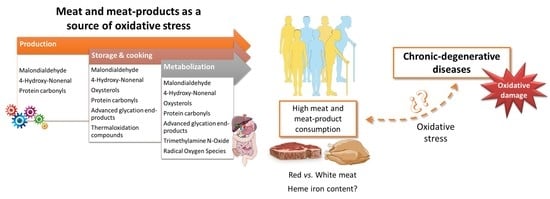
Can Meat and Meat-Products Induce Oxidative Stress?
Abstract
:1. Introduction
2. Meat and Meat-Products
3. Production, Consumption, and Nutritional Importance of Meat and Meat-Products
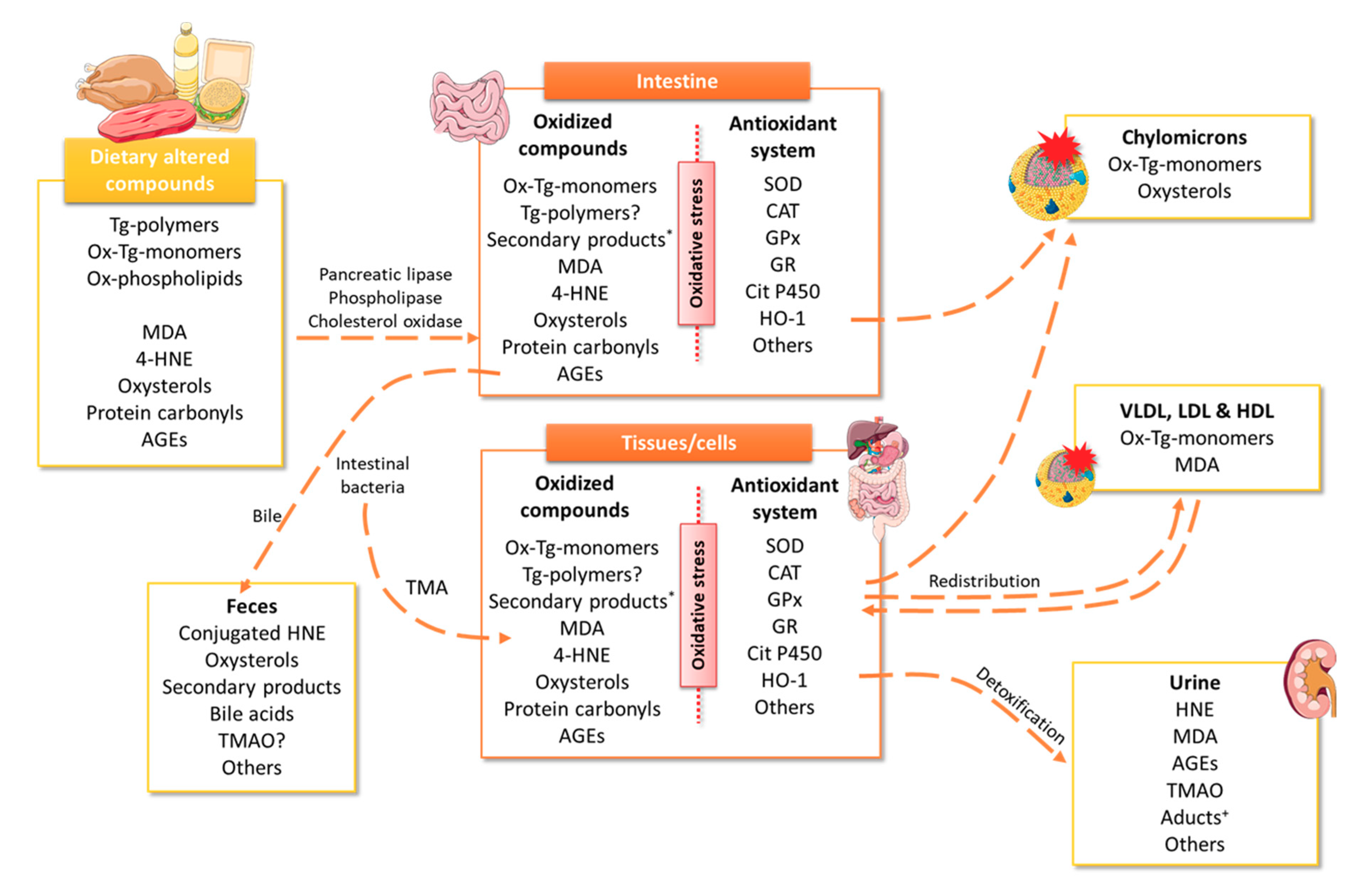
4. Meat Consumption as a Source of Oxidative Stress
4.1. Heme Iron
4.2. Malondialdehyde and Polar Triglycerides
4.3. 4-Hydroxy-Nonenal
4.4. Oxysterols
4.5. Protein Carbonyls
4.6. Advanced Glycation End-Products
4.7. Trimethylamine N-Oxide
5. Practical Implications and Future Works
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of personalized nutrition in chronic-degenerative diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef] [Green Version]
- Celada, P.; Bastida, S.; Sánchez-Muniz, F.J. To eat or not to eat meat. That is the question. Nutr. Hosp. 2016, 33, 177–181. [Google Scholar] [CrossRef] [Green Version]
- Celada, P.; Sánchez-Muniz, F.J. Are meat and meat product consumptions harmful? Their relationship with the risk of colorectal cancer and other degenerative diseases. An. Real Acad. Nac. Farm. 2016, 82, 68–90. [Google Scholar]
- Johnston, B.; Zeraatkar, D.; Han, M.; Vernooij, R.; Valli, C.; Dib, R.; Marshall, C.; Stover, P.; Fairweather-Taitt, S.; Wójcik, G.; et al. Unprocessed red meat and processed meat consumption: Dietary guideline recommendations from the nutritional recommendations (NutriRECS) consortium. Ann. Intern. Med. 2019, 171, 756–764. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.B. The environment and disease: Association or causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef] [Green Version]
- McNeill, S.H. Inclusion of red meat in healthful dietary patterns. Meat Sci. 2014, 98, 452–460. [Google Scholar] [CrossRef]
- Turner, N.D.; Lloyd, S.K. Association between red meat consumption and colon cancer: A systematic review of experimental results. Exp. Biol. Med. 2017, 242, 813–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hecke, T.; Van Camp, J.; De Smet, S. Oxidation during digestion of meat: Interactions with the diet and Helicobacter pylori gastritis, and implications on human health. Compr. Rev. Food Sci. Food Saf. 2017, 16, 214–233. [Google Scholar] [CrossRef] [Green Version]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of oxidative processes in meat and toxicity induced by postprandial degradation products: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 96–123. [Google Scholar] [CrossRef]
- Bastida, S.; Sánchez-Muniz, F.J.; Olivero, R.; Pérez-Olleros, L.; Ruíz-Roso, B.; Jiménez-Colmenero, F. Antioxidant activity of carob fruit extracts in cooked pork meat systems during chilled and frozen storage. Food Chem. 2009, 116, 748–754. [Google Scholar] [CrossRef]
- Kanner, J.; Gorelik, S.; Roman, S.; Kohen, R. Protection by polyphenols of postprandial human plasma and low-density lipoprotein modification: The stomach as a bioreactor. J. Agric. Food Chem. 2012, 60, 8790–8796. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. A novel function of red wine polyphenols in humans: Prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. 2008, 22, 41–46. [Google Scholar] [CrossRef]
- Awada, M.; Soulage, C.O.; Meynier, A.; Debard, C.; Plaisancié, P.; Benoit, B.; Picard, G.; Loizon, E.; Chauvin, M.A.; Estienne, M.; et al. Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: Role of intestinal absorption of 4-HHE and reactivity in intestinal cells. J. Lipid Res. 2012, 53, 2069–2080. [Google Scholar] [CrossRef] [Green Version]
- Kanner, J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef]
- Van Hecke, T.; Jakobsen, L.M.; Vossen, E.; Guéraud, F.; De Vos, F.; Pierre, F.; Bertram, H.C.; De Smet, S. Short-term beef consumption promotes systemic oxidative stress, TMAO formation and inflammation in rats, and dietary fat content modulates these effects. Food Funct. 2016, 7, 3760–3771. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Coltell, O.; Portoles, O.; Sotos-Prieto, M.; Fernandez-Carrion, R.; Ramirez-Sabio, J.B.; Zanon-Moreno, V.; Mattei, J.; Sorli, J.V.; Ordovas, J.M. A guide to applying the sex-gender perspective to nutritional genomics. Nutrients 2019, 11, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.Q.; Smith, C.E.; Parnell, L.D.; Lee, Y.C.; Corella, D.; Hopkins, P.; Hidalgo, B.A.; Aslibekyan, S.; Province, M.A.; Absher, D.; et al. Epigenomics and metabolomics reveal the mechanism of the APOA2-saturated fat intake interaction affecting obesity. Am. J. Clin. Nutr. 2018, 108, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Ramos-López, O.; Cuervo, M.; Goni, L.; Milagro, F.I.; Riezu-Boj, J.I.; Martinez, J.A. Modeling of an integrative prototype based on genetic, phenotypic, and environmental information for personalized prescription of energy-restricted diets in overweight/obese subjects. Am. J. Clin. Nutr. 2020, 111, 459–470. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Sánchez-Muniz, F.J.; Olmedilla-Alonso, B.; Collaborators. Design and development of meat-based functional foods with walnut: Technological, nutritional and health impact. Food Chem. 2010, 123, 959–967. [Google Scholar] [CrossRef] [Green Version]
- Olmedilla-Alonso, B.; Jiménez-Colmenero, F.; Sánchez-Muniz, F.J. Development and assessment of healthy properties of meat and meat products designed as functional foods. Meat Sci. 2013, 95, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Cofrades, S.; Benedí, J.; Garcimartín, A.; Sánchez-Muniz, F.J.; Jiménez-Colmenero, F. A comprehensive approach to formulation of seaweed-enriched meat products: From technological development to assessment of healthy properties. Food Res. Int. 2017, 99, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Codex Committee Residues of Veterinary Drugs in Food. Glossary of Terms and Definitions (Codex Alimentarius). 1993. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/vetdrugs/glossary/en/ (accessed on 1 April 2020).
- Seman, D.; Boler, D.; Carr, C.; Dikeman, M.; Owens, C.; Keeton, J.; Pringle, T.; Sindelar, J.; Woerner, D.; De Mello, A.; et al. Meat Science Lexicon. Meat Muscle Biol. 2018, 2. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Hygiene Rules for Food of Animal Origin. Off. J. Eur. Union 2004, 47, 22–82. [Google Scholar]
- Code of Federal Regulations (CFR) Title 9: Animals and Animal Products; National Archives and Records Administration, Office of the Federal Register: Washington, DC, USA, 2020; Volume 9.
- OECD/FAO. OECD-FAO Agricultural Outlook 2019–2028; OECD Publishing: Rome, Italy, 2019. [Google Scholar] [CrossRef]
- Bernardi, E.; Capri, E.; Pulina, G. The Sustainability of Meat and Cured Meats in Italy: Nutritional Aspect, Food Safety, Environmental Impact, Animal Welfare, Circular Economy, Fight against Waste; FrancoAngeli: Milan, Italy, 2019. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/FBS (accessed on 1 April 2020).
- Chizzolini, R.; Zanardi, E.; Dorigoni, V.; Ghidini, S. Calorific value and cholesterol content of normal and low-fat meat and meat products. Trends Food Sci. Technol. 1999, 10, 119–128. [Google Scholar] [CrossRef]
- Dorado, M.; Gómez, E.M.M.; Jiménez-Colmenero, F.; Masoud, T.A. Cholesterol and fat contents of Spanish commercial pork cuts. Meat Sci. 1999, 51, 321–323. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global and Regional Food Consumption Patterns and Trends. 2003. Available online: https://www.who.int/nutrition/topics/3_foodconsumption/en/ (accessed on 13 April 2020).
- Sharma, S.; Sheehy, T.; Kolonel, L.N. Contribution of meat to vitamin B12, iron and zinc intakes in five ethnic groups in the USA: Implications for developing food-based dietary guidelines. J. Hum. Nutr. Diet. 2013, 26, 156–168. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, W.; Górska-Warsewicz, H.; Kulykovets, O. Meat, meat products and seafood as sources of energy and nutrients in the average polish diet. Nutrients 2018, 10, 1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicklas, T.A.; O’Neil, C.E.; Zanovec, M.; Keast, D.R.; Fulgoni, V.L., 3rd. Contribution of beef consumption to nutrient intake, diet quality, and food patterns in the diets of the US population. Meat Sci. 2012, 90, 152–158. [Google Scholar] [CrossRef]
- Derbyshire, E. Associations between red meat intakes and the micronutrient intake and status of UK females: A secondary analysis of the UK national diet and nutrition survey. Nutrients 2017, 9, 768. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Klurfeld, D.M. Research gaps in evaluating the relationship of meat and health. Meat Sci. 2015, 109, 86–95. [Google Scholar] [CrossRef]
- GBD. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Pando, G. Diseño y Desarrollo de Productos Cárnicos con Perfil Lipídico Optimizado. Evaluación del Efecto Funcional en Humanos. Ph.D. Thesis, Complutense University of Madrid, Madrid, Spain, 2012. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsen, C.U.; Moller, J.K.S.; Skibsted, L.H. Heme-iron in lipid oxidation. Coord. Chem. Rev. 2005, 249, 485–498. [Google Scholar] [CrossRef]
- Fang, X.; An, P.; Wang, H.; Wang, X.; Shen, X.; Li, X.; Min, J.; Liu, S.; Wang, F. Dietary intake of heme iron and risk of cardiovascular disease: A dose-response meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovas. 2015, 25, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, J.; Wolk, A.; Larsson, S.C. Heme iron intake and risk of stroke a prospective study of men. Stroke 2013, 44, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Hori, A.; Mizoue, T.; Kasai, H.; Kawai, K.; Matsushita, Y.; Nanri, A.; Sato, M.; Ohta, M. Body iron store as a predictor of oxidative DNA damage in healthy men and women. Cancer Sci. 2010, 101, 517–522. [Google Scholar] [CrossRef]
- Keeton, J.T.; Dikeman, M.E. ‘Red’ and ‘white’ meats—Terms that lead to confusion. Anim. Front. 2017, 7, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Guéraud, F.; Taché, S.; Steghens, J.P.; Milkovic, L.; Borovic-Sunjic, S.; Zarkovic, N.; Gaultier, E.; Naud, N.; Héliès-Toussaint, C.; Pierre, F.; et al. Dietary polyunsaturated fatty acids and heme iron induce oxidative stress biomarkers and a cancer promoting environment in the colon of rats. Free Radic. Biol. Med. 2015, 83, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, T.; Wouters, A.; Rombouts, C.; Izzati, T.; Berardo, A.; Vossen, E.; Claeys, E.; Van Camp, J.; Raes, K.; Vanhaecke, L.; et al. Reducing compounds equivocally influence oxidation during digestion of a high-fat beef product, which promotes cytotoxicity in colorectal carcinoma cell lines. J. Agric. Food Chem. 2016, 64, 1600–1609. [Google Scholar] [CrossRef]
- Steppeler, C.; Haugen, J.E.; Rodbotten, R.; Kirkhus, B. Formation of Malondialdehyde, 4-hydroxynonenal, and 4-hydroxyhexenal during in vitro digestion of cooked beef, pork, chicken, and salmon. J. Agric. Food Chem. 2016, 64, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P. Nutrients and Oxidative Stress: Friend or Foe? Oxid. Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.G.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanner, J.; Selhub, J.; Shpaizer, A.; Rabkin, B.; Shacham, I.; Tirosh, O. Redox homeostasis in stomach medium by foods: The Postprandial Oxidative Stress Index (POSI) for balancing nutrition and human health. Redox Biol. 2017, 12, 929–936. [Google Scholar] [CrossRef]
- Hooda, J.; Shah, A.; Zhang, L. Heme, an essential nutrient from dietary proteins, critically impacts diverse physiological and pathological processes. Nutrients 2014, 6, 1080–1102. [Google Scholar] [CrossRef] [Green Version]
- Emerit, J.; Beaumont, C.; Trivin, F. Iron metabolism, free radicals, and oxidative injury. Biomed. Pharmacother. 2001, 55, 333–339. [Google Scholar] [CrossRef]
- Chiabrando, D.; Vinchi, F.; Fiorito, V.; Mercurio, S.; Tolosano, E. Heme in pathophysiology: A matter of scavenging, metabolism and trafficking across cell membranes. Front. Pharmacol. 2014, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- Wolk, A. Potential health hazards of eating red meat. J. Intern. Med. 2017, 281, 106–122. [Google Scholar] [CrossRef]
- West, A.R.; Oates, P.S. Mechanisms of heme iron absorption: Current questions and controversies. World J. Gastroenterol. 2008, 14, 4101–4110. [Google Scholar] [CrossRef]
- Duvigneau, J.C.; Esterbauer, H.; Kozlov, A.V. Role of heme oxygenase as a modulator of heme-mediated pathways. Antioxidants 2019, 8, 475. [Google Scholar] [CrossRef] [Green Version]
- Olivero David, R.; Sánchez-Muniz, F.J.; Bastida, S.; Benedi, J.; González-Muñoz, M.J. Gastric emptying and short-term digestibility of thermally oxidized sunflower oil used for frying in fasted and nonfasted rats. J. Agric. Food Chem. 2010, 58, 9242–9248. [Google Scholar] [CrossRef]
- Kanner, J. Oxidative processes in meat and meat products: Quality implications. Meat Sci. 1994, 36, 169–189. [Google Scholar] [CrossRef]
- Wills, T.M.; Dewitt, C.A.M.; Sigfusson, H.; Bellmer, D. Effect of cooking method and ethanolic tocopherol on oxidative stability and quality of beef patties during refrigerated storage (oxidative stability of cooked patties). J. Food Sci. 2006, 71, C109–C114. [Google Scholar] [CrossRef]
- Serrano, A.; Librelotto, J.; Cofrades, S.; Sánchez-Muniz, F.J.; Jiménez-Colmenero, F. Composition and physicochemical beef steaks containing walnuts as characteristics of restructured affected by cooking method. Meat Sci. 2007, 77, 304–313. [Google Scholar] [CrossRef]
- Brenes, M.; García, A.; Dobarganes, M.C.; Velasco, J.; Romero, C. Influence of thermal treatments simulating cooking processes on the polyphenol content in virgin olive oil. J. Agric. Food Chem. 2002, 50, 5962–5967. [Google Scholar] [CrossRef] [PubMed]
- Librelotto, J.; Bastida, S.; Zulim-Botega, D.; Jiménez-Colmenero, F.; Sánchez-Muniz, F.J. Effect of long frozen storage on the formation of triglyceride alteration compounds of pan-fried functional restructured beef steaks. Meat Sci. 2009, 81, 726–730. [Google Scholar] [CrossRef]
- Bastida, S.; Sánchez-Muniz, F.J. Frying a cultural way of cooking in the Mediterranean diet. In The Mediterranean Diet: An Evidence-Based Approach; Preedy, V.R., Watson, R.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 217–234. ISBN 978-0-12-407849-9. [Google Scholar]
- Sánchez-Muniz, F.J. Oils and fats: Changes due to culinary and industrial processes. Int. J. Vitam. Nutr. Res. 2006, 76, 230–237. [Google Scholar] [CrossRef]
- Jung, S.; Nam, K.C.; Jo, C. Detection of malondialdehyde in processed meat products without interference from the ingredients. Food Chem. 2016, 209, 90–94. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid Peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Wang, Z.M.; He, Z.F.; Emara, A.M.; Gan, X.; Li, H.J. Effects of malondialdehyde as a byproduct of lipid oxidation on protein oxidation in rabbit meat. Food Chem. 2019, 288, 405–412. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Miranda, A.M.; Santos, F.A.; Loureiro, A.P.M.; Fisberg, R.M.; Marchioni, D.M. High intake of heterocyclic amines from meat is associated with oxidative stress. Br. J. Nutr. 2015, 113, 1301–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorrain, B.; Dangles, O.; Loonis, M.; Armand, M.; Dufour, C. Dietary iron-initiated lipid oxidation and its inhibition by polyphenols in gastric conditions. J. Agric. Food Chem. 2012, 60, 9074–9081. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Sánchez-Muniz, F.J.; Bastida, S.; Dobarganes, M. Effect of heating and frying on oil and food fatty acids. In Fatty Acids in Foods and Their Health Implications; CRC Press: Boca Raton, FL, USA, 2007; pp. 511–543. [Google Scholar]
- Gasc, N.; Tache, S.; Rathahao, E.; Bertrand-Michel, J.; Roques, V.; Gueraud, F. 4-Hydroxynonenal in foodstuffs: Heme concentration, fatty acid composition and freeze-drying are determining factors. Redox Rep. 2007, 12, 40–44. [Google Scholar] [CrossRef]
- Alderton, A.L.; Faustman, C.; Liebler, D.C.; Hill, D.W. Induction of redox instability of bovine myoglobin by adduction with 4-hydroxy-2-nonenal. Biochemistry 2003, 42, 4398–4405. [Google Scholar] [CrossRef] [PubMed]
- Alary, J.; Gueraud, F.; Cravedi, J.P. Fate of 4-hydroxynonenal in vivo: Disposition and metabolic pathways. Mol. Asp. Med. 2003, 24, 177–187. [Google Scholar] [CrossRef]
- Otaegui-Arrazola, A.; Menendez-Carreno, M.; Ansorena, D.; Astiasaran, I. Oxysterols: A world to explore. Food Chem. Toxicol. 2010, 48, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Leonarduzzi, G.; Sottero, B.; Poli, G. Oxidized products of cholesterol: Dietary and metabolic origin, and proatherosclerotic effects (review). J. Nutr. Biochem. 2002, 13, 700–710. [Google Scholar] [CrossRef]
- Sabolova, M.; Pohorela, B.; Fisnar, J.; Kourimska, L.; Chrpova, D.; Panek, J. Formation of oxysterols during thermal processing and frozen storage of cooked minced meat. J. Sci. Food Agric. 2017, 97, 5092–5099. [Google Scholar] [CrossRef]
- Echarte, M.; Ansorena, D.; Astiasaran, I. Consequences of microwave heating and frying on the lipid fraction of chicken and beef patties. J. Agric. Food Chem. 2003, 51, 5941–5945. [Google Scholar] [CrossRef]
- Derewiaka, D.; Mieczysław, O. Oxysterol content in selected meats and meat products. Acta Sci. Pol. Technol. Aliment. 2009, 8, 5–13. [Google Scholar]
- Eder, K.; Muller, G.; Kluge, H.; Hirche, F.; Brandsch, C. Concentrations of oxysterols in meat and meat products from pigs fed diets differing in the type of fat (palm oil or soybean oil) and vitamin E concentrations. Meat Sci. 2005, 70, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Grandgirard, A.; Demaison-Meloche, J.; Cordelet, C.; Demaison, L. Incorporation of oxyphytosterols in tissues of hamster. Reprod. Nutr. Dev. 2004, 44, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Vine, D.F.; Mamo, J.C.L.; Beilin, L.J.; Mori, T.A.; Croft, K.D. Dietary oxysterols are incorporated in plasma triglyceride-rich lipoproteins, increase their susceptibility to oxidation and increase aortic cholesterol concentration of rabbits. J. Lipid Res. 1998, 39, 1995–2004. [Google Scholar]
- Staprans, I.; Pan, X.M.; Rapp, J.H.; Feingold, K.R. Oxidized cholesterol in the diet is a source of oxidized lipoproteins in human serum. J. Lipid Res. 2003, 44, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brzeska, M.; Szymczyk, K.; Szterk, A. Current knowledge about oxysterols: A review. J. Food Sci. 2016, 81, R2299–R2308. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Xiong, Y.L. Protein Oxidation and Implications for Muscle Food Quality; John Wiley and Sons: New York, NY, USA, 2000; pp. 85–111. [Google Scholar]
- Zhang, W.G.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Nieto, G.; Bañon, S.; Garrido, M.D. Administration of distillate thyme leaves into the diet of Segureña ewes: Effect on lamb meat quality. Animal 2012, 6, 2048–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005, 24, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Tornvall, U. Pinpointing oxidative modifications in proteins-recent advances in analytical methods. Anal. Methods 2010, 2, 1638–1650. [Google Scholar] [CrossRef]
- Soladoye, O.P.; Juárez, M.L.; Aalhus, J.L.; Shand, P.; Estévez, M. Protein oxidation in processed meat: Mechanisms and potential implications on human health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef]
- Fang, W.; Sun, J.; Lv, Z.; Le, G.; Shi, Y. Effect of oxidated food protein on mice gut flora and redox state. Chin. J. Microecol. 2012, 24, 193–196. [Google Scholar]
- Zhang, W.; Xiao, S.; Lee, E.J.; Ahn, D.U. Effects of Dietary Oxidation on the Quality of Broiler Breast Meat; Iowa State University Animal Industry Report; Iowa State University Digital Press: Ames, IA, USA, 2011; Volume 8. [Google Scholar]
- Sell, D.R.; Strauch, C.M.; Shen, W.; Monnier, V.M. 2-Aminoadipic acid is a marker of protein carbonyl oxidation in the aging human skin: Effects of diabetes, renal failure and sepsis. Biochem. J. 2007, 404, 269–277. [Google Scholar] [CrossRef]
- Wang, T.J.; Ngo, D.; Psychogios, N.; Dejam, A.; Larson, M.G.; Vasan, R.S.; Ghorbani, A.; O’Sullivan, J.; Cheng, S.; Rhee, E.P.; et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J. Clin. Investig. 2013, 123, 4309–4317. [Google Scholar] [CrossRef]
- Jiao, L.; Stolzenberg-Solomon, R.; Zimmerman, T.P.; Duan, Z.G.; Chen, L.; Kahle, L.; Risch, A.; Subar, A.F.; Cross, A.J.; Hollenbeck, A.; et al. Dietary consumption of advanced glycation end products and pancreatic cancer in the prospective NIH-AARP Diet and Health Study. Am. J. Clin. Nutr. 2015, 101, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, S.; Maeda, S.; Matsui, T.; Ueda, S.; Fukami, K.; Okuda, S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim. Biophys. Acta 2012, 1820, 663–671. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Hohn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, S.; Matsui, T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid. Med. Cell. Longev. 2010, 3, 101–108. [Google Scholar] [CrossRef]
- Ufnal, M.; Zadlo, A.; Ostaszewski, R. TMAO: A small molecule of great expectations. Nutrition 2015, 31, 1317–1323. [Google Scholar] [CrossRef]
- Abbasi, J. TMAO and heart disease: The new red meat risk? JAMA J. Am. Med. Assoc. 2019, 321, 2149–2151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.N.; Bergeron, N.; Levison, B.S.; Li, X.M.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.P.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.E.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.M.; Wu, Y.P.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.J.; Chen, Y.L.; Gua, C.J.; Li, X.D. Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front. Physiol. 2017, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Meng, Y.H.; Qi, H.X.; Zhu, W.F.; Wang, Z.E.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappa B. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.L.; Jiao, X.F.; Ma, Y.R.; Liu, Y.; Zhang, L.; He, Y.Z.; Chen, Y.H. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Y.; Zhao, Y.; Yuan, L.; Yang, X.B. Protective effects of tartary buckwheat flavonoids on high TMAO diet-induced vascular dysfunction and liver injury in mice. Food Funct. 2015, 6, 3359–3372. [Google Scholar] [CrossRef] [PubMed]
- Canales, A.; Benedí, J.; Nus, M.; Librelotto, J.; Sánchez-Montero, J.M.; Sánchez-Muniz, F.J. Effect of walnut-enriched restructured meat in the antioxidant status of overweight/obese senior subjects with at least one extra CHD-risk factor. J. Am. Coll. Nutr. 2007, 26, 225–232. [Google Scholar] [CrossRef]
- Bae, H.R.; Leung, P.S.C.; Hodge, D.L.; Fenimore, J.M.; Jeon, S.M.; Thovarai, V.; Dzutsev, A.; Welcher, A.A.; Boedigheimer, M.; Damore, M.A.; et al. Multi-omics: Differential expression of IFN-γ results in distinctive mechanistic features linking chronic inflammation, gut dysbiosis, and autoimmune diseases. J. Autoimmun. 2020, 111, 102436. [Google Scholar] [CrossRef]
- Ge, Y.; Lin, S.; Li, B.; Yang, Y.; Tang, X.; Shi, Y.; Sun, J.; Le, G. Oxidized pork induces oxidative stress and inflammation by altering gut microbiota in mice. Mol. Nutr. Food Res. 2020, 64, e1901012. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.I.; Zou, X.; Ijaz, M.U.; Hussain, M.; Liu, C.; Xu, X.; Zhou, G.; Li, C. Processed meat protein promoted inflammation and hepatic lipogenesis by upregulating Nrf2/Keap1 signaling pathway in glrx-deficient mice. J. Agric. Food Chem. 2019, 67, 8794–8809. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Ahmed, M.I.; Zou, X.; Hussain, M.; Zhang, M.; Zhao, F.; Xu, X.; Zhou, G.; Li, C. Beef, casein, and soy proteins differentially affect lipid metabolism, triglycerides accumulation and gut microbiota of high-fat diet-fed C57BL/6J mice. Front. Microbiol. 2018, 9, 2200. [Google Scholar] [CrossRef] [PubMed]
- Pierre, F.; Santarelli, R.; Taché, S.; Guéraud, F.; Corpet, D.E. Beef meat promotion of dimethylhydrazine-induced colorectal carcinogenesis biomarkers is suppressed by dietary calcium. Br. J. Nutr. 2008, 99, 1000–1006. [Google Scholar] [CrossRef] [Green Version]
- Corpet, D.E. Red meat and colon cancer: Should we become vegetarians, or can we make meat safer? Meat Sci. 2011, 89, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Sun, J.; Ding, Y.; Le, G.; Shi, Y. Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl. Microbiol. Biotechnol. 2013, 97, 1689–1697. [Google Scholar] [CrossRef]
- IJssennagger, N.; Derrien, M.; van Doorn, G.M.; Rijnierse, A.; van den Bogert, B.; Müller, M.; Dekker, J.; Kleerebezem, M.; van der Meer, R. Dietary heme alters microbiota and mucosa of mouse colon without functional changes in host-microbe cross-talk. PLoS ONE 2012, 7, e49868. [Google Scholar] [CrossRef] [Green Version]
- Omaye, A.T.; Omaye, S.T. Caveats for the good and bad of dietary red meat. Antioxidants 2019, 8, 544. [Google Scholar] [CrossRef] [Green Version]


| Type of Meat | Meat Consumption (kg/per capita/year) * | |||||
|---|---|---|---|---|---|---|
| World | Africa | America | Asia | Europe | Oceania | |
| Bovine | 9 | 5.63 | 27.83 | 4.68 | 14 | 31.22 |
| Mutton and goat | 1.86 | 2.49 | 0.62 | 1.93 | 1.75 | 10.79 |
| Pork | 15.7 | 1.48 | 18.65 | 15.18 | 35.75 | 24.24 |
| Poultry | 15.18 | 6.21 | 41.94 | 9.71 | 24.59 | 43.96 |
| Others | 0.84 | 1.43 | 0.65 | 0.55 | 1.84 | 2.1 |
| Total | 42.58 | 17.24 | 89.69 | 32.05 | 77.93 | 112.31 |
| Beef | Pork | |||||
|---|---|---|---|---|---|---|
| USA | UK | Spain | USA | UK | Spain | |
| Energy (kcal) | 126 | 129 | 131 | 144 | 124 | 155 |
| Protein (g) | 21.0 | 22.5 | 20.7 | 21.2 | 21.8 | 20.0 |
| Fat (g) | 4.0 | 4.3 | 5.4 | 5.9 | 4.0 | 8.3 |
| SFA (g) | 1.4 | 1.7 | 2.2 | 2.0 | 1.4 | 3.2 |
| MUFA (g) | 1.6 | 1.9 | 2.5 | 2.7 | 1.5 | 3.6 |
| PUFA (g) | 0.2 | 0.2 | 0.2 | 0.6 | 0.7 | 0.6 |
| Niacin (mg) | 6.2 | 9.7 | 8.1 | 4.8 | 6.9 | 8.7 |
| Tiamin (mg) | 0.1 | 0.1 | 0.1 | 1.0 | 1.0 | 0.9 |
| Vitamin B12 (μg) | 1.5 | 2.0 | 2.0 | 0.7 | 1.0 | 3.0 |
| Iron (mg) | 1.8 | 2.7 | 2.7 | 0.9 | 0.7 | 1.5 |
| Zinc (mg) | 3.9 | 4.1 | 3.8 | 2.0 | 2.1 | 2.5 |
| Selenium (mg) | 26.0 | 7.0 | 3.0 | 32.4 | 13.0 | 14.0 |
| Sodium (mg) | 54.0 | 63.0 | 61.0 | 54.0 | 63.0 | 76.0 |
| Potasium (mg) | 323.0 | 350.0 | 350.0 | 384.0 | 380.0 | 370.0 |
| Food and Nutrient Database for Dietary Studies | Base de Datos Española de Composición de Alimentos | |||||||
|---|---|---|---|---|---|---|---|---|
| Iron (mg) | Total Fat (g) | SFA (g) | PUFA (g) | Iron (mg) | Total Fat (g) | SFA (g) | PUFA (g) | |
| Iberian ham | 14.29 | 25.0 | 7.14 | 1.31 | 4.3 | 19.2 | 7.81 | 1.18 |
| Pâté | 9.19 | 13.1 | 4.0 | 2.46 | 5.5 | 29.5 | 10.48 | 3.59 |
| Ground beef | 1.97 | 19.07 | 7.29 | 0.51 | 1.9 | 21 | 8.51 | 1.25 |
| Ground lamb | 1.78 | 19.49 | 8.05 | 1.39 | 1.12 * | 14.55 | 5.34 | 0.74 |
| Ground Pork | 1.28 | 20.6 | 7.66 | 1.85 | 1.3 | 23 | 7.43 | 3.51 |
| Pork sausage | 1.2 | 27.25 | 8.83 | 5.12 | 1.44 | 28.1 | 10.55 | 2.60 |
| Cooked ham | 1.0 | 17.46 | 6.42 | 1.67 | 1.2 | 5.1 | 1.9 | 0.6 |
| Turkey leg | 0.83 | 3.38 | 1.0 | 0.91 | 1.5 | 8.36 | 2.6 | 2.3 |
| Chicken nuggets | 0.83 | 20.36 | 3.57 | 6.51 | 1.39 | 15.12 | 2.88 | 2.3 |
| Chicken breast | 0.51 | 7.67 | 1.96 | 0.95 | 1.5 | 1.2 | 0.33 | 0.28 |
 Red meat and meat-products;
Red meat and meat-products;  White meat and meat-products. * mostly suckling lamb; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; Source: Food and Nutrient Database for Dietary Studies (FNDDS) and Base de Datos Española de Composición de Alimentos (BEDCA). Compositions are expressed either per 100 g edible portion of different meat.
White meat and meat-products. * mostly suckling lamb; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; Source: Food and Nutrient Database for Dietary Studies (FNDDS) and Base de Datos Española de Composición de Alimentos (BEDCA). Compositions are expressed either per 100 g edible portion of different meat.© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macho-González, A.; Garcimartín, A.; López-Oliva, M.E.; Bastida, S.; Benedí, J.; Ros, G.; Nieto, G.; Sánchez-Muniz, F.J. Can Meat and Meat-Products Induce Oxidative Stress? Antioxidants 2020, 9, 638. https://doi.org/10.3390/antiox9070638
Macho-González A, Garcimartín A, López-Oliva ME, Bastida S, Benedí J, Ros G, Nieto G, Sánchez-Muniz FJ. Can Meat and Meat-Products Induce Oxidative Stress? Antioxidants. 2020; 9(7):638. https://doi.org/10.3390/antiox9070638
Chicago/Turabian StyleMacho-González, Adrián, Alba Garcimartín, María Elvira López-Oliva, Sara Bastida, Juana Benedí, Gaspar Ros, Gema Nieto, and Francisco José Sánchez-Muniz. 2020. "Can Meat and Meat-Products Induce Oxidative Stress?" Antioxidants 9, no. 7: 638. https://doi.org/10.3390/antiox9070638
APA StyleMacho-González, A., Garcimartín, A., López-Oliva, M. E., Bastida, S., Benedí, J., Ros, G., Nieto, G., & Sánchez-Muniz, F. J. (2020). Can Meat and Meat-Products Induce Oxidative Stress? Antioxidants, 9(7), 638. https://doi.org/10.3390/antiox9070638