Unraveling the Lipidome and Antioxidant Activity of Native Bifurcaria bifurcata and Invasive Sargassum muticum Seaweeds: A Lipid Perspective on How Systemic Intrusion May Present an Opportunity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sampling
2.3. Lipid Extraction
2.4. Phospholipid and Glycolipid Quantification
2.5. Fatty Acid Analysis by Gas Chromatography-Mass Spectrometry (GC–MS)
2.6. Polar Lipid Analysis by Hydrophilic Interaction Liquid Chromatography-High Resolution Mass Spectrometry (HILIC−MS) and Tandem Mass Spectrometry (MS/MS)
2.7. Data Analysis
2.9. 2.2-Diphenyl-1-Picrylhydrazyl Radical Assay (DPPH) Radical Scavenging Activity
3. Results
3.1. Total Lipid Content
3.1.1. Bifurcaria bifurcata and S. muticum Fatty Acid Profiles
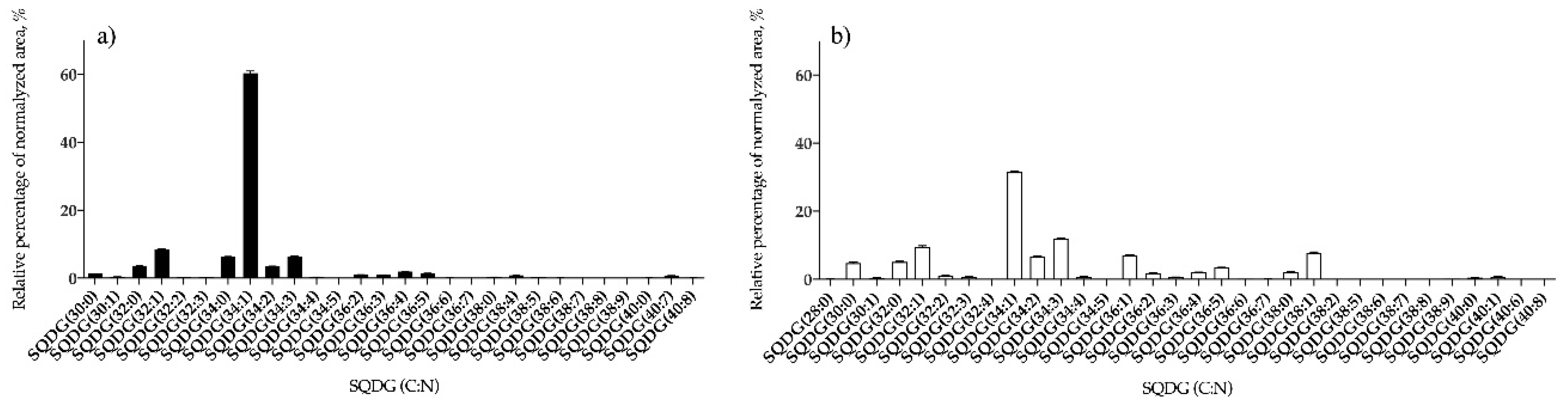
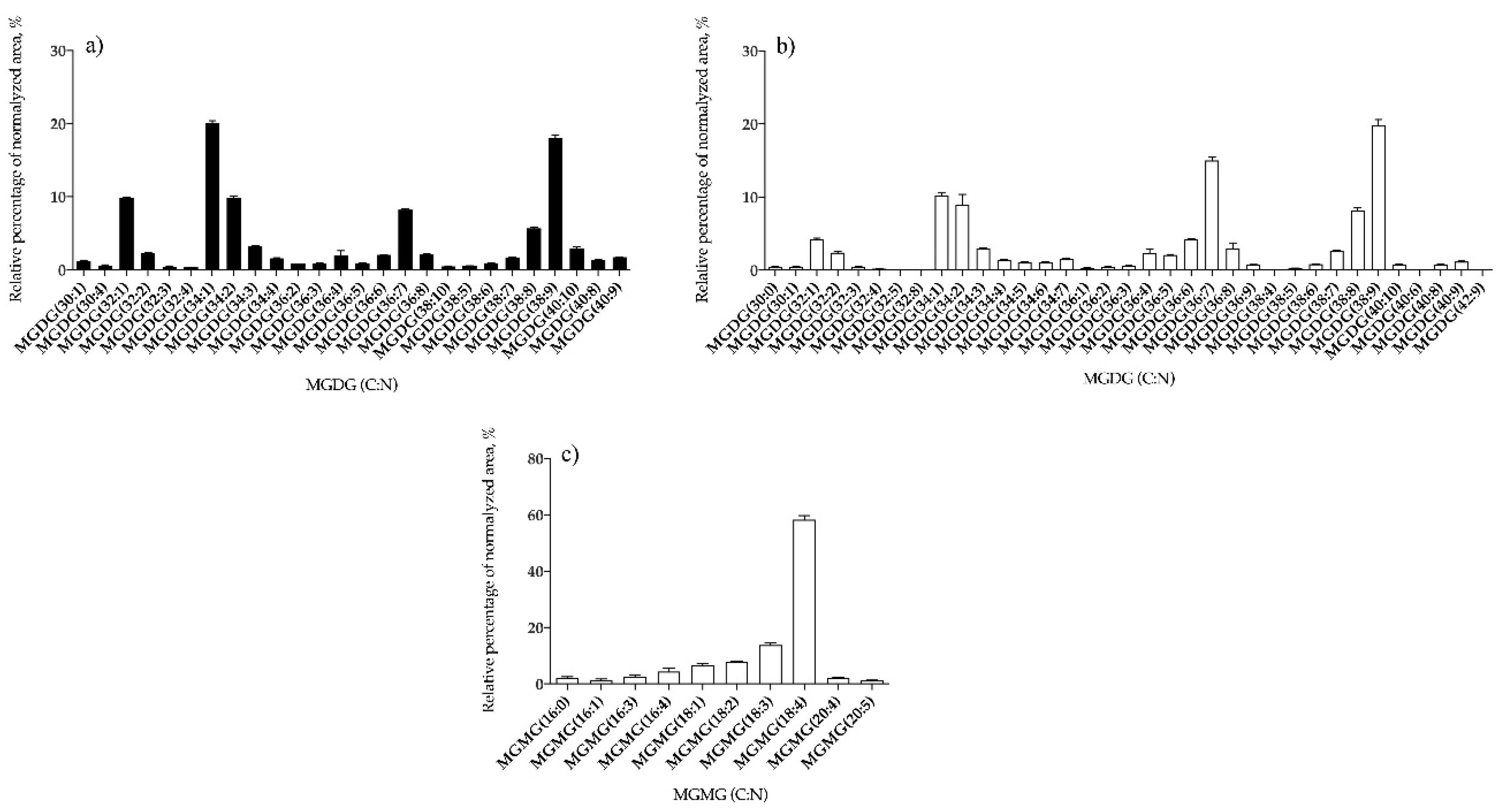
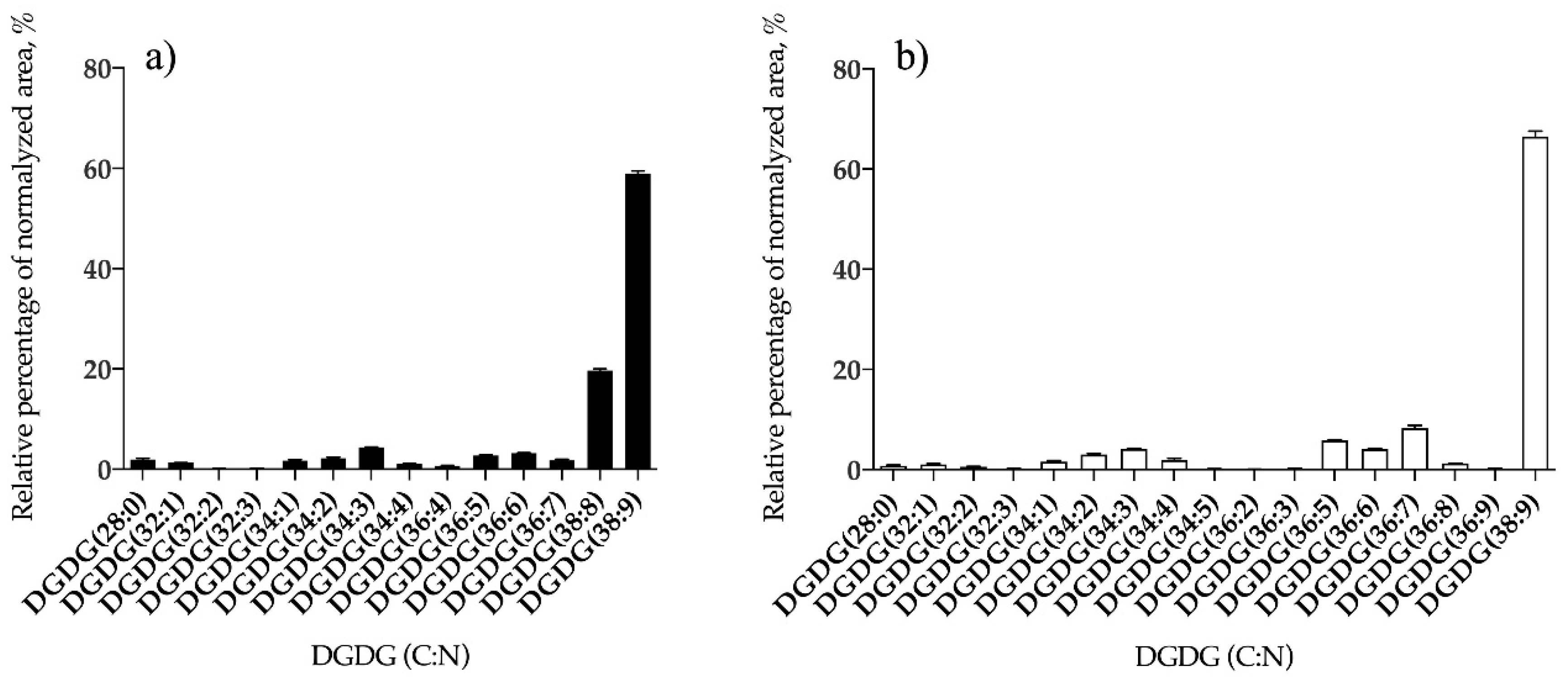
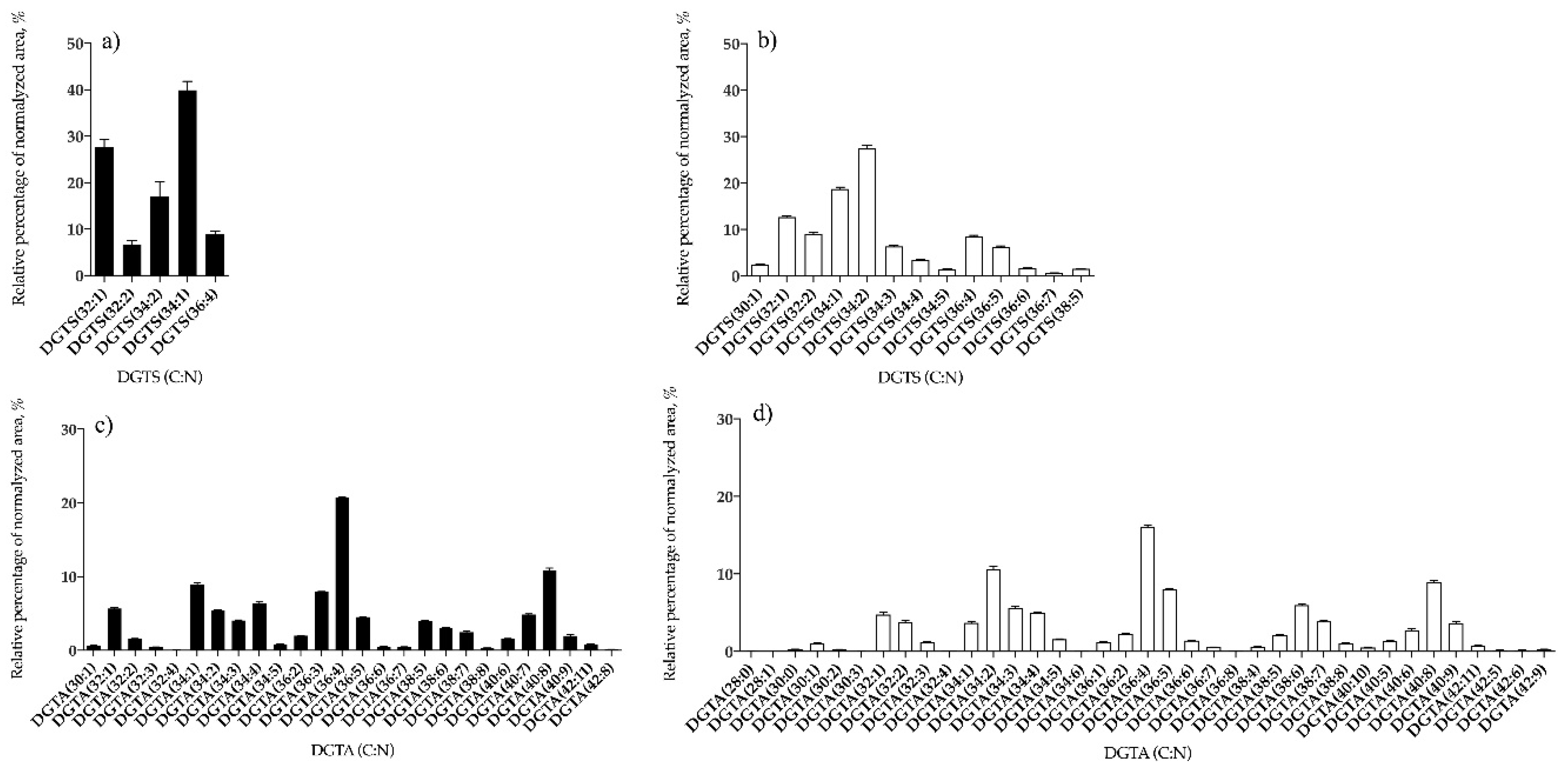
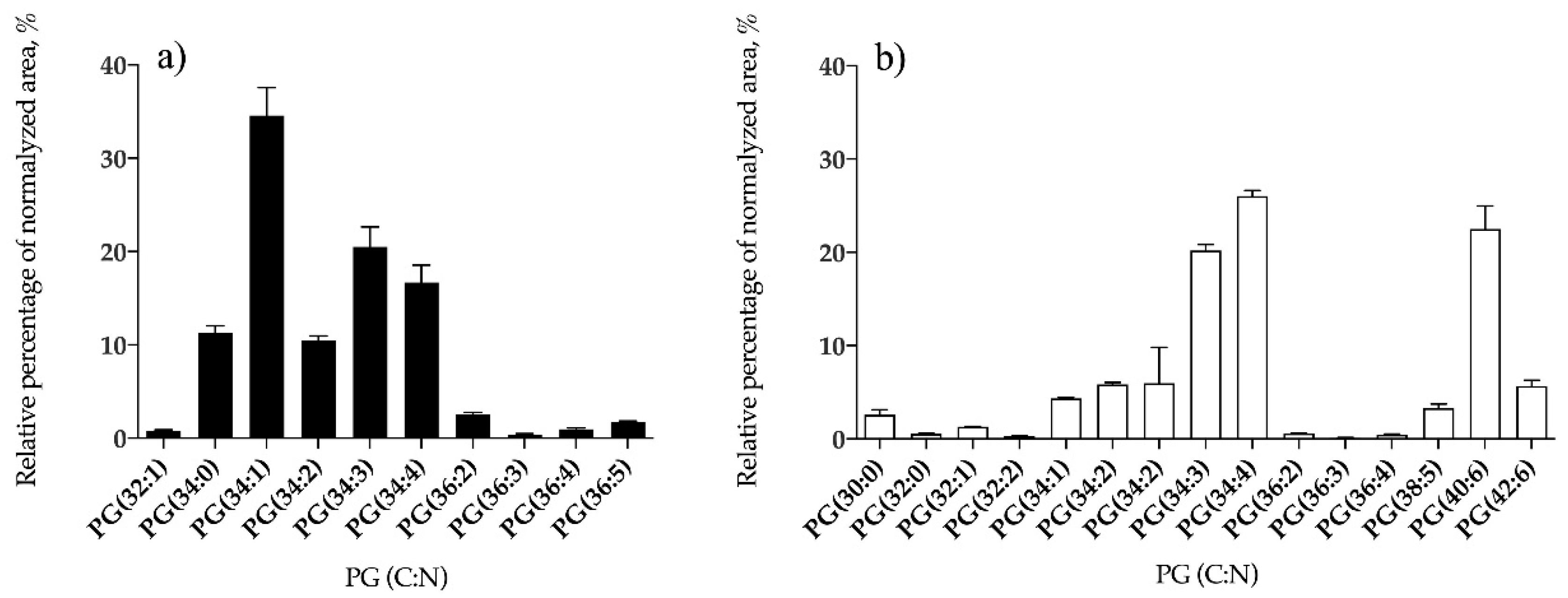
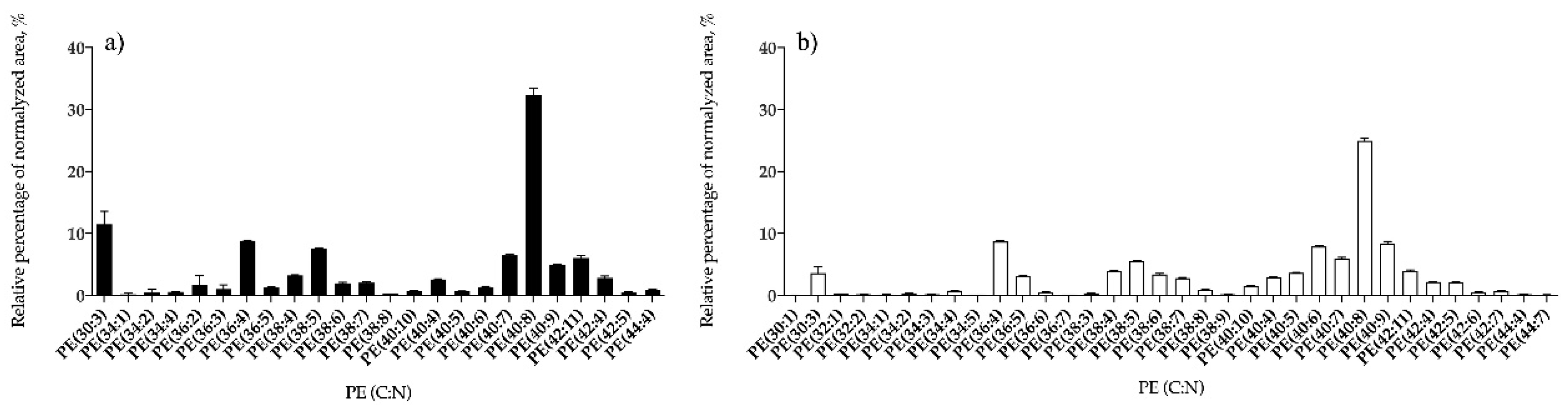
3.1.2. Bifurcaria bifurcata and S. muticum Polar Lipid Profiles
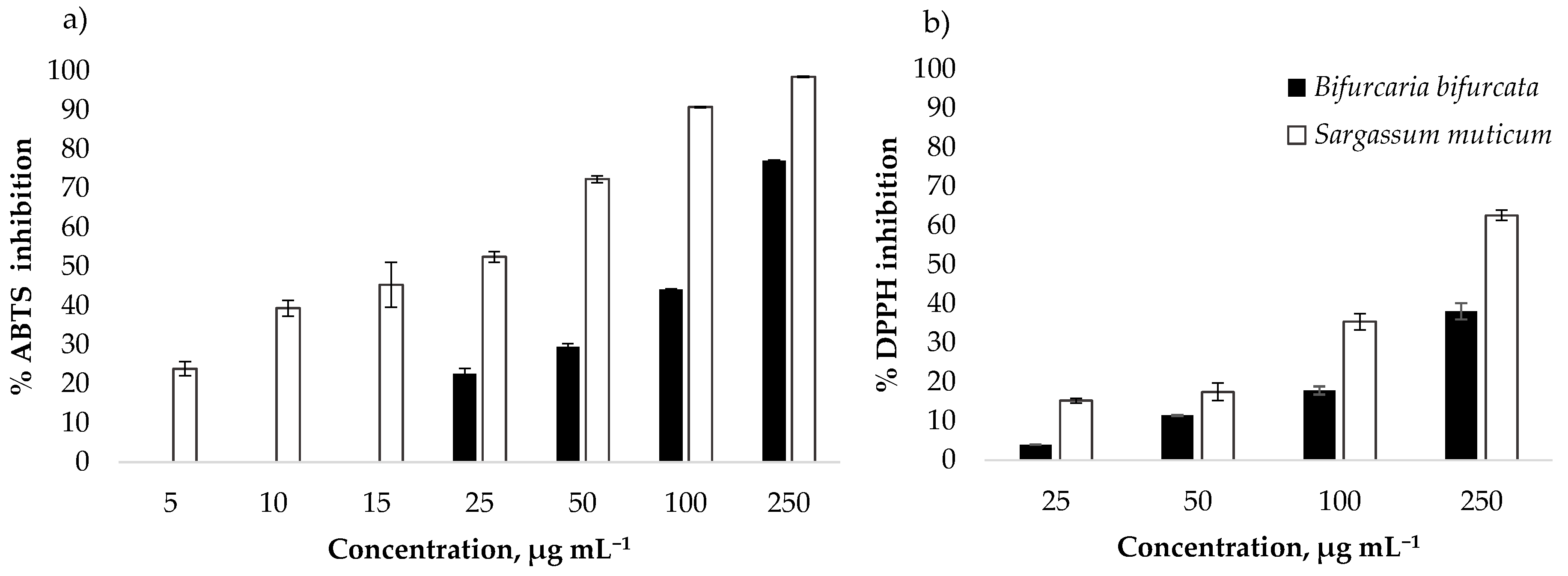
3.2. Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nie, C.; Zepeda, L. Lifestyle segmentation of US food shoppers to examine organic and local food consumption. Appetite 2011, 57, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Rachel, D.; Thangaraju, N.; Anantharaman, P. Potential of marine algae (seaweeds) as source of medicinally important compounds. Plant Genet. Resour. Characterisation Util. 2016, 14, 303–313. [Google Scholar] [CrossRef]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Cornish, M.L.; Garbary, D.J. Antioxidants from macroalgae: Potential applications in human health and nutrition. ALGAE 2010, 25, 155–171. [Google Scholar] [CrossRef]
- Zubia, M.; Robledo, D.; Freile-Pelegrin, Y. Antioxidant activities in tropical marine macroalgae from the Yucatan Peninsula, Mexico. J. Appl. Phycol. 2007, 19, 449–458. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaBase. 2019. Available online: https://www.algaebase.org/about/ (accessed on 1 May 2019).
- Alves, C.; Pinteus, S.; Simões, T.; Horta, A.; Silva, J.; Tecelão, C.; Pedrosa, R. Bifurcaria bifurcata: A key macro-alga as a source of bioactive compounds and functional ingredients. Int. J. Food Sci. Technol. 2016, 51, 1638–1646. [Google Scholar] [CrossRef]
- Le Lann, K.; Rumin, J.; Cérantola, S.; Culioli, G.; Stiger-Pouvreau, V. Spatiotemporal variations of diterpene production in the brown macroalga Bifurcaria bifurcata from the western coasts of Brittany (France). J. Appl. Phycol. 2014, 26, 1207–1214. [Google Scholar] [CrossRef]
- Briggs, H. Sargassum: The biggest seaweed bloom in the world. BBC News, 4 July 2019. [Google Scholar]
- Gower, J.F.R.; King, S.A. Distribution of floating Sargassum in the Gulf of Mexico and the Atlantic ocean mapped using MERIS. Int. J. Remote Sens. 2011, 32, 1917–1929. [Google Scholar] [CrossRef]
- Sánchez, Í.; Fernández, C.; Arrontes, J. Long-term changes in the structure of intertidal assemblages after invasion by Sargassum muticum (Phaeophyta). J. Phycol. 2005, 41, 942–949. [Google Scholar] [CrossRef]
- Stæhr, P.A.; Pedersen, M.F.; Thomsen, M.S.; Wernberg, T.; Krause-Jensen, D. Invasion of Sargassum muticum in Limfjorden (Denmark) and its possible impact on the indigenous macroalgal community. Mar. Ecol. Prog. Ser. 2000, 207, 79–88. [Google Scholar] [CrossRef]
- Britton-Simmons, K.H. Direct and indirect effects of the introduced alga Sargassum muticum on benthic, subtidal communities of Washington State, USA. Mar. Ecol. Prog. Ser. 2004, 277, 61–78. [Google Scholar] [CrossRef]
- Critchley, A.T.; Farnham, W.F.; Morrell, S.L. An account of the attempted control of an introduced marine alga, Sargassum muticum, in Southern England. Biol. Conserv. 1986, 35, 313–332. [Google Scholar] [CrossRef]
- DeAmicis, S.; Foggo, A. Long-term field study reveals subtle effects of the invasive alga Sargassum muticum upon the epibiota of Zostera marina. PLoS ONE 2015, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, P.; Balboa, E.M.; González-García, S.; Domínguez, H.; Feijoo, G.; Moreira, M.T. Comparative environmental assessment of valorization strategies of the invasive macroalgae Sargassum muticum. Bioresour. Technol. 2014, 161, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Nielsen, B.V.; Bailey, D. High-value products from macroalgae: The potential uses of the invasive brown seaweed, Sargassum muticum. Rev. Environ. Sci. Biotechnol. 2016, 15, 67–88. [Google Scholar] [CrossRef]
- Infinium Global Research, Marine-derived Drugs Market (Type - Phenol, Steroid, Ether, Peptide, and Other; Source - Algae, Invertebrates, and Microorganisms; Mode of Delivery - Anti-microbial, Anti-tumor, Anti-cardiovascular, Anti-viral, Anti-inflammatory, and Others): Global Industry Analysis, Trends, Size, Share and Forecasts to 2025. 2019. Available online: https://www.medgadget.com/2019/07/marinederived-dr (accessed on 4 July 2019).
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef]
- Yoza, B.A.; Masutani, E.M. The analysis of macroalgae biomass found around Hawaii for bioethanol production. Environ. Technol. 2013, 34, 1859–1867. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 1–27. [Google Scholar] [CrossRef]
- Chojnacka, K. Biologically Active compounds in seaweed extracts - the prospects for the application. Open Conf. Proc. J. 2012, 3, 20–28. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef]
- Santos, S.; Trindade, S.; Oliveira, C.; Parreira, P.; Rosa, D.; Duarte, M.; Ferreira, I.; Cruz, M.; Rego, A.; Abreu, M.; et al. Lipophilic Fraction of Cultivated Bifurcaria bifurcata R. Ross: Detailed composition and In vitro prospection of current challenging bioactive properties. Mar. Drugs 2017, 15, 340. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F.J. Proximate composition and nutritional value of three macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef] [PubMed]
- Plouguerné, E.; Ioannou, E.; Georgantea, P.; Vagias, C.; Roussis, V.; Hellio, C.; Kraffe, E.; Stiger-Pouvreau, V. Anti-microfouling activity of lipidic metabolites from the invasive brown alga Sargassum muticum (Yendo) Fensholt. Mar. Biotechnol. 2010, 12, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Conde, E.; Moure, A.; Domínguez, H. Supercritical CO2 extraction of fatty acids, phenolics and fucoxanthin from freeze-dried Sargassum muticum. J. Appl. Phycol. 2015, 27, 957–964. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids in wild plants, nuts and seeds. Asia Pac. J. Clin. Nutr. 2002, 11, S163–S173. [Google Scholar] [CrossRef]
- Newton, I.S. Long chain fatty acids in health and nutrition. J. Food Lipids 1996, 233–249. [Google Scholar] [CrossRef]
- Miyashita, K.; Mikami, N.; Hosokawa, M. Chemical and nutritional characteristics of brown seaweed lipids: A review. J. Funct. Foods 2013, 5, 1507–1517. [Google Scholar] [CrossRef]
- Lim, S.; Choi, A.H.; Kwon, M.; Joung, E.J.; Shin, T.; Lee, S.G.; Kim, N.G.; Kim, H.R. Evaluation of antioxidant activities of various solvent extract from Sargassum serratifolium and its major antioxidant components. Food Chem. 2019, 278, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Alves, C.; Pinteus, S.; Horta, A.; Pedrosa, R. High antioxidant activity of Sargassum muticum and Padina pavonica collected from Peniche coast (Portugal). Curr. Opin. Biotechnol. 2013, 24, S116. [Google Scholar] [CrossRef]
- Terme, N.; Boulho, R.; Kucma, J.-P.; Bourgougnon, N.; Bedoux, G.; Terme, N.; Bourgougnon, N.; Kucma, J.-P.; Bedoux, G. Radical scavenging activity of lipids from seaweeds isolated by solid-liquid extraction and supercritical fluids. OCL 2018, 25, D505. [Google Scholar] [CrossRef]
- Airanthi, M.K.W.-A.; Sasaki, N.; Iwasaki, S.; Baba, N.; Abe, M.; Hosokawa, M.; Miyashita, K. Effect of brown seaweed lipids on fatty acid composition and lipid hydroperoxide levels of mouse liver. J. Agric. Food Chem. 2011, 59, 4156–4163. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Airanthi, M.K.; Hosokawa, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J. Food Sci. Technol. 2010, 47, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Lehtimaa, T.; Tarvo, V.; Mortha, G.; Kuitunen, S.; Vuorinen, T. Reactions and kinetics of Cl(III) decomposition. Ind. Eng. Chem. Res. 2008, 47, 5284–5290. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rozentsvet, O.A.; Pechenkina, E.E. Glycolipids, phospholipids and fatty acids of brown algae species. Phytochemistry 1990, 29, 3417–3421. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current trends on seaweeds: Looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, E.; Domingues, P.; Melo, T.; Coelho, E.; Pereira, R.; Calado, R.; Abreu, M.H.; Domingues, M.R. Lipidomic signatures reveal seasonal shifts on the relative abundance of high-valued lipids from the brown algae Fucus vesiculosus. Mar. Drugs 2019, 17, 335. [Google Scholar] [CrossRef]
- Rey, F.; Lopes, D.; Maciel, E.; Monteiro, J.; Skjermo, J.; Funderud, J.; Raposo, D.; Domingues, P.; Calado, R.; Domingues, M.R. Polar lipid profile of Saccharina latissima, a functional food from the sea. Algal Res. 2019, 39, 1–8. [Google Scholar] [CrossRef]
- da Costa, E.; Ricardo, F.; Melo, T.; Mamede, R.; Abreu, M.H.; Domingues, P.; Domingues, M.R.; Calado, R. Site-specific lipidomic signatures of sea lettuce (Ulva spp., chlorophyta) hold the potential to trace their geographic origin. Biomolecules 2020, 10, 489. [Google Scholar] [CrossRef]
- Lopes, D.; Melo, T.; Meneses, J.; Abreu, M.H.; Pereira, R.; Domingues, P.; Lillebø, A.I.; Calado, R.; Rosário Domingues, M. A new look for the red macroalga Palmaria palmata: A seafood with polar lipids rich in EPA and with antioxidant properties. Mar. Drugs 2019, 17, 533. [Google Scholar] [CrossRef]
- Da Costa, E.; Melo, T.; Moreira, A.S.P.A.; Bernardo, C.; Helguero, L.; Ferreira, I.; Cruz, M.T.M.; Rego, A.M.A.; Domingues, P.; Calado, R.; et al. Valorization of lipids from Gracilaria sp. through lipidomics and decoding of antiproliferative and anti-Inflammatory activity. Mar. Drugs 2017, 15, 62. [Google Scholar] [CrossRef]
- Bell, B.M.; Daniels, D.G.H.; Fearn, T.; Stewart, B.A. Lipid compositions, baking qualities and other characteristics of wheat varieties grown in the U.K. J. Cereal Sci. 1987, 5, 277–286. [Google Scholar] [CrossRef]
- Melo, T.; Alves, E.; Azevedo, V.; Martins, A.S.; Neves, B.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Lipidomics as a new approach for the bioprospecting of marine macroalgae—Unraveling the polar lipid and fatty acid composition of Chondrus crispus. Algal Res. 2015, 8, 181–191. [Google Scholar] [CrossRef]
- Lopes, D.; Moreira, A.S.P.; Rey, F.; da Costa, E.; Melo, T.; Maciel, E.; Rego, A.; Abreu, M.H.; Domingues, P.; Calado, R.; et al. Lipidomic signature of the green macroalgae Ulva rigida farmed in a sustainable integrated multi-trophic aquaculture. J. Appl. Phycol. 2018, 1369–1381. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Automatic method for determination of total antioxidant capacity using 2,2-diphenyl-1-picrylhydrazyl assay. Anal. Chim. Acta 2006, 558, 310–318. [Google Scholar] [CrossRef]
- Balboa, E.M.; Gallego-fábrega, C.; Moure, A.; Domínguez, H. Study of the seasonal variation on proximate composition of oven-dried Sargassum muticum biomass collected in Vigo Ria, Spain. J. Appl. Phycol. 2016, 28, 1943–1953. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Kamat, S.Y.; Wahidulla, S.; D’Souza, L.; Naik, C.G.; Ambiye, V.; Bhakuni, D.S.; Goel, A.K.; Garg, H.S.; Srimal, R.C. Bioactivity of Marine Organisms: VI. Antiviral evaluation of marine algal extracts from the Indian Coast. Bot. Mar. 1992, 35, 161–164. [Google Scholar] [CrossRef]
- Plouguerné, E.; da Gama, B.A.P.; Pereira, R.C.; Barreto-Bergter, E. Glycolipids from seaweeds and their potential biotechnological applications. Front. Cell. Infect. Microbiol. 2014, 4, 1–3. [Google Scholar] [CrossRef]
- da Costa, E.; Azevedo, V.; Melo, T.; Rego, A.M.; Evtuguin, D.V.; Domingues, P.; Calado, R.; Pereira, R.; Abreu, M.H.; Domingues, M.R. High-resolution lipidomics of the early life stages of the red seaweed Porphyra dioica. Molecules 2018, 23, 187. [Google Scholar] [CrossRef]
- Murakami, H.; Nobusawa, T.; Hori, K.; Shimojima, M.; Ohta, H. Betaine lipid is crucial for adapting to low temperature and phosphate deficiency in Nannochloropsis. Plant Physiol. 2018, 177, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Kunzler, K.; Eichenberger, W. Betaine lipids and zwitterionic phospholipids in plants and fungi. Phytochemistry 1997, 46, 883–892. [Google Scholar] [CrossRef]
- Sato, N. Betaine Lipids. Bot. Mag. Tokyo 1992, 1, 185–197. [Google Scholar] [CrossRef]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive compounds and properties of seaweeds—A Review. Open Access Libr. J. 2014, e752, 1–17. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Freitas, R.; Martins, A.; Pinteus, S.; Ribeiro, J.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Antioxidant and neuroprotective potential of the brown seaweed Bifurcaria bifurcata in an In vitro Parkinson’s disease model. Mar. Drugs 2019, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Fino, N.; Rodrigues, A.I.; Mendes, S.; Pedrosa, R. Cytoprotective effect of seaweeds with high antioxidant activity from the Peniche coast (Portugal). Food Chem. 2017, 218, 591–599. [Google Scholar] [CrossRef]
- Gerasimenko, N.; Menchinskaya, E.; Esipov, A.; Aminin, D.; Logvinov, S.; Pislyagin, E. Application of glyceroglycolipids, photosynthetic pigments and extracts of brown algae for suppression ROS. Open J. Mar. Sci. 2016, 6, 371–385. [Google Scholar] [CrossRef]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar]
- Matschke, V.; Theiss, C.; Matschke, J. Oxidative stress: The lowest common denominator of multiple diseases. Neural Regen. Res. 2019, 14, 238–241. [Google Scholar] [CrossRef]
- Christ, A.; Latz, E. The Western lifestyle has lasting effects on metaflammation. Nat. Rev. Immunol. 2019, 19, 267–268. [Google Scholar] [CrossRef]
- Chatterjee, S. Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. [Google Scholar]


| Fatty Acid | B. bifurcata (%) | S. muticum (%) |
|---|---|---|
| 14:0 | 4.04 ± 1.18% | 3.00 ± 0.17% |
| 15:0 | 0.90 ± 0.36% | 0.77 ± 0.05% |
| 16:0 | 30.07 ± 2.78% | 24.18 ± 0.48% |
| 16:1 | 2.84 ± 0.56% | 2.34 ± 0.43% |
| 16:2 | – | 1.65 ± 0.86% |
| 18:0 | 10.42 ± 7.06% | 3.29 ± 0.33% |
| 18:1 n-9 | 12.12 ± 1.22% | 7.91 ± 0.14% |
| 18:2 n-6 | 3.22 ± 0.45% | 5.61 ± 0.26% |
| 18:3 n-6 | 0.37 ± 0.26% | 0.67 ± 0.09% |
| 18:3 n-3 | 3.84 ± 0.51% | 7.07 ± 0.07% |
| 18:4 n-3 | 5.02 ± 0.73% | 8.03 ± 0.15% |
| 20:0 | 0.67 ± 0.18% | 0.64 ± 0.12% |
| 20:1 | 2.23 ± 0.33% | 1.56 ± 0.10% |
| 20:2 | – | 1.46 ± 0.14% |
| 20:3 | 4.08 ± 1.31% (a) | 3.57 ± 0.33% (a) |
| 20:4 n-3 | 1.19 ± 0.20% | 0.72 ± 0.09% |
| 20:4 n-6 | 14.28 ± 0.72% | 12.53 ± 0.72% |
| 20:5 n-3 | 4.72 ± 0.26% | 9.71 ± 0.34% |
| 22:0 | – | 0.74 ± 0.10% |
| 22:1 | – | 4.56 ± 1.05% |
| ∑ SFA | 46.09 ± 4.30% | 32.62 ± 0.92% |
| ∑ MUFA | 17.19 ± 1.95% | 16.36 ± 0.49% |
| ∑ PUFA | 36.72 ± 2.55% | 51.02 ± 4.79% |
| ∑ (n-3) | 14.77 ± 1.28% | 25.53 ± 0.49% |
| ∑ (n-6) | 17.87 ± 0.74% | 18.81 ± 0.94% |
| n-6/n-3 | 1.22 ± 0.09 | 0.74 ± 0.03 |
| Lipid Classes | Bifurcaria bifurcata | Sargassum muticum | ||
|---|---|---|---|---|
| Lipid Species Number | Major Species | Lipid Species Number | Major Species | |
| MGDG | 26 | MGDG (34:1) | 35 | MGDG (38:9) |
| MGMG | – | – | 10 | MGMG (18:4) |
| DGDG | 14 | DGDG (38:9) | 17 | DGDG (38:9) |
| SQDG | 28 | SQDG (34:1) | 32 | SQDG (34:1) |
| LPC | – | – | 3 | LPC (16:0) |
| PC | 3 | PC (30:3) | 10 | PC (30:3) |
| LPE | – | – | 2 | LPE (20:4) |
| PE | 24 | PE (40:8) | 34 | PE (40:8) |
| LPG | – | – | 1 | LPG (16:0) |
| PG | 10 | PG (34:1) | 15 | PG (34:4) |
| PI | 7 | PI (38:8) | 8 | PI (34:2) |
| DGTS | 5 | DGTS (34:1) | 13 | DGTS (34:2) |
| DGTA | 26 | DGTA (36:4) | 37 | DGTA (36:4) |
| Glycolipids | 68 | – | 94 | – |
| Phospholipids | 44 | – | 73 | – |
| Betaine lipids | 31 | – | 50 | – |
| Total | 143 | – | 217 | – |
| Species | ABTS Assay | DPPH Assay | ||
|---|---|---|---|---|
| IC50 (μg mL−1) | TE (µmol g−1) | IC * (μg mL−1) | TE (µmol g−1) | |
| B. bifurcata | 134.19 ± 2.12 | 100.33 ± 1.57 | 155.00 ± 3.96 | 74.89 ± 1.93 |
| S. muticum | 23.42 ± 0.79 | 648.35 ± 21.43 | 188.43 ± 20.55 | 124.19 ± 14.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, F.; Monteiro, J.P.; Duarte, D.; Melo, T.; Lopes, D.; da Costa, E.; Domingues, M.R. Unraveling the Lipidome and Antioxidant Activity of Native Bifurcaria bifurcata and Invasive Sargassum muticum Seaweeds: A Lipid Perspective on How Systemic Intrusion May Present an Opportunity. Antioxidants 2020, 9, 642. https://doi.org/10.3390/antiox9070642
Santos F, Monteiro JP, Duarte D, Melo T, Lopes D, da Costa E, Domingues MR. Unraveling the Lipidome and Antioxidant Activity of Native Bifurcaria bifurcata and Invasive Sargassum muticum Seaweeds: A Lipid Perspective on How Systemic Intrusion May Present an Opportunity. Antioxidants. 2020; 9(7):642. https://doi.org/10.3390/antiox9070642
Chicago/Turabian StyleSantos, Fábio, João P. Monteiro, Daniela Duarte, Tânia Melo, Diana Lopes, Elisabete da Costa, and Maria Rosário Domingues. 2020. "Unraveling the Lipidome and Antioxidant Activity of Native Bifurcaria bifurcata and Invasive Sargassum muticum Seaweeds: A Lipid Perspective on How Systemic Intrusion May Present an Opportunity" Antioxidants 9, no. 7: 642. https://doi.org/10.3390/antiox9070642
APA StyleSantos, F., Monteiro, J. P., Duarte, D., Melo, T., Lopes, D., da Costa, E., & Domingues, M. R. (2020). Unraveling the Lipidome and Antioxidant Activity of Native Bifurcaria bifurcata and Invasive Sargassum muticum Seaweeds: A Lipid Perspective on How Systemic Intrusion May Present an Opportunity. Antioxidants, 9(7), 642. https://doi.org/10.3390/antiox9070642






