebensm. Unters. Forsch. 1993, 196, 423–429. [Google Scholar]
- Polyakov, N.; Leshina, T.; Salakhutdinov, N.; Konovalova, T.; Kispert, L. Antioxidant and redox properties of supramolecular complexes of carotenoids with β-glycyrrhizic acid. Free Radic. Biol. Med. 2006, 40, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidants and human disease: A general introduction. Nutr. Rev. 1997, 55, S44–S49. [Google Scholar] [CrossRef]
- Cross, C.E.; Halliwell, B.; Borish, E.T.; Pryor, W.A.; Ames, B.N.; Saul, R.L.; McCord, J.M.; Harman, D. Oxygen radicals and human disease. Ann. Intern. Med. 1987, 107, 526–545. [Google Scholar] [CrossRef]
- Grollier, J.F.; Cotteret, J.; Rosenbaum, G. Light-Stable Screening Cosmetic Composition Containing Bixin Combined with a Lipid-Soluble UV Filter and its Use for Protecting the Human Epidermis against Ultra-Violet Radiation. U.S. Patent 5,032,382, 16 July 1991. [Google Scholar]
- Hallagan, J.; Allen, D.; Borzelleca, J. The safety and regulatory status of food, drug and cosmetics colour additives exempt from certification. Food Chem. Toxicol. 1995, 33, 515–528. [Google Scholar] [CrossRef]
- Soudant, E.; Koulbanis, C. Cosmetic or Dermatological Composition with Controlled Release of Active Principle Containing a Photoconvertible Carotenoid. U.S. Patent 5,712,311, 27 January 1998. [Google Scholar]
- Burke, M.; Edge, R.; Land, E.J.; McGarveya, D.J.; Truscott, T.G. One-electron reduction potentials of dietary carotenoid radical cations in aqueous micellar environments. FEBS Lett. 2001, 500, 132–136. [Google Scholar] [CrossRef]
- Edge, R.; Land, E.J.; McGarveya, D.J.; Burke, M.; Truscott, T.G. The reduction potential of the β-carotene•+/β-carotene couple in an aqueous micro-heterogeneous environment. FEBS Lett. 2000, 471, 125–127. [Google Scholar] [CrossRef]
- Mairanovsky, V.G.; Engovatov, A.A.; Ioffe, N.T.; Samokhvalov, G.I. Electron-donor and electron-acceptor properties of carotenoids: Electrochemical study of carotenes. J. Electroanal. Chem. Interfacial Electrochem. 1975, 66, 123–137. [Google Scholar] [CrossRef]
- Niedzwiedzki, D.; Rusling, J.F.; Frank, H.A. Voltammetric redox potentials of carotenoids associated with the xanthophyll cycle in photosynthesis. Chem. Phys. Lett. 2005, 415, 308–312. [Google Scholar] [CrossRef]
- Soffers, A.E.M.F.; van Haandel, M.J.H.; Boersma, M.G.; Tyrakowska, B.; Laane, C.; Rietjens, I.M.C.M. Antioxidant activities of carotenoids: Quantitative relationships between theoretical calculations and experimental literature data. Free Radic. Res. 1999, 30, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Edge, R.; Land, E.J.; McGarvey, D.; Mulroy, L.; Truscott, T.J. Relative one-electron reduction potentials of carotenoid radical cations and the interactions of carotenoids with the vitamin E radical cation. J. Am. Chem. Soc. 1998, 120, 4087–4090. [Google Scholar] [CrossRef]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Woodall, A.A.; Lee, S.W.-M.; Weesie, R.J.; Jackson, M.J.; Britton, G. Oxidation of carotenoids by free radicals: Relationship between structure and reactivity. Biochim. Biophys. Acta 1997, 1336, 33–42. [Google Scholar] [CrossRef]
- Kleinová, M.; Hewitt, M.; Brezová, V.; Madden, J.C.; Cronin, M.T.D.; Valko, M. Antioxidant properties of carotenoids: QSAR prediction of their redox potentials. General Physiol. Biophys. 2007, 26, 97–103. [Google Scholar]
- Polyakov, N.E.; Leshina, T.V.; Konovalova, T.A.; Kispert, L.D. Carotenoids as scavengers of free radicals in a Fenton reaction: Antioxidants or pro-oxidants? Free Radic. Biol. Med. 2001, 31, 398–404. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Kruppa, A.I.; Leshina, T.V.; Konovalova, T.A.; Kispert, L.D. Carotenoids as antioxidants: Spin trapping EPR and optical study. Free Radic. Biol. Med. 2001, 31, 43–52. [Google Scholar] [CrossRef]
- Tay-Agbozo, S.; Street, S.; Kispert, L. The carotenoid bixin found to exhibit the highest measured carotenoid oxidation potential to date consistent with its practical protective use in cosmetics, drugs and food. J. Photochem. Photobiol. B Biol. 2018, 186, 1–8. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens Jr, F.L.; Valanis, B.; Williams Jr, J.H. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef]
- Yeh, S.-L.; Hu, M.-L. Antioxidant and pro-oxidant effects of lycopene in comparison with β-carotene on oxidant-induced damage in Hs68 cells. J. Nutr. Biochem. 2000, 11, 548–554. [Google Scholar] [CrossRef]
- Siems, W.; Wiswedel, I.; Salerno, C.; Crifò, C.; Augustin, W.; Schild, L.; Langhans, C.-D.; Sommerburg, O. β-Carotene breakdown products may impair mitochondrial functions—potential side effects of high-dose β-carotene supplementation. J. Nutr. Biochem. 2005, 16, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.E.; Magyar, A.; Kispert, L.D. Photochemical and optical properties of water-soluble xanthophyll antioxidants: Aggregation vs complexation. J. Phys. Chem. B 2013, 117, 10173–10182. [Google Scholar] [CrossRef] [PubMed]
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Supramolecular carotenoid complexes of enhanced solubility and stability-the way of bioavailability improvement. Molecules 2019, 24, 3947. [Google Scholar]
- Yuan, C.; Du, L.; Jin, Z.; Xu, X. Storage stability and antioxidant activity of complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2013, 91, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.E.; Leshina, T.V.; Meteleva, E.S.; Dushkin, A.V.; Konovalova, T.A.; Kispert, L.D. Water soluble complexes of carotenoids with arabinogalactan. J. Phys. Chem. B 2009, 113, 275–282. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Kispert, L.D. Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohydr. Polym. 2015, 128, 207–219. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Leshina, T.V.; Salakhutdinov, N.F.; Kispert, L.D. Host−guest complexes of carotenoids with β-glycyrrhizic acid. J. Phys. Chem. B 2006, 110, 6991–6998. [Google Scholar] [CrossRef]
- Kispert, L.D.; Polyakov, N.E. Carotenoid radicals: Cryptochemistry of natural colorants. Chem. Lett. 2010, 39, 148–155. [Google Scholar] [CrossRef]
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146. [Google Scholar] [CrossRef]
- Katouzian, I.; Esfanjani, A.F.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Xia, Z.; McClements, D.J.; Xiao, H. Influence of lipid content in a corn oil preparation on the bioaccessibility of β-Carotene: A Comparison of low-fat and high-fat samples. J. Food Sci. 2017, 82, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; McClements, D.J.; Cao, Y.; Xiao, H. Chemical and physical stability of astaxanthin-enriched emulsion-based delivery systems. Food Biophys. 2016, 11, 302–310. [Google Scholar] [CrossRef]
- Zhao, C.; Shen, X.; Guo, M. Stability of lutein encapsulated whey protein nano-emulsion during storage. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Gu, K. Nanoliposomes as vehicles for astaxanthin: Characterization, in vitro release evaluation and structure. Molecules 2018, 23, 2822. [Google Scholar] [CrossRef]
- Tan, C.; Xue, J.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Liposome as a delivery system for carotenoids: Comparative antioxidant activity of carotenoids as measured by ferric reducing antioxidant power, DPPH assay and lipid peroxidation. J. Agric. Food Chem. 2014, 62, 6726–6735. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Modulation of the carotenoid bioaccessibility through liposomal encapsulation. Colloids Surf. B Biointerfaces 2014, 123, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.C.; Srivastava, P.; Pandey, P.; Khan, W.; Panda, B.P. Nose to brain delivery of astaxanthin-loaded solid lipid nanoparticles: Fabrication, radio labeling, optimization and biological studies. RSC Adv. 2016, 6, 10001–10010. [Google Scholar] [CrossRef]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Cellular uptake of β-carotene from protein stabilized solid lipid nanoparticles prepared by homogenization–evaporation method. J. Agric. Food Chem. 2014, 62, 1096–1104. [Google Scholar] [CrossRef]
- Rao, M.P.; Manjunath, K.; Bhagawati, S.T.; Thippeswamy, B.S. Bixin loaded solid lipid nanoparticles for enhanced hepatoprotection–preparation, characterisation and in vivo evaluation. Int. J. Pharm. 2014, 473, 485–492. [Google Scholar] [CrossRef]
- Zirak, M.B.; Pezeshki, A. Effect of surfactant concentration on the particle size, stability and potential zeta of beta carotene nano lipid carrier. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 924–932. [Google Scholar]
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int. J. Pharm. 2015, 478, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, V.; Salatti-Dorado, J.Á.; Barzegari, A.; Nicolas-Boluda, A.; Houaoui, A.; Caballo, C.; Caballero-Casero, N.; Sicilia, D.; Bastias Venegas, J.; Pauthe, E. Astaxanthin-loaded nanostructured lipid carriers for preservation of antioxidant activity. Molecules 2018, 23, 2601. [Google Scholar] [CrossRef]
- Focsan, A.L.; Pan, S.; Kispert, L.D. Electrochemical study of astaxanthin and astaxanthin n-octanoic monoester and diester: Tendency to form radicals. J. Phys. Chem. B 2014, 118, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gao, Y.; Kispert, L.D. Electrochemical properties of natural carotenoids. J. Electroanal. Chem. 2000, 488, 140–150. [Google Scholar] [CrossRef]
- Hapiot, P.; Kispert, L.D.; Konovalov, V.V.; Savéant, J.-M. Single two-electron transfers vs successive one-electron transfers in polyconjugated systems illustrated by the electrochemical oxidation and reduction of carotenoids. J. Am. Chem. Soc. 2001, 123, 6669–6677. [Google Scholar] [CrossRef]
- Jeevarajan, J.A.; Jeevarajan, A.S.; Kispert, L.D. Electrochemical, EPR and AM1 studies of acetylenic and ethylenic carotenoids. J. Chem. Soc. Faraday Trans. 1996, 92, 1757–1765. [Google Scholar] [CrossRef]
- Méndez-Hernández, D.D.; Tarakeshwar, P.; Gust, D.; Moore, T.A.; Moore, A.L.; Mujica, V. Simple and accurate correlation of experimental redox potentials and DFT-calculated HOMO/LUMO energies of polycyclic aromatic hydrocarbons. J. Mol. Model. 2013, 19, 2845–2848. [Google Scholar] [CrossRef]
- Gao, Y.; Ligia Focsan, A.; Kispert, L.D. Improved modifications to supramolecular complexes of carotenoids deduced from DFT calculations of carotenoid oxidation potentials in various solvents. Chem. Phys. Lett. 2020, Accepted. [Google Scholar]
- Katritzky, A.R.; Fara, D.C.; Yang, H.; Tämm, K.; Tamm, T.; Karelson, M. Quantitative measures of solvent polarity. Chem. Rev. 2004, 104, 175–198. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Focsan, A.L.; Bowman, M.K.; Shamshina, J.; Krzyaniak, M.D.; Magyar, A.; Polyakov, N.E.; Kispert, L.D. EPR study of the astaxanthin n-octanoic acid monoester and diester radicals on silica–alumina. J. Phys. Chem. B 2012, 116, 13200–13210. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.E.; Leshina, T.V.; Konovalova, T.A.; Hand, E.O.; Kispert, L.D. Inclusion complexes of carotenoids with cyclodextrins: 1HNMR, EPR, and optical studies. Free Radic. Biol. Med. 2004, 36, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Woodall, A.A.; Britton, G.; Jackson, M.J. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: Relationship between carotenoid structure and protective ability. Biochim. Biophys. Acta (BBA) Gen. Subj. 1997, 1336, 575–586. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; Mccullen, S.B.; et al. A new family of mesoporous molecular-sieves prepared with liquid-crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, H.; Li, Y.; Li, L.; Lang, M.; Shi, J. Uniform rattle-type hollow magnetic mesoporous spheres as drug delivery carriers and their sustained-release property. Adv. Funct. Mater. 2008, 18, 2780–2788. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Ramila, A.; Del Real, R.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Horcajada, P.; Ramila, A.; Perez-Pariente, J.; Vallet-Regı, M. Influence of pore size of MCM-41 matrices on drug delivery rate. Microporous Mesoporous Mater. 2004, 68, 105–109. [Google Scholar] [CrossRef]
- Munoz, B.; Ramila, A.; Perez-Pariente, J.; Diaz, I.; Vallet-Regi, M. MCM-41 organic modification as drug delivery rate regulator. Chem. Mater. 2003, 15, 500–503. [Google Scholar] [CrossRef]
- Manzano, M.; Aina, V.; Arean, C.; Balas, F.; Cauda, V.; Colilla, M.; Delgado, M.; Vallet-Regi, M. Studies on MCM-41 mesoporous silica for drug delivery: Effect of particle morphology and amine functionalization. Chem. Eng. J. 2008, 137, 30–37. [Google Scholar] [CrossRef]
- Rámila, A.; Munoz, B.; Pérez-Pariente, J.; Vallet-Regí, M. Mesoporous MCM-41 as drug host system. J. Sol-Gel Sci. Technol. 2003, 26, 1199–1202. [Google Scholar] [CrossRef]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Wang, S. Ordered mesoporous materials for drug delivery. Microporous Mesoporous Mater. 2009, 117, 1–9. [Google Scholar] [CrossRef]
- Doadrio, J.C.; Sousa, E.M.; Izquierdo-Barba, I.; Doadrio, A.L.; Perez-Pariente, J.; Vallet-Regí, M. Functionalization of mesoporous materials with long alkyl chains as a strategy for controlling drug delivery pattern. J. Mater. Chem. 2006, 16, 462–466. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Quan, Z.; Li, C.; Lian, H.; Huang, S.; Lin, J. Fabrication, characterization of spherical CaWO4: Ln@ MCM-41 (Ln = Eu3+, Dy3+, Sm3+, Er3+) composites and their applications as drug release systems. Microporous Mesoporous Mater. 2008, 116, 524–531. [Google Scholar] [CrossRef]
- Gao, Y.L.; Konovalova, T.A.; Xu, T.; Kispert, L.A. Electron transfer of carotenoids imbedded in MCM-41 and Ti-MCM-41: EPR, ENDOR, and UV-Vis studies. J. Phys. Chem. B 2002, 106, 10808–10815. [Google Scholar] [CrossRef]
- Gao, Y.L.; Konovalova, T.A.; Lawrence, J.N.; Smitha, M.A.; Nunley, J.; Schad, R.; Kispert, L.D. Interaction of carotenoids and Cu2+ in Cu-MCM-41: Distance-dependent reversible electron transfer. J. Phys. Chem. B 2003, 107, 2459–2465. [Google Scholar] [CrossRef]
- Gao, Y.L.; Xu, D.Y.; Kispert, L.D. Hydrogen bond formation between the carotenoid canthaxanthin and the silanol group on MCM-41 surface. J. Phys. Chem. B 2015, 119, 10488–10495. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, H.; Tay-Agbozo, S.; Kispert, L.D. Photo-induced electron transfer of carotenoids in mesoporous sieves (MCM-41) and surface modified MCM-41: The role of hydrogen bonds on the electron transfer. J. Photochem. Photobiol. A Chem. 2017, 341, 1–11. [Google Scholar] [CrossRef]
- Konovalova, T.A.; Gao, Y.L.; Schad, R.; Kispert, L.D.; Saylor, C.A.; Brunel, L.C. Photooxidation of carotenoids in mesoporous MCM-41, Ni-MCM-41 and Al-MCM-41 molecular sieves. J. Phys. Chem. B 2001, 105, 7459–7464. [Google Scholar] [CrossRef]
- Konovalova, T.A.; Gao, Y.L.; Kispert, L.D.; van Tol, J.; Brunel, L.C. Characterization of Fe-MCM-41 molecular sieves with incorporated carotenoids by multifrequency electron paramagnetic resonance. J. Phys. Chem. B 2003, 107, 1006–1011. [Google Scholar] [CrossRef]
- Krishna, R.M.; Prakash, A.M.; Kevan, L. Photoionization of N-alkylphenothiazines in mesoporous SiMCM-41, AlMCM-41, and TiMCM-41 molecular sieves. J. Phys. Chem. B 2000, 104, 1796–1801. [Google Scholar] [CrossRef]
- SungSuh, H.M.; Luan, Z.H.; Kevan, L. Photoionization of porphyrins in mesoporous siliceous MCM-41, AlMCM-41, and TiMCM-41 molecular sieves. J. Phys. Chem. B 1997, 101, 10455–10463. [Google Scholar] [CrossRef]
- Kimura, T.; Kuroda, K.; Sugahara, Y.; Kuroda, K. Esterification of the silanol groups in the mesoporous silica derived from kanemite. J. Porous Mater. 1998, 5, 127–132. [Google Scholar] [CrossRef]
- Closs, G.L.; Miller, J.R. Intramolecular Long-Distance Electron-Transfer in Organic-Molecules. Science 1988, 240, 440–447. [Google Scholar] [CrossRef]
- Guarr, T.; Mclendon, G. Quantum-mechanical effects in inorganic and bioinorganic electron-transfer. Coord. Chem. Rev. 1985, 68, 1–52. [Google Scholar] [CrossRef]
- Hush, N.S. Distance dependence of electron-transfer rates. Coord. Chem. Rev. 1985, 64, 135–157. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta 1985, 811, 265–322. [Google Scholar] [CrossRef]
- Frank, S.N.; Bard, A.J. Semiconductor electrodes. 12. Photoassisted oxidations and photoelectrosynthesis at polycrystalline titanium dioxide electrodes. J. Am. Chem. Soc. 1977, 99, 4667–4675. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Song, Y.-Y.; Schmidt-Stein, F.; Bauer, S.; Schmuki, P. Amphiphilic TiO2 nanotube arrays: An actively controllable drug delivery system. J. Am. Chem. Soc. 2009, 131, 4230–4232. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, H.; Wan, L.; Zhao, Q.; Jiang, T.; Wang, B.; Wang, S. Potential application of functional porous TiO2 nanoparticles in light-controlled drug release and targeted drug delivery. Acta Biomater. 2015, 13, 354–363. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.-Y.; Li, H.-Q.; Chen, Z.; Zhao, A.Z.-J.; Wang, Y.; Zhang, K.-Q.; Sun, H.-T.; Al-Deyab, S.S.; Lai, Y.-K. TiO2 nanotube platforms for smart drug delivery: A review. Int. J. Nanomed. 2016, 11, 4819. [Google Scholar]
- Yin, M.; Ju, E.; Chen, Z.; Li, Z.; Ren, J.; Qu, X. Upconverting nanoparticles with a mesoporous TiO2 shell for Near-Infrared-triggered drug delivery and synergistic targeted cancer therapy. Chem. A Eur. J. 2014, 20, 14012–14017. [Google Scholar] [CrossRef]
- Signoretto, M.; Ghedini, E.; Nichele, V.; Pinna, F.; Crocellà, V.; Cerrato, G. Effect of textural properties on the drug delivery behaviour of nanoporous TiO2 matrices. Microporous Mesoporous Mater. 2011, 139, 189–196. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, X.; Gao, Z.; Song, Y.Y.; Schmuki, P. Visible-light-triggered drug release from TiO2 nanotube arrays: A controllable antibacterial platform. Angew. Chem. Int. Ed. 2016, 55, 593–597. [Google Scholar] [CrossRef]
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol. 2011, 6, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Weng, Z.; Liu, X.; Yeung, K.; Chu, P.K. Functionalized TiO2 based nanomaterials for biomedical applications. Adv. Funct. Mater. 2014, 24, 5464–5481. [Google Scholar] [CrossRef]
- von Wilmowsky, C.; Bauer, S.; Lutz, R.; Meisel, M.; Neukam, F.W.; Toyoshima, T.; Schmuki, P.; Nkenke, E.; Schlegel, K.A. In vivo evaluation of anodic TiO2 nanotubes: An experimental study in the pig. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 89, 165–171. [Google Scholar] [CrossRef]
- Jaroenworaluck, A.; Sunsaneeyametha, W.; Kosachan, N.; Stevens, R. Characteristics of silica-coated TiO2 and its UV absorption for sunscreen cosmetic applications. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Films 2006, 38, 473–477. [Google Scholar] [CrossRef]
- Leong, H.; Jang, I.; Hyun, K.S.; Jung, S.K.; Hong, G.H.; Jeong, H.A.; Oh, S.G. Preparation of alpha-bisabolol and phenylethyl resorcinol/TiO2 hybrid composites for potential applications in cosmetics. Int. J. Cosmet. Sci. 2016, 38, 524–534. [Google Scholar] [CrossRef]
- De la Calle, I.; Menta, M.; Klein, M.; Séby, F. Screening of TiO2 and Au nanoparticles in cosmetics and determination of elemental impurities by multiple techniques (DLS, SP-ICP-MS, ICP-MS and ICP-OES). Talanta 2017, 171, 291–306. [Google Scholar] [CrossRef]
- Gao, Y.; Lockart, M.; Kispert, L.D.; Bowman, M.K. Photoinduced charge separation in retinoic acid on TiO2: Comparison of three anchoring modes. J. Phys. Chem. C 2019, 123, 24634–24642. [Google Scholar] [CrossRef]
- Lundqvist, M.J.; Nilsing, M.; Persson, P.; Lunell, S. DFT study of bare and dye-sensitized TiO2 clusters and nanocrystals. Int. J. Quantum Chem. 2006, 106, 3214–3234. [Google Scholar] [CrossRef]
- Gao, Y.; Lockart, M.; Kispert, L.; Bowman, M. Photo-induced charge separation in hydroxycoumarins on TiO2 and F-TiO2. Dalton Trans. 2019, 48. [Google Scholar] [CrossRef]
- Amat, A.; De Angelis, F. Challenges in the simulation of dye-sensitized ZnO solar cells: Quantum confinement, alignment of energy levels and excited state nature at the dye/semiconductor interface. Phys. Chem. Chem. Phys. 2012, 14, 10662–10668. [Google Scholar] [CrossRef][Green Version]
- Persson, P.; Lundqvist, M.J.; Ernstorfer, R.; Goddard, W.; Willig, F. Quantum chemical calculations of the influence of anchor-cum-spacer groups on femtosecond electron transfer times in dye-sensitized semiconductor nanocrystals. J. Chem. Theory Comput. 2006, 2, 441–451. [Google Scholar] [CrossRef]
- Muscat, J.; Newns, D. Chemisorption on metals. Prog. Surf. Sci. 1978, 9, 1–43. [Google Scholar] [CrossRef]
- Marotta, G.; Reddy, M.A.; Singh, S.P.; Islam, A.; Han, L.; De Angelis, F.; Pastore, M.; Chandrasekharam, M. Novel carbazole-phenothiazine dyads for dye-sensitized solar cells: A combined experimental and theoretical study. ACS Appl. Mater. Interfaces 2013, 5, 9635–9647. [Google Scholar] [CrossRef]
- Sokolow, J.D.; Trzop, E.; Chen, Y.; Tang, J.; Allen, L.J.; Crabtree, R.H.; Benedict, J.B.; Coppens, P. Binding modes of carboxylate-and acetylacetonate-linked chromophores to homodisperse polyoxotitanate nanoclusters. J. Am. Chem. Soc. 2012, 134, 11695–11700. [Google Scholar] [CrossRef]
- Katoh, R.; Furube, A. Electron injection efficiency in dye-sensitized solar cells. J. Photochem. Photobiol. C 2014, 20, 1–16. [Google Scholar] [CrossRef]

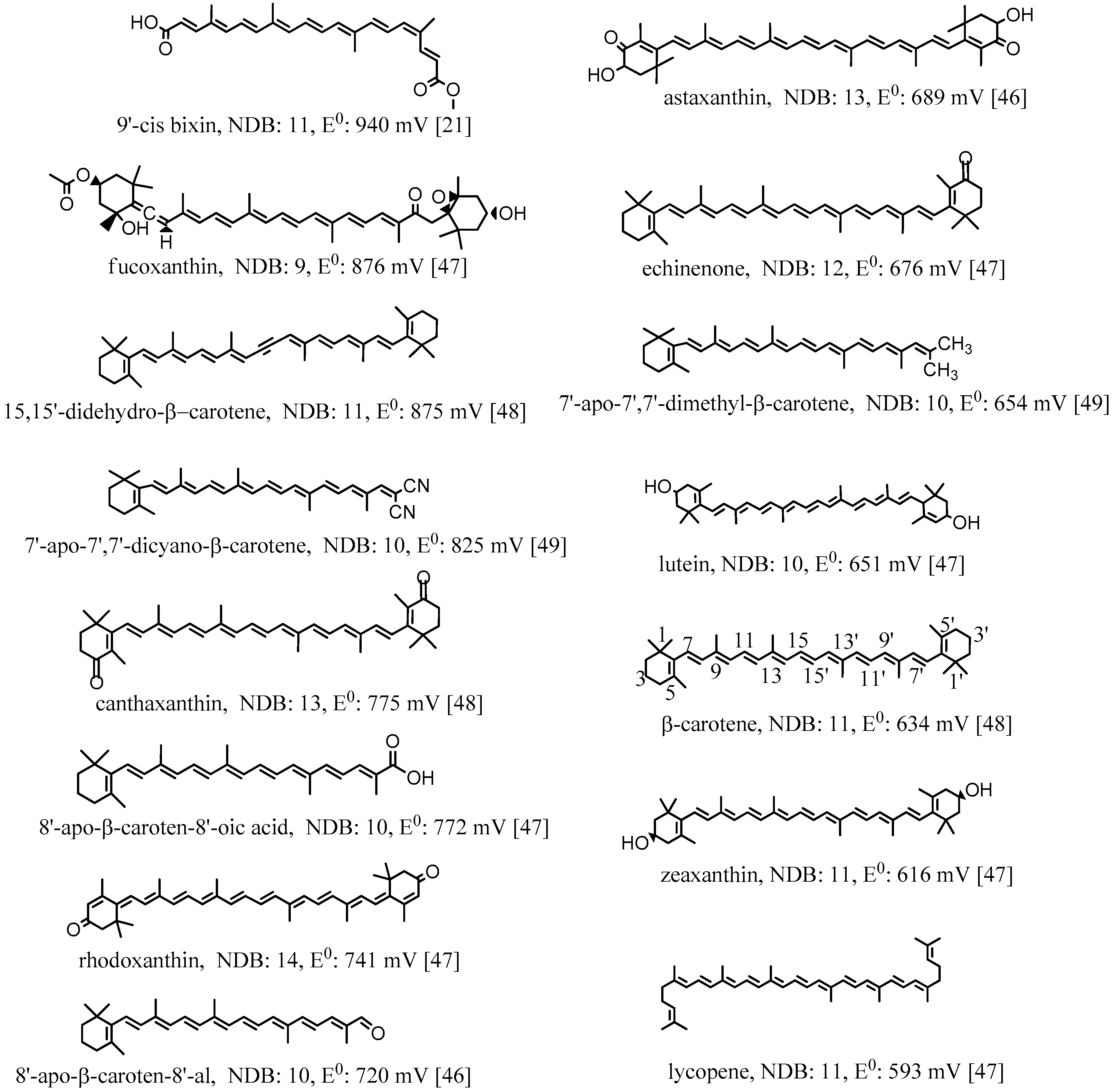
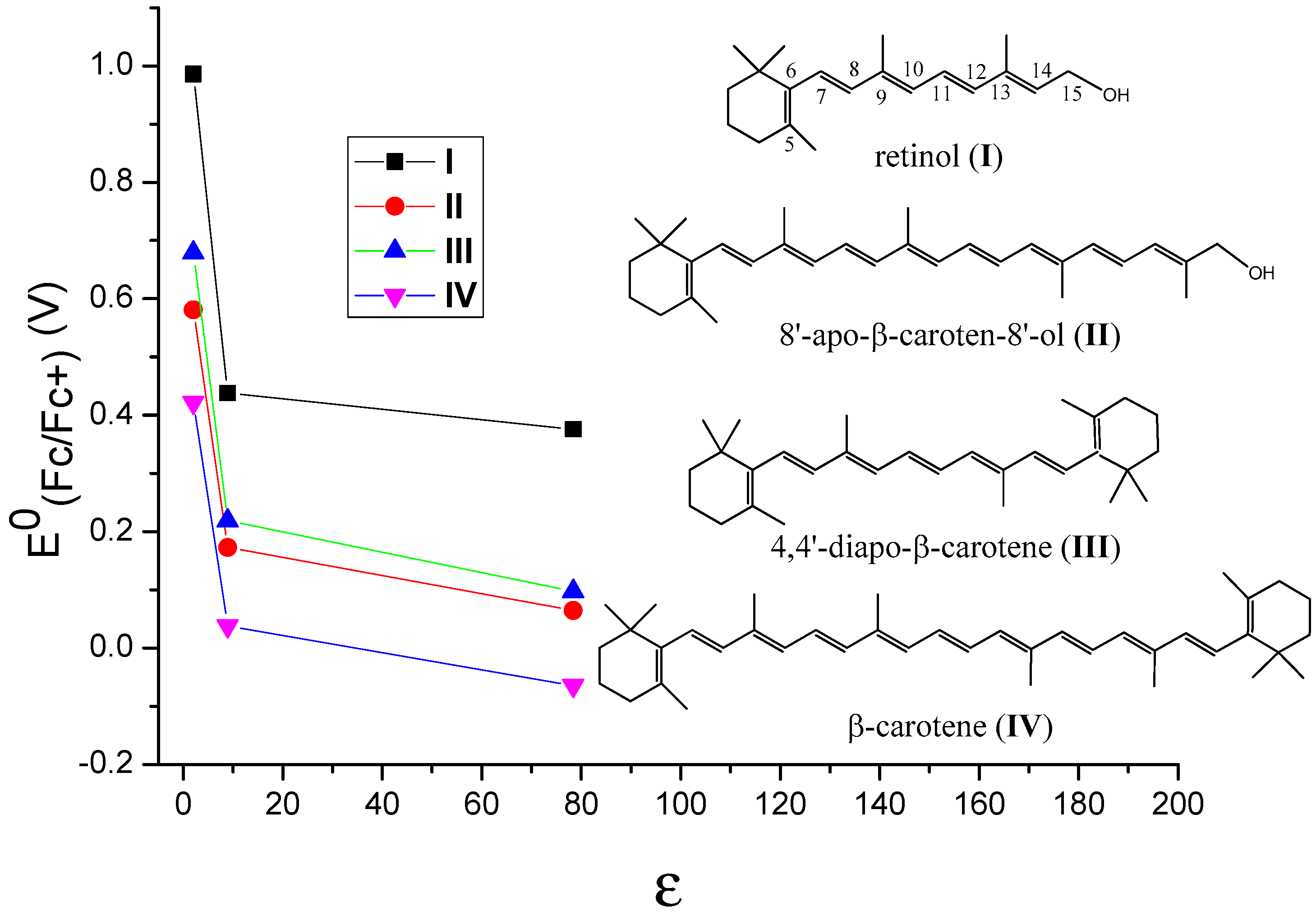

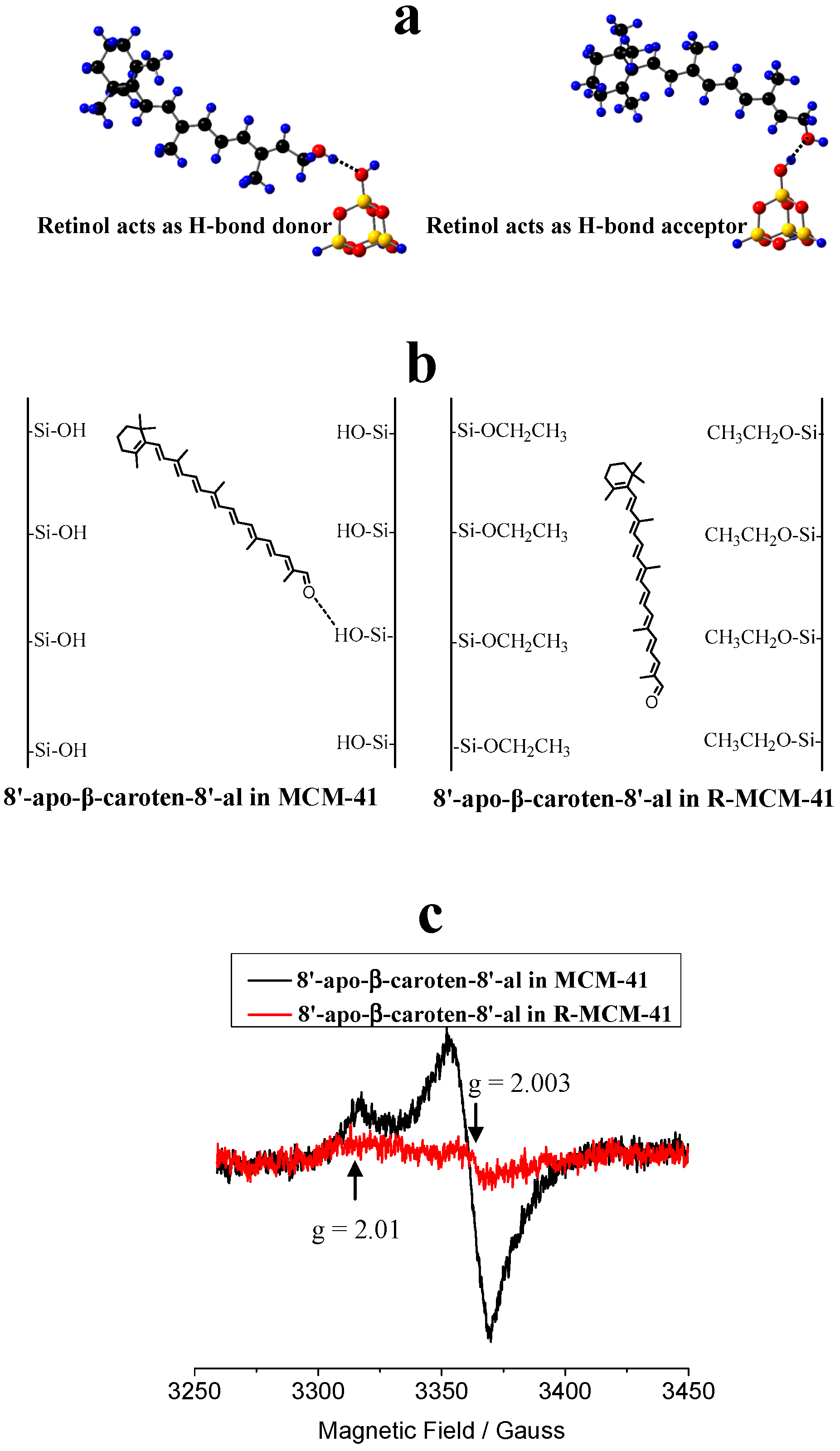
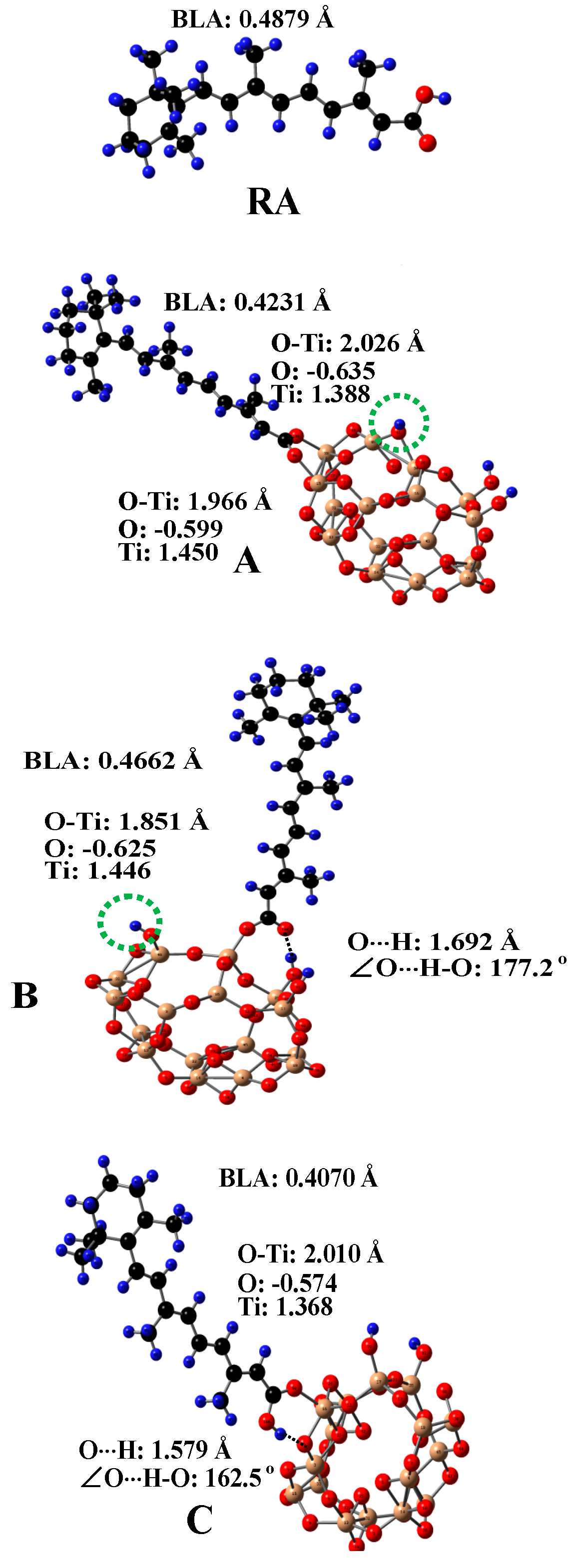
| Car | I | II | III | IV | |
|---|---|---|---|---|---|
| Solvent | |||||
| C6H12 | 0.986 | 0.581 | 0.679 | 0.422 | |
| CH2Cl2 | 0.438 | 0.173 | 0.219 | 0.0376 | |
| H2O | 0.376 | 0.0645 | 0.0975 | −0.0647 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Focsan, A.L.; Kispert, L.D. Antioxidant Activity in Supramolecular Carotenoid Complexes Favored by Nonpolar Environment and Disfavored by Hydrogen Bonding. Antioxidants 2020, 9, 625. https://doi.org/10.3390/antiox9070625
Gao Y, Focsan AL, Kispert LD. Antioxidant Activity in Supramolecular Carotenoid Complexes Favored by Nonpolar Environment and Disfavored by Hydrogen Bonding. Antioxidants. 2020; 9(7):625. https://doi.org/10.3390/antiox9070625
Chicago/Turabian StyleGao, Yunlong, A. Ligia Focsan, and Lowell D. Kispert. 2020. "Antioxidant Activity in Supramolecular Carotenoid Complexes Favored by Nonpolar Environment and Disfavored by Hydrogen Bonding" Antioxidants 9, no. 7: 625. https://doi.org/10.3390/antiox9070625
APA StyleGao, Y., Focsan, A. L., & Kispert, L. D. (2020). Antioxidant Activity in Supramolecular Carotenoid Complexes Favored by Nonpolar Environment and Disfavored by Hydrogen Bonding. Antioxidants, 9(7), 625. https://doi.org/10.3390/antiox9070625






