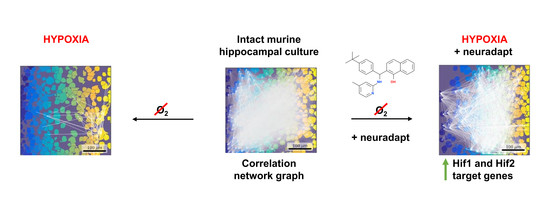
Neuroprotective Effect of HIF Prolyl Hydroxylase Inhibition in an In Vitro Hypoxia Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Reporter Assay
2.2. Real Time PCR
2.3. Ethics Statement
2.4. Isolation of Murine Primary Hippocampal Cultures
2.5. Acute Normobaric Hypoxia Model
2.6. Pharmacological Treatment
- PHD inhibitor neuradapt cytotoxicity assessment. The following drug concentrations were analyzed: 0.5 µM, 1 µM, 2 µM, 5 µM, 10 µM, 15 µM, 20 µM, 30 µM. The solvent, dimethyl sulfoxide (DMSO) (Sigma-Aldrich, D8418, Steinheim, Germany), was used in the control group;
- Immediate treatment. To study the effect of neuradapt on cell viability and functional parameters of neuron-glial networks in an in vitro hypoxia model, 0.5 µM, 1 µM, 2 µM, 5 µM, 10 µM, 15 µM, and 20 µM were added to the hypoxic culture medium and to the normoxic medium used for re-oxygenation;
- Post-treatment. To evaluate the post-hypoxia neuroprotective action of neuradapt, 1 µM or 15 µM drug was applied to the culture medium daily starting 2 h post-hypoxia modeling and continued for the following 7 days.
2.7. Cell Viability Assay
2.8. Calcium Imaging
2.9. Network Characteristics of Primary Hippocampal Cultures
2.10. Statistical Analysis
3. Results
3.1. HIF Activating Properties of Neuradapt
3.2. Evaluation of Neuradapt Cytotoxicity
3.3. Evaluation of Neuradapt Neuroprotective Effects upon Immediate Administration
3.4. Neuradapt Effects in Post-Treatment Regime
3.5. Features of Functional Network Activity in Primary Hippocampal Cultures in the Post-Hypoxic Period
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dukoff, D.J.; Hogg, D.W.; Hawrysh, P.J.; Buck, L.T. Scavenging ROS dramatically increase NMDA receptor whole-cell currents in painted turtle cortical neurons. J. Exp. Biol. 2014, 217, 3346–3355. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Oxygen Sensing, Hypoxia-Inducible Factors, and Disease Pathophysiology. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 47–71. [Google Scholar] [CrossRef]
- Bertout, J.A.; Patel, S.A.; Simon, M.C. The impact of O2 availability on human cancer. Nat. Rev. Cancer 2008, 8, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Bruick, R.K. A Conserved Family of Prolyl-4-Hydroxylases That Modify HIF. Science 2001, 294, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factor 1: Master regulator of O2 homeostasis. Curr. Opin. Genet. Dev. 1998, 8, 588–594. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Williams, B.B.; Battaglioli, E.J.; Whitaker, W.R.; Till, L.; Grover, M.; Linden, D.R.; Akiba, Y.; Kandimalla, K.K.; Zachos, N.C.; et al. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe 2018, 23, 775–785.e5. [Google Scholar] [CrossRef]
- Cerychova, R.; Pavlinkova, G. HIF-1, Metabolism, and Diabetes in the Embryonic and Adult Heart. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Wacker, B.K.; Perfater, J.L.; Gidday, J.M. Hypoxic preconditioning induces stroke tolerance in mice via a cascading HIF, sphingosine kinase, and CCL2 signaling pathway. J. Neurochem. 2012, 123, 954–962. [Google Scholar] [CrossRef]
- Engelhardt, S.; Al-Ahmad, A.J.; Gassmann, M.; Ogunshola, O.O. Hypoxia Selectively Disrupts Brain Microvascular Endothelial Tight Junction Complexes Through a Hypoxia-Inducible Factor-1 (HIF-1) Dependent Mechanism. J. Cell. Physiol. 2014, 229, 1096–1105. [Google Scholar] [CrossRef]
- Chen, R.L.; Ogunshola, O.O.; Yeoh, K.K.; Jani, A.; Papadakis, M.; Nagel, S.; Schofield, C.J.; Buchan, A.M. HIF prolyl hydroxylase inhibition prior to transient focal cerebral ischaemia is neuroprotective in mice. J. Neurochem. 2014, 131, 177–189. [Google Scholar] [CrossRef]
- Chen, R.; Lai, U.H.; Zhu, L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive Oxygen Species Formation in the Brain at Different Oxygen Levels: The Role of Hypoxia Inducible Factors. Front. Cell Dev. Biol. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, S.S.; Alim, I.; Khim, S.J.; Bourassa, M.W.; Sleiman, S.F.; John, R.; Thinnes, C.C.; Yeh, T.-L.; Demetriades, M.; Neitemeier, S.; et al. Therapeutic targeting of oxygen-sensing prolyl hydroxylases abrogates ATF4-dependent neuronal death and improves outcomes after brain hemorrhage in several rodent models. Sci. Transl. Med. 2016, 8, 328ra29. [Google Scholar] [CrossRef] [PubMed]
- Poloznikov, A.A.; Khristichenko, A.Y.; Smirnova, N.A.; Hushpulian, D.M.; Gaisina, I.N.; Osipyants, A.I.; Tishkov, V.I.; Gazaryan, I.G. Structural optimization of adaptaquin, a HIF prolyl hydroxylase inhibitor. Russ. Chem. Bull. 2019, 68, 168–173. [Google Scholar] [CrossRef]
- Smirnova, N.A.; Rakhman, I.; Moroz, N.; Basso, M.; Payappilly, J.; Kazakov, S.; Hernandez-Guzman, F.; Gaisina, I.N.; Kozikowski, A.P.; Ratan, R.R.; et al. Utilization of an in vivo reporter for high throughput identification of branched small molecule regulators of hypoxic adaptation. Chem. Biol. 2010, 17, 380–391. [Google Scholar] [CrossRef]
- Primer3 software. Available online: http://bioinfo.ut.ee/primer3-0.4.0/ (accessed on 29 June 2020).
- Primer-BLAST software. Available online: https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 29 June 2020).
- Pfaffl, M.W. Relative expression software tool (REST(C)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Vedunova, M.; Sakharnova, T.; Mitroshina, E.; Perminova, M.; Pimashkin, A.; Zakharov, Y.; Dityatev, A.; Mukhina, I. Seizure-like activity in hyaluronidase-treated dissociated hippocampal cultures. Front. Cell. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Lurie, U.U. Standard Methods for Water Analysis; Chimia: Moscow, Russia, 1973. [Google Scholar]
- Kasischke, K.A.; Lambert, E.M.; Panepento, B.; Sun, A.; Gelbard, H.A.; Burgess, R.W.; Foster, T.H.; Nedergaard, M. Two-Photon NADH Imaging Exposes Boundaries of Oxygen Diffusion in Cortical Vascular Supply Regions. J. Cereb. Blood Flow Metab. 2011, 31, 68–81. [Google Scholar] [CrossRef]
- Johnston, A.J.; Steiner, L.A.; Gupta, A.K.; Menon, D.K. Cerebral oxygen vasoreactivity and cerebral tissue oxygen reactivity. Br. J. Anaesth. 2003, 90, 774–786. [Google Scholar] [CrossRef]
- Ngwenya, L.B.; Burke, J.F.; Manley, G.T. Brain Tissue Oxygen Monitoring and the Intersection of Brain and Lung: A Comprehensive Review. Respir. Care 2016, 61, 1232–1244. [Google Scholar] [CrossRef]
- Vedunova, M.V.; Sakharnova, T.A.; Mitroshina, E.V.; Shishkina, T.V.; Astrakhanova, T.A.; Mukhina, I.V. Antihypoxic and Neuroprotective Properties of BDNF and GDNF in vitro and in vivo Under Hypoxic Conditions. Sovrem. Tehnol. Med. 2014, 6, 38–45. [Google Scholar]
- Vedunova, M.V.; Mishchenko, T.A.; Mitroshina, E.V.; Mukhina, I.V. TrkB-Mediated Neuroprotective and Antihypoxic Properties of Brain-Derived Neurotrophic Factor. Oxid. Med. Cell. Longev. 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kustikova, V.; Krivonosov, M.; Pimashkin, A.; Denisov, P.; Zaikin, A.; Ivanchenko, M.; Meyerov, I.; Semyanov, A. CalciumCV: Computer Vision Software for Calcium Signaling in Astrocytes. In Proceedings of the 7th International Conference, AIST 2018, Moscow, Russia, 5–7 July 2018; pp. 168–179. [Google Scholar]
- Semenza, G.L.; Roth, P.H.; Fang, H.M.; Wang, G.L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 1994, 269, 23757–23763. [Google Scholar] [PubMed]
- Fukuda, R.; Zhang, H.; Kim, J.; Shimoda, L.; Dang, C.V.; Semenza, G.L. HIF-1 Regulates Cytochrome Oxidase Subunits to Optimize Efficiency of Respiration in Hypoxic Cells. Cell 2007, 129, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, P.J. HIF-1 and HIF-2: Working alone or together in hypoxia? J. Clin. Investig. 2007, 117, 862–865. [Google Scholar] [CrossRef]
- Obach, M.; Navarro-Sabaté, A.; Caro, J.; Kong, X.; Duran, J.; Gómez, M.; Perales, J.C.; Ventura, F.; Rosa, J.L.; Bartrons, R. 6-Phosphofructo-2-kinase (pfkfb3) gene promoter contains hypoxia-inducible factor-1 binding sites necessary for transactivation in response to hypoxia. J. Biol. Chem. 2004, 279, 53562–53570. [Google Scholar] [CrossRef]
- Hu, C.; Wang, L.; Chodosh, L.A.; Keith, B.; Simon, M.C. Differential Roles of Hypoxia-Inducible Factor 1 alpha ( HIF-1 alpha ) and HIF-2 alpha in Hypoxic Gene Regulat. Mol. Cell Biol. 2003, 23, 9361–9374. [Google Scholar] [CrossRef]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef]
- Ivan, M.; Kaelin, W.G. The EGLN-HIF O2-Sensing System: Multiple Inputs and Feedbacks. Mol. Cell 2017, 66, 772–779. [Google Scholar] [CrossRef]
- Ngo, J.K.; Pomatto, L.C.D.; Davies, K.J.A. Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol. 2013, 1, 258–264. [Google Scholar] [CrossRef]
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013, 27, 41–53. [Google Scholar] [CrossRef]
- Rankin, E.B.; Biju, M.P.; Liu, Q.; Unger, T.L.; Rha, J.; Johnson, R.S.; Simon, M.C.; Keith, B.; Haase, V.H.; Erinn, B.; et al. Regulates Hepatic Erythropoietin in Vivo. J. Clin. Investig. 2007, 117, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Pollard, P.J.; El-Bahrawy, M.; Poulsom, R.; Elia, G.; Killick, P.; Kelly, G.; Hunt, T.; Jeffery, R.; Seedhar, P.; Barwell, J.; et al. Expression of HIF-1α, HIF-2α (EPAS1), and their target genes in paraganglioma and pheochromocytoma with VHL and SDH mutations. J. Clin. Endocrinol. Metab. 2006, 91, 4593–4598. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, H.; Zhang, J.; Zhao, E. Knockdown of hypoxia inducible factor-2α inhibits cell invasion via the downregulation of MMP-2 expression in breast cancer cells. Oncol. Lett. 2016, 11, 3743–3748. [Google Scholar] [CrossRef] [PubMed]
- Yamano, N.; Ikeda, Y.; Sakama, M.; Izawa-Ishizawa, Y.; Kihira, Y.; Ishizawa, K.; Miyamoto, L.; Tomita, S.; Tsuchiya, K.; Tamaki, T. A long-term high-fat diet changes iron distribution in the body, increasing iron accumulation specifically in the mouse spleen. J. Nutr. Sci. Vitaminol. 2015, 61, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Masson, N.; Keeley, T.P.; Giuntoli, B.; White, M.D.; Lavilla Puerta, M.; Perata, P.; Hopkinson, R.J.; Flashman, E.; Licausi, F.; Ratcliffe, P.J. Conserved N-terminal cysteine dioxygenases transduce responses to hypoxia in animals and plants. Science 2019, 364, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.; Ilott, N.E.; Schödel, J.; Sims, D.; Tumber, A.; Lippl, K.; Mole, D.R.; Pugh, C.W.; Ratcliffe, P.J.; Ponting, C.P.; et al. Tuning the Transcriptional Response to Hypoxia by Inhibiting Hypoxia-inducible Factor (HIF) Prolyl and Asparaginyl Hydroxylases. J. Biol. Chem. 2016, 291, 20661–20673. [Google Scholar] [CrossRef]
- Mukhina, I.V.; Kazantsev, V.B.; Khaspeckov, L.G.; Zakharov, Y.N.; Vedunova, M.V.; Mitroshina, E.V.; Korotchenko, S.A.; Koryagina, E.A. Multielectrode matrices—new possibilities in investigation of the neuronal network plasticity. Sovrem. Tehnol. Med. 2009, 1, 8–15. [Google Scholar]
- Shirokova, O.M.; Frumkina, L.A.; Vedunova, M.V.; Mitroshina, E.V.; Zakharov, Y.N.; Khaspekov, L.G.; Mukhina, I.V. Morphofunctional patterns of neuronal network developing in dissociated hippocampal cell cultures. Sovrem. Tehnol. Med. 2013, 5, 6–12. [Google Scholar]
- Mishchenko, T.A.; Mitroshina, E.V.; Usenko, A.V.; Voronova, N.V.; Astrakhanova, T.A.; Shirokova, O.M.; Kastalskiy, I.A.; Vedunova, M.V. Features of Neural Network Formation and Their Functions in Primary Hippocampal Cultures in the Context of Chronic TrkB Receptor System Influence. Front. Physiol. 2019, 9. [Google Scholar] [CrossRef]
- Carrillo-Reid, L.; Yang, W.; Kang Miller, J.; Peterka, D.S.; Yuste, R. Imaging and Optically Manipulating Neuronal Ensembles. Annu. Rev. Biophys. 2017, 46, 271–293. [Google Scholar] [CrossRef]
- Jha, N.K.; Jha, S.K.; Sharma, R.; Kumar, D.; Ambasta, R.K.; Kumar, P. Hypoxia-Induced Signaling Activation in Neurodegenerative Diseases: Targets for New Therapeutic Strategies. J. Alzheimer’s Dis. 2018, 62, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Mitroshina, E.V.; Mishchenko, T.A.; Shirokova, O.M.; Astrakhanova, T.A.; Loginova, M.M.; Epifanova, E.A.; Babaev, A.A.; Tarabykin, V.S.; Vedunova, M.V. Intracellular Neuroprotective Mechanisms in Neuron-Glial Networks Mediated by Glial Cell Line-Derived Neurotrophic Factor. Oxid. Med. Cell. Longev. 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.K.; Jain, S.A.; Bae, O.-N.; Majid, A.; Rajanikant, G.K. Hypoxia Mimetic Agents for Ischemic Stroke. Front. Cell Dev. Biol. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Wilson, J.W.; Schofield, C.J.; Chen, R. Author Correction: Hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitors induce autophagy and have a protective effect in an in-vitro ischaemia model. Sci. Rep. 2020, 10, 6041. [Google Scholar] [CrossRef]
- Li, K.; Li, T.; Wang, Y.; Xu, Y.; Zhang, S.; Culmsee, C.; Wang, X.; Zhu, C. Sex differences in neonatal mouse brain injury after hypoxia-ischemia and adaptaquin treatment. J. Neurochem. 2019, 150, 759–775. [Google Scholar] [CrossRef]
- Reischl, S.; Li, L.; Walkinshaw, G.; Flippin, L.A.; Marti, H.H.; Kunze, R. Inhibition of HIF prolyl-4-hydroxylases by FG-4497 Reduces Brain Tissue Injury and Edema Formation during Ischemic Stroke. PLoS ONE 2014, 9, e84767. [Google Scholar] [CrossRef]
- Ratan, R.R. The Chemical Biology of Ferroptosis in the Central Nervous System. Cell Chem. Biol. 2020. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Lu, Y.; Chen, J.; Jiang, H. Repeated Administration of Ketamine can Induce Hippocampal Neurodegeneration and Long-Term Cognitive Impairment via the ROS/HIF-1a Pathway in Developing Rats. Cell. Physiol. Biochem. 2014, 33, 1715–1732. [Google Scholar] [CrossRef]
- Sun, R.; Meng, X.; Pu, Y.; Sun, F.; Man, Z.; Zhang, J.; Yin, L.; Pu, Y. Overexpression of HIF-1a could partially protect K562 cells from 1,4-benzoquinone induced toxicity by inhibiting ROS, apoptosis and enhancing glycolysis. Toxicol. In Vitro 2019, 55, 18–23. [Google Scholar] [CrossRef]
- Guo, Y. Role of HIF-1a in regulating autophagic cell survival during cerebral ischemia reperfusion in rats. Oncotarget 2017, 8. [Google Scholar] [CrossRef]
- Luo, C.; Ouyang, M.-W.; Fang, Y.-Y.; Li, S.-J.; Zhou, Q.; Fan, J.; Qin, Z.-S.; Tao, T. Dexmedetomidine Protects Mouse Brain from Ischemia-Reperfusion Injury via Inhibiting Neuronal Autophagy through Up-Regulating HIF-1α. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Khan, S.A.; Luo, W.; Nanduri, J.; Semenza, G.L.; Prabhakar, N.R. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J. Cell. Physiol. 2011, 226, 2925–2933. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Yuan, L.; Ping, L.; Hu, L. Neonatal bronchopulmonary dysplasia increases neuronal apoptosis in the hippocampus through the HIF-1α and p53 pathways. Respir. Physiol. Neurobiol. 2016, 220, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, J.; Shi, H. Role of Hypoxia Inducible Factor 1 in Hyperglycemia-Exacerbated Blood-Brain Barrier Disruption in Ischemic Stroke. Neurobiol. Dis. 2016, 95, 82–92. [Google Scholar] [CrossRef]
- Yeh, T.-L.; Leissing, T.M.; Abboud, M.I.; Thinnes, C.C.; Atasoylu, O.; Holt-Martyn, J.P.; Zhang, D.; Tumber, A.; Lippl, K.; Lohans, C.T.; et al. Molecular and cellular mechanisms of HIF prolyl hydroxylase inhibitors in clinical trials. Chem. Sci. 2017, 8, 7651–7668. [Google Scholar] [CrossRef]
- Miyake, S.; Muramatsu, R.; Hamaguchi, M.; Yamashita, T. Prolyl hydroxylase regulates axonal rewiring and motor recovery after traumatic brain injury. Cell Death Dis. 2015, 6, e1638. [Google Scholar] [CrossRef]
- Siddiq, A.; Aminova, L.R.; Troy, C.M.; Suh, K.; Messer, Z.; Semenza, G.L.; Ratan, R.R. Selective Inhibition of Hypoxia-Inducible Factor (HIF) Prolyl-Hydroxylase 1 Mediates Neuroprotection against Normoxic Oxidative Death via HIF- and CREB-Independent Pathways. J. Neurosci. 2009, 29, 8828–8838. [Google Scholar] [CrossRef]
- Neitemeier, S.; Dolga, A.M.; Honrath, B.; Karuppagounder, S.S.; Alim, I.; Ratan, R.R.; Culmsee, C. Inhibition of HIF-prolyl-4-hydroxylases prevents mitochondrial impairment and cell death in a model of neuronal oxytosis. Cell Death Dis. 2016, 7, e2214. [Google Scholar] [CrossRef]
- Mitroshina, E.; Mishchenko, T.; Usenko, A.; Epifanova, E.; Yarkov, R.; Gavrish, M.; Babaev, A.; Vedunova, M. AAV-Syn-BDNF-EGFP Virus Construct Exerts Neuroprotective Action on the Hippocampal Neural Network during Hypoxia In Vitro. Int. J. Mol. Sci. 2018, 19, 2295. [Google Scholar] [CrossRef]
- Vecchio, F.; Miraglia, F.; Rossini, P.M. Tracking Neuronal Connectivity from Electric Brain Signals to Predict Performance. Neuroscientist 2019, 25, 86–93. [Google Scholar] [CrossRef]
- Crepel, V.; Epsztein, J.; Ben-Ari, Y. Ischemia induces short- and long-term remodeling of synaptic activity in the hippocampus. J. Cell. Mol. Med. 2003, 7, 401–407. [Google Scholar] [CrossRef] [PubMed]
- le Feber, J.; Tzafi Pavlidou, S.; Erkamp, N.; van Putten, M.J.A.M.; Hofmeijer, J. Progression of Neuronal Damage in an In Vitro Model of the Ischemic Penumbra. PLoS ONE 2016, 11, e0147231. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The Science of Stroke: Mechanisms in Search of Treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [PubMed]
- York, E.M.; Bernier, L.-P.; MacVicar, B.A. Microglial modulation of neuronal activity in the healthy brain. Dev. Neurobiol. 2018, 78, 593–603. [Google Scholar] [CrossRef]
- Fedorovich, S.; Hofmeijer, J.; van Putten, M.J.A.M.; le Feber, J. Reduced Synaptic Vesicle Recycling during Hypoxia in Cultured Cortical Neurons. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef]








| Group | Number of Viable Cells, % |
|---|---|
| Intact | 91.25 ± 0.75 |
| Control (DMSO) | 89.71 ± 1.26 |
| Neuradapt 0.5 μM | 93.02 ± 1.65 |
| Neuradapt 1 μM | 93.40 ± 1.31 |
| Neuradapt 2 μM | 92.49 ± 1.54 |
| Neuradapt 5 μM | 90.01 ± 1.55 |
| Neuradapt 10 μM | 90.86 ± 1.80 |
| Neuradapt 15 μM | 92.15 ± 1.78 |
| Neuradapt 20 μM | 89.95 ± 1.23 |
| Neuradapt 30 μM | 81.06 ± 2.37 * |
| Group | Number of Viable Cells, % |
|---|---|
| Intact | 91.24 ± 0.75 |
| Control (DMSO) | 89.71 ± 1.26 |
| Hypoxia | 76.61 ± 2.67 * |
| Hypoxia + DMSO | 81.29 ± 3.82 * |
| Hypoxia + neuradapt 0.5 μM | 91.21 ± 1.97 # |
| Hypoxia + neuradapt 1 μM | 89.55 ± 1.75 # |
| Hypoxia + neuradapt 5 μM | 90.43 ± 1.73 # |
| Hypoxia + neuradapt 10 μM | 89.37 ± 2.37 # |
| Hypoxia + neuradapt 15 μM | 92.67 ± 0.49 # |
| Hypoxia + neuradapt 20 μM | 92.78 ± 1.13 # |
| Group | Number of Viable Cells, % |
|---|---|
| Intact | 91.06 ± 0.97 |
| Hypoxia | 73.11 ± 1.76 * |
| Hypoxia + Neuradapt 1 μM | 80.85 ± 1.41 *,# |
| Hypoxia + Neuradapt 15 μM | 80.70 ± 1.19 *,# |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savyuk, M.; Krivonosov, M.; Mishchenko, T.; Gazaryan, I.; Ivanchenko, M.; Khristichenko, A.; Poloznikov, A.; Hushpulian, D.; Nikulin, S.; Tonevitsky, E.; et al. Neuroprotective Effect of HIF Prolyl Hydroxylase Inhibition in an In Vitro Hypoxia Model. Antioxidants 2020, 9, 662. https://doi.org/10.3390/antiox9080662
Savyuk M, Krivonosov M, Mishchenko T, Gazaryan I, Ivanchenko M, Khristichenko A, Poloznikov A, Hushpulian D, Nikulin S, Tonevitsky E, et al. Neuroprotective Effect of HIF Prolyl Hydroxylase Inhibition in an In Vitro Hypoxia Model. Antioxidants. 2020; 9(8):662. https://doi.org/10.3390/antiox9080662
Chicago/Turabian StyleSavyuk, Maria, Mikhail Krivonosov, Tatiana Mishchenko, Irina Gazaryan, Mikhail Ivanchenko, Anna Khristichenko, Andrey Poloznikov, Dmitry Hushpulian, Sergey Nikulin, Evgeny Tonevitsky, and et al. 2020. "Neuroprotective Effect of HIF Prolyl Hydroxylase Inhibition in an In Vitro Hypoxia Model" Antioxidants 9, no. 8: 662. https://doi.org/10.3390/antiox9080662
APA StyleSavyuk, M., Krivonosov, M., Mishchenko, T., Gazaryan, I., Ivanchenko, M., Khristichenko, A., Poloznikov, A., Hushpulian, D., Nikulin, S., Tonevitsky, E., Abuzarova, G., Mitroshina, E., & Vedunova, M. (2020). Neuroprotective Effect of HIF Prolyl Hydroxylase Inhibition in an In Vitro Hypoxia Model. Antioxidants, 9(8), 662. https://doi.org/10.3390/antiox9080662