Antioxidant Potential of Psychotropic Drugs: From Clinical Evidence to In Vitro and In Vivo Assessment and toward a New Challenge for in Silico Molecular Design
Abstract
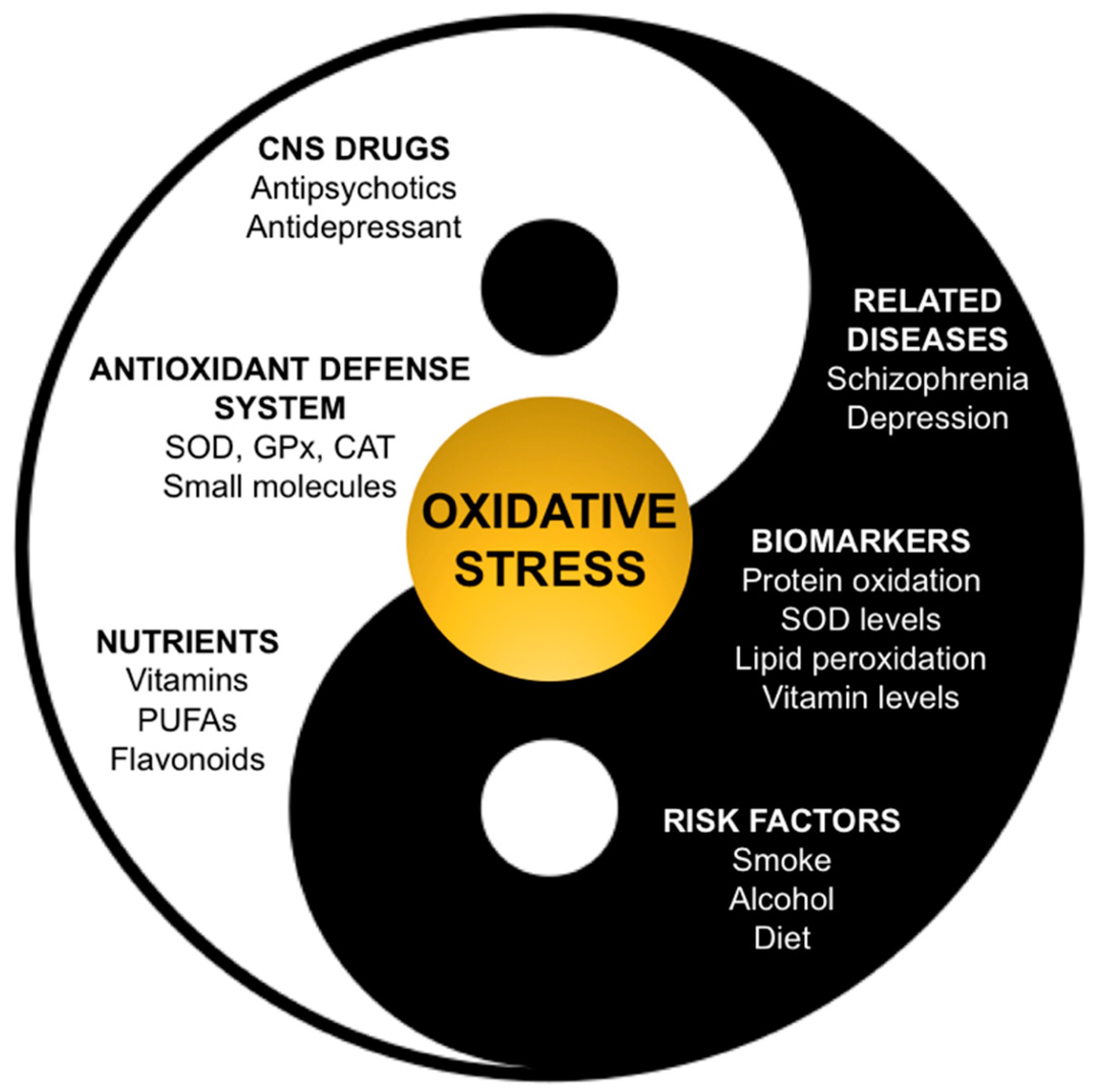
:1. Introduction
Oxidative Stress in Schizophrenia and Depression
2. Antipsychotic Drugs
2.1. First Generation (Typical) Antipsychotics
2.2. Second Generation (Atypical) Antipsychotics
2.3. Aripiprazole
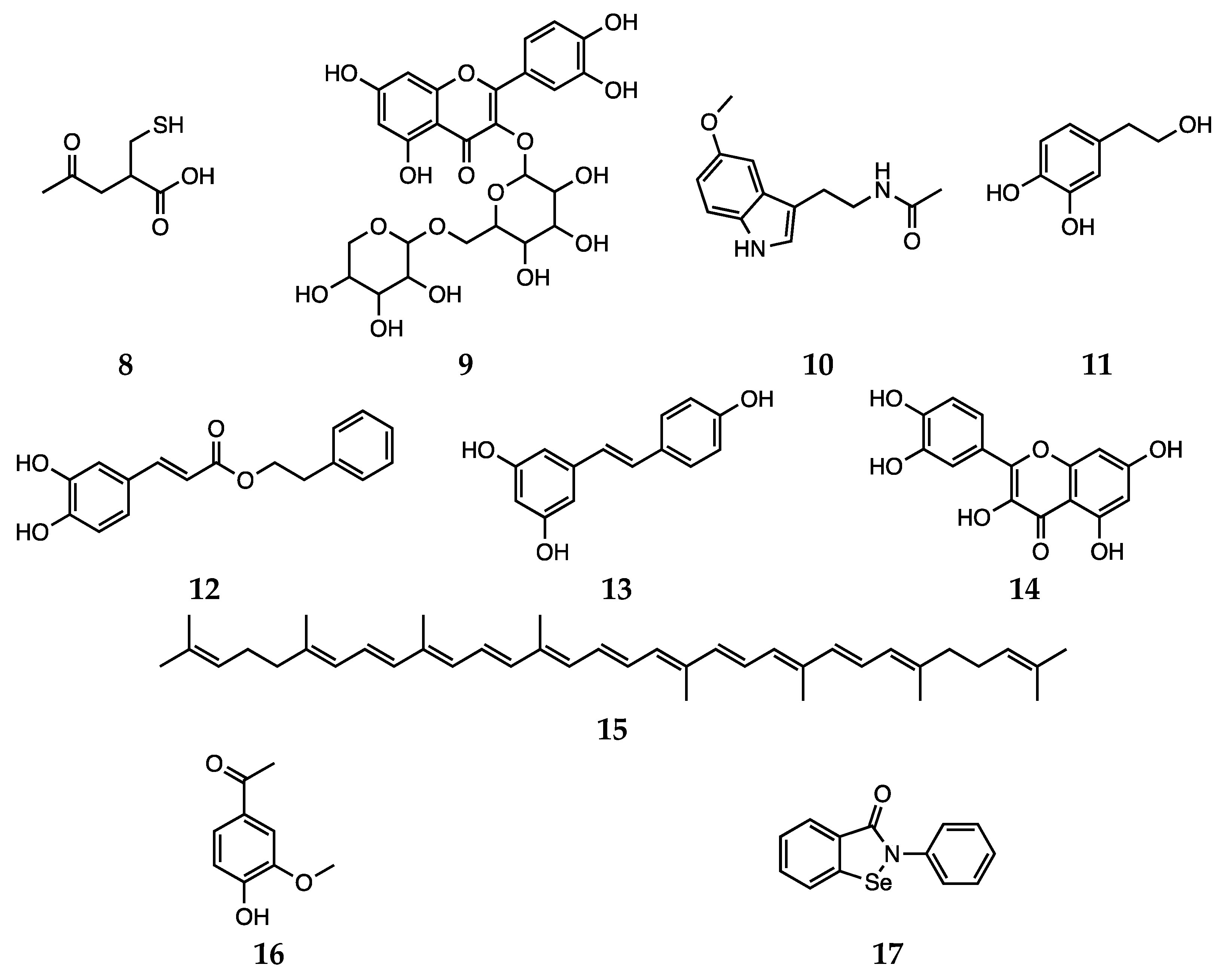
2.4. Other Agents against Oxidative Stress: Natural and Dietary Compounds
3. Antidepressant Drugs
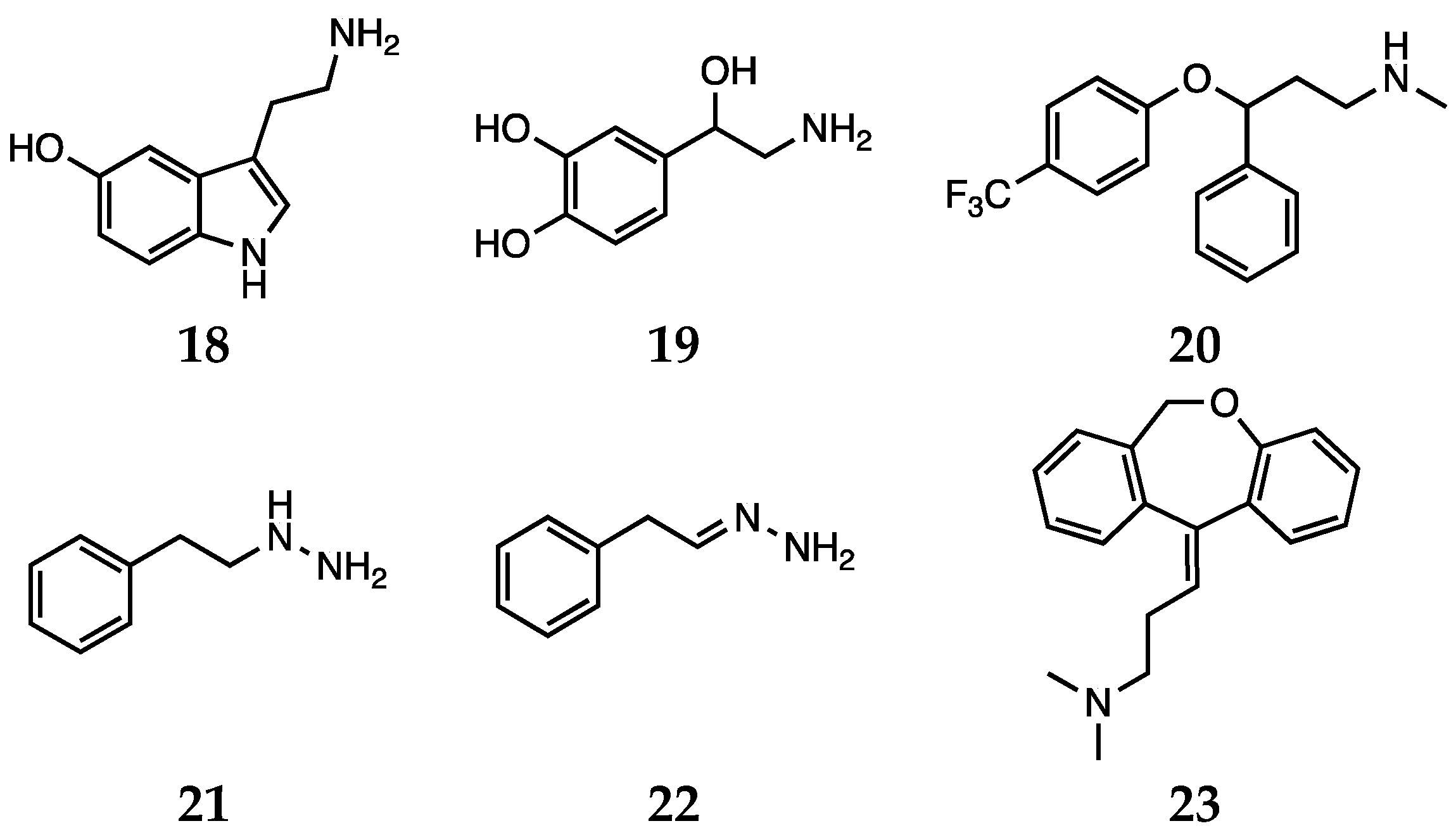
3.1. Conventional Antidepressants
3.2. Natural Compounds
4. In Silico Approaches
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Acronyms
| Central nervous system | CNS |
| Reactive oxygen species | ROS |
| Reactive nitrogen species | RNS |
| Deoxyribonucleic acid DNA | DNA |
| Polyunsaturated fatty acids | PUFAs |
| Superoxide dismutase | SOD |
| Glutathione peroxidase | GPx |
| Catalase | CAT |
| Glutathione | GSH |
| Thiobarbituric acid related substances | TBARS |
| Monoamine oxidase A | MAO-A |
| Monoamine oxidase B | MAO-B |
| Dopamine | DA |
| Catechol-O-methyl transferase | COMT |
| 6-hydroxydopamine | 6-OHDA |
| 8-hydroxy-2′-deoxyguanosine | 8-OHdG |
| Dihydrorhodamine 123 | DHR123 |
| 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) | ABTS |
| 2,2-diphenyl-1-picryl-hydrazyl-hydrate | DPPH |
| Ferric reducing antioxidant power | FRAP |
| Ultra-performance liquid chromatography-tandem mass spectrometry | UPLC-MS/MS |
| N-methyl-4-phenylpyridinium | MPP+ |
| Nicotinamide adenine dinucleotide phosphate | NADPH |
| Glutamate-cysteine ligase modifier subunit knockout | GCLM-KO |
| Γ-aminobutyric acid | GABA |
| Extracellular-signal-regulated kinase | ERK |
| Protein kinase B | AKT |
| area under the curve | AUC |
| Quantitative structure activity relationship | QSAR |
| Quantum mechanics | QM |
| Density functional theory | DFT |
| Hydrogen atom transfer | HAT |
| Integral equation formalism polarizable continuum model | IEFPCM |
| Sequential electron proton transfer | SEPT |
| Sequential proton loss electron transfer | SPLET |
| Iodothyronine deiodinase | DIO |
| Thioredoxin reductases | TrxR |
| Methionine sulfoxide reductases | Msr |
References
- Halliwell, B. Drug antioxidant effects. Drugs 1991, 42, 569–605. [Google Scholar] [CrossRef]
- Ambade, A.; Mandrekar, P. Oxidative stress and inflammation: Essential partners in alcoholic liver disease. Int. J. Hepatol. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kabuto, H.; Amakawa, M.; Mankura, M.; Yamanushi, T.T.; Mori, A. Docosahexaenoic acid ethyl ester enhances 6-hydroxydopamine-induced neuronal damage by induction of lipid peroxidation in mouse striatum. Neurochem. Res. 2009, 34, 1299–1303. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Klabnik, J.J.; O’Donnell, J.M. Novel therapeutic targets in depression and anxiety: Antioxidants as a candidate treatment. Curr. Neuropharmacol. 2014, 12, 108–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, F.; Su, N.; Li, X.; Fei, Z. Neuroprotection mediated by natural products and their chemical derivatives. Neural Regen. Res. 2020, 15, 2008. [Google Scholar] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Abed, M.N.; Alassaf, F.A.; Jasim, M.H.M.; Alfahad, M.; Qazzaz, M.E. Comparison of antioxidant effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole. Pharmacology 2020, 1–7. [Google Scholar] [CrossRef]
- Choi, S.W.; Ho, C.K. Antioxidant properties of drugs used in Type 2 diabetes management: Could they contribute to, confound or conceal effects of antioxidant therapy? Redox Rep. 2018, 23, 1–24. [Google Scholar] [CrossRef]
- Neganova, M.E.; Klochkov, S.G.; Shevtsova, E.F.; Bogatyrenko, T.N.; Mishchenko, D.V. Antioxidant properties of a pharmaceutical substance hypocard, a potential drug for ischemic disease. Bull. Exp. Biol. Med. 2018, 166, 46–49. [Google Scholar] [CrossRef]
- Aline, d.A.O.; Maria, I.L.; Adriano, J.M.C.F.; Emiliano, R.V.R.; Camila, N.d.C.L.; Edith, T.V.; Alana, G.d.S.; Klistenes, A.d.L.; Francisca, C.F.d.S.; Danielle, M.G.; et al. Antioxidant properties of antiepileptic drugs levetiracetam and clonazepam in mice brain after in vitro-induced oxidative stress. African J. Pharm. Pharmacol. 2016, 10, 278–288. [Google Scholar] [CrossRef] [Green Version]
- Osowole, A. Synthesis, physicochemical and antioxidant properties of some metal(II) complexes of mixed drugs, aspirin and nicotinamide. Lett. Health Biol. Sci. 2016, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Redaelli, M.; Mucignat-Caretta, C.; Isse, A.A.; Gennaro, A.; Pezzani, R.; Pasquale, R.; Pavan, V.; Crisma, M.; Ribaudo, G.; Zagotto, G. New naphthoquinone derivatives against glioma cells. Eur. J. Med. Chem. 2015, 96, 458–466. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Wu, S.; He, Y.; Dai, Z.; Ma, S.; Liu, B. Studies of the structure-antioxidant activity relationships and antioxidant activity mechanism of iridoid valepotriates and their degradation products. PLoS ONE 2017, 12, e0189198. [Google Scholar] [CrossRef] [Green Version]
- Zanforlin, E.; Zagotto, G.; Ribaudo, G. The medicinal chemistry of natural and semisynthetic compounds against Parkinson’s and Huntington’s diseases. ACS Chem. Neurosci. 2017, 8, 2356–2368. [Google Scholar] [CrossRef]
- Pavan, V.; Ribaudo, G.; Zorzan, M.; Redaelli, M.; Pezzani, R.; Mucignat-Caretta, C.; Zagotto, G. Antiproliferative activity of Juglone derivatives on rat glioma. Nat. Prod. Res. 2017, 31, 632–638. [Google Scholar] [CrossRef]
- Pohl, F.; Kong Thoo Lin, P. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef] [Green Version]
- Povolo, C.; Foschini, A.; Ribaudo, G. Optimization of the extraction of bioactive molecules from Lycium barbarum fruits and evaluation of the antioxidant activity: A combined study. Nat. Prod. Res. 2019, 33, 2694–2698. [Google Scholar] [CrossRef]
- Ribaudo, G.; Ongaro, A.; Zorzan, M.; Pezzani, R.; Redaelli, M.; Zagotto, G.; Memo, M.; Gianoncelli, A. Investigation of the molecular reactivity of bioactive oxiranylmethyloxy anthraquinones. Arch. Pharm. (Weinh.) 2019, 352, 1900030. [Google Scholar] [CrossRef]
- Mastinu, A.; Ribaudo, G.; Ongaro, A.; Bonini, S.A.; Memo, M.; Gianoncelli, A. Critical review on the Chemical Aspects of Cannabidiol (CBD) and harmonization of computational bioactivity data. Curr. Med. Chem. 2020, 27. [Google Scholar] [CrossRef]
- Godos, J.; Currenti, W.; Angelino, D.; Mena, P.; Castellano, S.; Caraci, F.; Galvano, F.; Del Rio, D.; Ferri, R.; Grosso, G. Diet and mental health: Review of the recent updates on molecular mechanisms. Antioxidants 2020, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Singh, E.; Devasahayam, G. Neurodegeneration by oxidative stress: A review on prospective use of small molecules for neuroprotection. Mol. Biol. Rep. 2020, 47, 3133–3140. [Google Scholar] [CrossRef]
- Bošković, M.; Vovk, T.; Kores Plesničar, B.; Grabnar, I. Oxidative stress in schizophrenia. Curr. Neuropharmacol. 2011, 9, 301–312. [Google Scholar]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008, 11, 851–876. [Google Scholar] [CrossRef] [Green Version]
- Kaur, T.; Cadenhead, K.S. Treatment implications of the schizophrenia prodrome. Curr. Top. Behav. Neurosci. 2010, 4, 97–121. [Google Scholar]
- Kriisa, K.; Haring, L.; Vasar, E.; Koido, K.; Janno, S.; Vasar, V.; Zilmer, K.; Zilmer, M. Antipsychotic treatment reduces indices of oxidative stress in first-episode psychosis patients. Oxid. Med. Cell. Longev. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; Natarajan, R.; Ziedonis, D.; Fan, X. Antioxidant and anti-inflammatory nutrient status, supplementation, and mechanisms in patients with schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 78, 1–11. [Google Scholar] [CrossRef]
- Fedoce, A.d.G.; Ferreira, F.; Bota, R.G.; Bonet-Costa, V.; Sun, P.Y.; Davies, K.J.A. The role of oxidative stress in anxiety disorder: Cause or consequence? Free Radic. Res. 2018, 52, 737–750. [Google Scholar] [CrossRef]
- Halliwell, B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Harris, E.D. Regulation of antioxidant enzymes. FASEB J. 1992, 6, 2675–2683. [Google Scholar] [CrossRef]
- Dorfman-Etrog, P.; Hermesh, H.; Prilipko, L.; Weizman, A.; Munitz, H. The effect of vitamin E addition to acute neuroleptic treatment on the emergence of extrapyramidal side effects in schizophrenic patients: An open label study. Eur. Neuropsychopharmacol. 1999, 9, 475–477. [Google Scholar] [CrossRef]
- Mukerjee, S.; Mahadik, S.P.; Scheffer, R.; Correnti, E.E.; Kelkar, H. Impaired antioxidant defense at the onset of psychosis. Schizophr. Res. 1996, 19, 19–26. [Google Scholar] [CrossRef]
- Cai, H.L.; Jiang, P.; Tan, Q.Y.; Dang, R.L.; Tang, M.M.; Xue, Y.; Deng, Y.; Zhang, B.K.; Fang, P.F.; Xu, P.; et al. Therapeutic efficacy of atypical antipsychotic drugs by targeting multiple stress-related metabolic pathways. Transl. Psychiatry 2017, 7, e1130. [Google Scholar] [CrossRef] [Green Version]
- Koga, M.; Serritella, A.V.; Sawa, A.; Sedlak, T.W. Implications for reactive oxygen species in schizophrenia pathogenesis. Schizophr. Res. 2016, 176, 52–71. [Google Scholar] [CrossRef]
- Mahadik, S.P.; Evans, D.; Lal, H. Oxidative stress and role of antioxidant and omega-3 essential fatty acid supplementation in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 463–493. [Google Scholar] [CrossRef]
- Young, J.; McKinney, S.B.; Ross, B.M.; Wahle, K.W.J.; Boyle, S.P. Biomarkers of oxidative stress in schizophrenic and control subjects. Prostaglandins. Leukot. Essent. Fatty Acids 2007, 76, 73–85. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [Green Version]
- Nishioka, N.; Arnold, S.E. Evidence for oxidative DNA damage in the hippocampus of elderly patients with chronic schizophrenia. Am. J. Geriatr. Psychiatry 2004, 12, 167–175. [Google Scholar] [CrossRef]
- Dadheech, G.; Mishra, S.; Gautam, S.; Sharma, P. Oxidative stress, α-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Indian J. Clin. Biochem. 2006, 21, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Ranjekar, P.K.; Hinge, A.; Hegde, M.V.; Ghate, M.; Kale, A.; Sitasawad, S.; Wagh, U.V.; Debsikdar, V.B.; Mahadik, S.P. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003, 121, 109–122. [Google Scholar] [CrossRef]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar] [PubMed]
- Mousseau, D.D.; Baker, B.G. Recent developments in the regulation of monoamine oxidase form and function: Is the current model restricting our understanding of the breadth of contribution of monoamine oxidase to brain [dys]function? Curr. Top. Med. Chem. 2013, 12, 2163–2176. [Google Scholar] [CrossRef] [Green Version]
- Hritcu, L.; Ionita, R.; Postu, P.A.; Gupta, G.K.; Turkez, H.; Lima, T.C.; Carvalho, C.U.S.; de Sousa, D.P. Antidepressant flavonoids and their relationship with oxidative stress. Oxid. Med. Cell. Longev. 2017, 2017, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribaudo, G.; Zanforlin, E.; Canton, M.; Bova, S.; Zagotto, G. Preliminary studies of berberine and its semi-synthetic derivatives as a promising class of multi-target anti-parkinson agents. Nat. Prod. Res. 2018, 32, 1395–1401. [Google Scholar] [CrossRef]
- Juárez Olguín, H.; Calderón Guzmán, D.; Hernández García, E.; Barragán Mejía, G. The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxid. Med. Cell. Longev. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Pandya, C.D.; Howell, K.R.; Pillai, A. Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Rawdin, B.J.; Mellon, S.H.; Dhabhar, F.S.; Epel, E.S.; Puterman, E.; Su, Y.; Burke, H.M.; Reus, V.I.; Rosser, R.; Hamilton, S.P.; et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 2013, 31, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W.J.H. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Glassman, A.H.; Helzer, J.E.; Covey, L.S.; Cottler, L.B.; Stetner, F.; Tipp, J.E.; Johnson, J. Smoking, smoking cessation, and major depression. JAMA 1990, 264, 1546. [Google Scholar] [CrossRef]
- Andreazza, A.C.; Kauer-Sant’Anna, M.; Frey, B.N.; Bond, D.J.; Kapczinski, F.; Young, L.T.; Yatham, L.N. Oxidative stress markers in bipolar disorder: A meta-analysis. J. Affect. Disord. 2008, 111, 135–144. [Google Scholar] [CrossRef]
- Palta, P.; Samuel, L.J.; Miller, E.R.; Szanton, S.L. Depression and oxidative stress: Results from a meta-analysis of observational studies. Psychosom. Med. 2014, 76, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parikh, V.; Khan, M.M.; Mahadik, S.P. Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J. Psychiatr. Res. 2003, 37, 43–51. [Google Scholar] [CrossRef]
- Brinholi, F.F.; de Farias, C.C.; Bonifácio, K.L.; Higachi, L.; Casagrande, R.; Moreira, E.G.; Barbosa, D.S. Clozapine and olanzapine are better antioxidants than haloperidol, quetiapine, risperidone and ziprasidone in in vitro models. Biomed. Pharmacother. 2016, 81, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Dazzan, P.; Morgan, K.D.; Orr, K.; Hutchinson, G.; Chitnis, X.; Suckling, J.; Fearon, P.; McGuire, P.K.; Mallett, R.M.; Jones, P.B.; et al. Different effects of typical and atypical antipsychotics on grey matter in first episode psychosis: The ÆSOP study. Neuropsychopharmacology 2005, 30, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Aringhieri, S.; Carli, M.; Kolachalam, S.; Verdesca, V.; Cini, E.; Rossi, M.; McCormick, P.J.; Corsini, G.U.; Maggio, R.; Scarselli, M. Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences. Pharmacol. Ther. 2018, 192, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Marder, S.R.; Cannon, T.D. Schizophrenia. N. Engl. J. Med. 2019, 381, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Galiniak, S.; Bartosz, G.; Zuberek, M.; Grzelak, A.; Dietrich-Muszalska, A. Antioxidant properties of atypical antipsychotic drugs used in the treatment of schizophrenia. Schizophr. Res. 2016, 176, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Altuntas, I.; Aksoy, H.; Coskun, I.; Cayköylü, A.; Akçay, F. Erythrocyte superoxide dismutase and glutathione peroxidase activities, and malondialdehyde and reduced glutathione levels in schizophrenic patients. Clin. Chem. Lab. Med. 2000, 38, 1277–1281. [Google Scholar] [CrossRef]
- Yao, J.K.; Reddy, R.; van Kammen, D.P. Abnormal age-related changes of plasma antioxidant proteins in schizophrenia. Psychiatry Res. 2000, 97, 137–151. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tan, Y.L.; Cao, L.Y.; Wu, G.Y.; Xu, Q.; Shen, Y.; Zhou, D.F. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr. Res. 2006, 81, 291–300. [Google Scholar] [CrossRef]
- Kropp, S.; Kern, V.; Lange, K.; Degner, D.; Hajak, G.; Kornhuber, J.; Rüther, E.; Emrich, H.M.; Schneider, U.; Bleich, S. Oxidative stress during treatment with first- and second-generation antipsychotics. J. Neuropsychiatry Clin. Neurosci. 2005, 17, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Sagara, Y. Induction of reactive oxygen species in neurons by haloperidol. J. Neurochem. 1998, 71, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, K.; Anemo, K.; Nakamura, K.; Fukunishi, I.; Igarashi, K. Analysis of the metabolism of haloperidol and its neurotoxic pyridinium metabolite in patients with drug-induced parkinsonism. Neuropsychobiology 2001, 44, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Padurariu, M.; Ciobica, A.; Hritcu, L.; Stoica, B.; Bild, W.; Stefanescu, C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci. Lett. 2010, 469, 6–10. [Google Scholar] [CrossRef]
- Akyol, Ö.; Herken, H.; Uz, E.; Fadıllıoǧlu, E.; Ünal, S.; Söǧüt, S.; Özyurt, H.; Savaş, H.A. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients: The possible role of oxidant/antioxidant imbalance. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2002, 26, 995–1005. [Google Scholar] [CrossRef]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Invest. 2002, 62, 231–236. [Google Scholar] [CrossRef]
- Dakhale, G.; Khanzode, S.; Khanzode, S.; Saoji, A.; Khobragade, L.; Turankar, A. Oxidative damage and schizophrenia: The potential benefit by atypical antipsychotics. Neuropsychobiology 2004, 49, 205–209. [Google Scholar] [CrossRef]
- Evans, D.R.; Parikh, V.V.; Khan, M.M.; Coussons, C.; Buckley, P.F.; Mahadik, S.P. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins. Leukot. Essent. Fatty Acids 2003, 69, 393–399. [Google Scholar] [CrossRef]
- Blakely, R.D.; Wages, S.A.; Justice, J.B.; Herndon, J.G.; Neill, D.B. Neuroleptics increase striatal catecholamine metabolites but not ascorbic acid in dialyzed perfusate. Brain Res. 1984, 308, 1–8. [Google Scholar] [CrossRef]
- Eftekhari, A.; Azarmi, Y.; Parvizpur, A.; Eghbal, M.A. Involvement of oxidative stress and mitochondrial/lysosomal cross-talk in olanzapine cytotoxicity in freshly isolated rat hepatocytes. Xenobiotica 2016, 46, 369–378. [Google Scholar] [CrossRef]
- Dietrich-Muszalska, A.; Kolińska-Łukaszuk, J. Comparative effects of aripiprazole and selected antipsychotic drugs on lipid peroxidation in plasma. Psychiatry Clin. Neurosci. 2018, 72, 329–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Bai, O.; Richardson, J.S.; Mousseau, D.D.; Li, X.-M. Olanzapine protects PC12 cells from oxidative stress induced by hydrogen peroxide. J. Neurosci. Res. 2003, 73, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhou, D.F.; Shen, Y.C.; Zhang, P.Y.; Zhang, W.F.; Liang, J.; Chen, D.C.; Xiu, M.H.; Kosten, T.A.; Kosten, T.R. Effects of risperidone and haloperidol on superoxide dismutase and nitric oxide in schizophrenia. Neuropharmacology 2012, 62, 1928–1934. [Google Scholar] [CrossRef] [PubMed]
- Al-Chalabi, B.M.; Thanoon, I.A.J.; Ahmed, F.A. Potential effect of olanzapine on total antioxidant status and lipid peroxidation in schizophrenic patients. Neuropsychobiology 2009, 59, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Dalla Libera, A.; Scutari, G.; Boscolo, R.; Rigobello, M.P.; Bindoli, A. Antioxidant properties of clozapine and related neuroleptics. Free Radic. Res. 1998, 29, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kracmarova, A.; Pohanka, M. The impact of clozapine on regulation of inflammation in murine macrophage cells. Neuro Endocrinol. Lett. 2014, 35 (Suppl. 2), 175–179. [Google Scholar]
- Singh, O.P.; Chakraborty, I.; Dasgupta, A.; Datta, S. A comparative study of oxidative stress and interrelationship of important antioxidants in haloperidol and olanzapine treated patients suffering from schizophrenia. Indian J. Psychiatry 2008, 50, 171–176. [Google Scholar] [CrossRef]
- Fehsel, K.; Loeffler, S.; Krieger, K.; Henning, U.; Agelink, M.; Kolb-Bachofen, V.; Klimke, A. Clozapine induces oxidative stress and proapoptotic gene expression in neutrophils of schizophrenic patients. J. Clin. Psychopharmacol. 2005, 25, 419–426. [Google Scholar] [CrossRef]
- de Farias, C.C.; Bonifácio, K.L.; Matsumoto, A.K.; Higachi, L.; Casagrande, R.; Moreira, E.G.; Barbosa, D.S. Comparison of the antioxidant potential of antiparkinsonian drugs in different in vitro models. Braz. J. Pharm. Sci. 2014, 50, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Mailman, R.B.; Murthy, V. Third generation antipsychotic drugs: Partial agonism or receptor functional selectivity? Curr. Pharm. Des. 2010, 16, 488–501. [Google Scholar] [CrossRef] [Green Version]
- Park, S.W.; Lee, C.H.; Lee, J.G.; Kim, L.W.; Shin, B.S.; Lee, B.J.; Kim, Y.H. Protective effects of atypical antipsychotic drugs against MPP+-induced oxidative stress in PC12 cells. Neurosci. Res. 2011, 69, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.A.; Monji, A.; Yasukawa, K.; Mizoguchi, Y.; Horikawa, H.; Seki, Y.; Hashioka, S.; Han, Y.-H.; Kasai, M.; Sonoda, N.; et al. Aripiprazole inhibits superoxide generation from phorbol-myristate-acetate (PMA)-stimulated microglia in vitro: Implication for antioxidative psychotropic actions via microglia. Schizophr. Res. 2011, 129, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Songur, A.; Sarsilmaz, M.; Sogut, S.; Ozyurt, B.; Ozyurt, H.; Zararsiz, I.; Turkoglu, A.O. Hypothalamic superoxide dismutase, xanthine oxidase, nitric oxide, and malondialdehyde in rats fed with fish ω-3 fatty acids. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, M.; Sourgens, H.; Arolt, V.; Erfurth, A. Severe tardive dyskinesia in affective disorders: Treatment with vitamin E and C. Neuropsychobiology 2002, 46, 28–30. [Google Scholar]
- Singh, S.; Barreto, G.; Aliev, G.; Echeverria, V. Ginkgo biloba as an alternative medicine in the treatment of anxiety in dementia and other psychiatric disorders. Curr. Drug Metab. 2017, 18, 112–119. [Google Scholar] [CrossRef]
- Schiavone, S.; Trabace, L. The use of antioxidant compounds in the treatment of first psychotic episode: Highlights from preclinical studies. CNS Neurosci. Ther. 2018, 24, 465–472. [Google Scholar] [CrossRef]
- das Neves Duarte, J.M.; Kulak, A.; Gholam-Razaee, M.M.; Cuenod, M.; Gruetter, R.; Do, K.Q. N-acetylcysteine normalizes neurochemical changes in the glutathione-deficient schizophrenia mouse model during development. Biol. Psychiatry 2012, 71, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Cabungcal, J.-H.; Steullet, P.; Kraftsik, R.; Cuenod, M.; Do, K.Q. Early-life insults impair parvalbumin interneurons via oxidative stress: Reversal by N-acetylcysteine. Biol. Psychiatry 2013, 73, 574–582. [Google Scholar] [CrossRef]
- Phensy, A.; Duzdabanian, H.E.; Brewer, S.; Panjabi, A.; Driskill, C.; Berz, A.; Peng, G.; Kroener, S. Antioxidant treatment with n-acetyl cysteine prevents the development of cognitive and social behavioral deficits that result from perinatal ketamine treatment. Front. Behav. Neurosci. 2017, 11, 106. [Google Scholar] [CrossRef] [Green Version]
- Cabungcal, J.-H.; Counotte, D.S.; Lewis, E.M.; Tejeda, H.A.; Piantadosi, P.; Pollock, C.; Calhoon, G.G.; Sullivan, E.M.; Presgraves, E.; Kil, J.; et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron 2014, 83, 1073–1084. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Pereira, V.S.; Hiroaki-Sato, V.A. A brief history of antidepressant drug development: From tricyclics to beyond ketamine. Acta Neuropsychiatr. 2018, 30, 307–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative stress and anxiety: Relationship and cellular pathways. Oxid. Med. Cell. Longev. 2009, 2, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Battal, D.; Yalin, S.; Eker, E.D.; Aktas, A.; Sahin, N.O.; Cebo, M.; Berköz, M. Possible role of selective serotonin reuptake inhibitor sertraline on oxidative stress responses. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 477–484. [Google Scholar] [PubMed]
- da Silva, A.I.; Braz, G.R.F.; Silva-Filho, R.; Pedroza, A.A.; Ferreira, D.S.; Manhães de Castro, R.; Lagranha, C. Effect of fluoxetine treatment on mitochondrial bioenergetics in central and peripheral rat tissues. Appl. Physiol. Nutr. Metab. 2015, 40, 565–574. [Google Scholar] [CrossRef]
- Mendez-David, I.; Tritschler, L.; El Ali, Z.; Damiens, M.-H.; Pallardy, M.; David, D.J.; Kerdine-Römer, S.; Gardier, A.M. Nrf2-signaling and BDNF: A new target for the antidepressant-like activity of chronic fluoxetine treatment in a mouse model of anxiety/depression. Neurosci. Lett. 2015, 597, 121–126. [Google Scholar] [CrossRef]
- Baker, G.; Matveychuk, D.; MacKenzie, E.M.; Holt, A.; Wang, Y.; Kar, S. Attenuation of the effects of oxidative stress by the MAO-inhibiting antidepressant and carbonyl scavenger phenelzine. Chem. Biol. Interact. 2019, 304, 139–147. [Google Scholar] [CrossRef]
- Thase, M.E. Atypical depression: Useful concept, but it’s time to revise the DSM-IV criteria. Neuropsychopharmacology 2009, 34, 2633–2641. [Google Scholar] [CrossRef] [Green Version]
- Kulbe, J.R.; Singh, I.N.; Wang, J.A.; Cebak, J.E.; Hall, E.D. Continuous infusion of phenelzine, cyclosporine A, or their combination: Evaluation of mitochondrial bioenergetics, oxidative damage, and cytoskeletal degradation following severe controlled cortical impact traumatic brain injury in rats. J. Neurotrauma 2018, 35, 1280–1293. [Google Scholar] [CrossRef]
- Baker, G.B.; Matveychuk, D.; MacKenzie, E.M.; Dursun, S.M.; Mousseau, D.D. Monoamine oxidase inhibitors and neuroprotective mechanisms. Klin. Psikofarmakol. Bülteni-Bull. Clin. Psychopharmacol. 2012, 22, 293–296. [Google Scholar] [CrossRef]
- Clineschmidt, B.V.; Horita, A. The monoamine oxidase catalyzed degradation of phenelzine-1-14C, an irreversible inhibitor of monoamine oxidase—I: Studies in vitro. Biochem. Pharmacol. 1969, 18, 1011–1020. [Google Scholar] [CrossRef]
- Tipton, K.F. The reaction of monoamine oxidase with phenethylhydrazine. Biochem. J. 1971, 121, 33P–34P. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.S.; Han, E.S.; Lee, W.B. Antioxidant effect of phenelzine on MPP+-induced cell viability loss in differentiated PC12 cells. Neurochem. Res. 2003, 28, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Palchoudhuri, S.; Mukhopadhyay, D.; Roy, D.S.; Ghosh, B.; Das, S.; Dastidar, S.G. The antidepressant drug doxepin: A promising antioxidant. Asian J. Pharm. Clin. Res. 2017, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zanforlin, E.; Zagotto, G.; Ribaudo, G. An overview of new possible treatments of alzheimer’s disease, based on natural products and semi-synthetic compounds. Curr. Med. Chem. 2017, 24, 3749–3773. [Google Scholar] [CrossRef]
- Ribaudo, G.; Coghi, P.; Zanforlin, E.; Law, B.Y.K.; Wu, Y.Y.J.; Han, Y.; Qiu, A.C.; Qu, Y.Q.; Zagotto, G.; Wong, V.K.W. Semi-synthetic isoflavones as BACE-1 inhibitors against Alzheimer’s disease. Bioorg. Chem. 2019, 87, 474–483. [Google Scholar] [CrossRef]
- Martini, L.H.; Jung, F.; Soares, F.A.; Rotta, L.N.; Vendite, D.A.; dos Santos Frizzo, M.E.; Yunes, R.A.; Calixto, J.B.; Wofchuk, S.; Souza, D.O. Naturally occurring compounds affect glutamatergic neurotransmission in rat brain. Neurochem. Res. 2007, 32, 1950–1956. [Google Scholar] [CrossRef]
- Pingili, R.; Vemulapalli, S.; Mullapudi, S.S.; Nuthakki, S.; Pendyala, S.; Kilaru, N. Pharmacokinetic interaction study between flavanones (hesperetin, naringenin) and rasagiline mesylate in wistar rats. Drug Dev. Ind. Pharm. 2016, 42, 1110–1117. [Google Scholar] [CrossRef]
- Ortmann, C.F.; Abelaira, H.M.; Réus, G.Z.; Ignácio, Z.M.; Chaves, V.C.; dos Santos, T.C.; de Carvalho, P.; Carlessi, A.S.; Bruchchen, L.; Danielski, L.G.; et al. LC/QTOF profile and preliminary stability studies of an enriched flavonoid fraction of Cecropia pachystachya Trécul leaves with potential antidepressant-like activity. Biomed. Chromatogr. 2017, 31, e3982. [Google Scholar] [CrossRef]
- Antunes, M.S.; Jesse, C.R.; Ruff, J.R.; de Oliveira Espinosa, D.; Gomes, N.S.; Altvater, E.E.T.; Donato, F.; Giacomeli, R.; Boeira, S.P. Hesperidin reverses cognitive and depressive disturbances induced by olfactory bulbectomy in mice by modulating hippocampal neurotrophins and cytokine levels and acetylcholinesterase activity. Eur. J. Pharmacol. 2016, 789, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Lin, J.; Li, Y.; Wang, T.; Jiang, Q.; Chen, D. Sarcandra glabra (Caoshanhu) protects mesenchymal stem cells from oxidative stress: A bioevaluation and mechanistic chemistry. BMC Complement. Altern. Med. 2016, 16, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabirifar, R.; Ghoreshi, Z.-A.-S.; Safari, F.; Karimollah, A.; Moradi, A.; Eskandari-Nasab, E. Quercetin protects liver injury induced by bile duct ligation via attenuation of Rac1 and NADPH oxidase1 expression in rats. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 88–95. [Google Scholar] [CrossRef]
- Alam, M.A.; Zaidul, I.S.M.; Ghafoor, K.; Sahena, F.; Hakim, M.A.; Rafii, M.Y.; Abir, H.M.; Bostanudin, M.F.; Perumal, V.; Khatib, A. In vitro antioxidant and, α-glucosidase inhibitory activities and comprehensive metabolite profiling of methanol extract and its fractions from Clinacanthus nutans. BMC Complement. Altern. Med. 2017, 17, 181. [Google Scholar] [CrossRef] [Green Version]
- Minarini, A.; Ferrari, S.; Galletti, M.; Giambalvo, N.; Perrone, D.; Rioli, G.; Galeazzi, G.M. N-acetylcysteine in the treatment of psychiatric disorders: Current status and future prospects. Expert Opin. Drug Metab. Toxicol. 2017, 13, 279–292. [Google Scholar] [CrossRef]
- Ooi, S.L.; Green, R.; Pak, S.C. N-Acetylcysteine for the treatment of psychiatric disorders: A review of current evidence. Biomed Res. Int. 2018, 2018, 2469486. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.-Z.; Wang, J.; Sheridan, S.D.; Perlis, R.H.; Rasenick, M.M. N-3 polyunsaturated fatty acids promote astrocyte differentiation and neurotrophin production independent of cAMP in patient-derived neural stem cells. Mol. Psychiatry 2020, 1–11. [Google Scholar] [CrossRef]
- Zhao, K.; So, H.-C. Drug repositioning for schizophrenia and depression/anxiety disorders: A machine learning approach leveraging expression data. IEEE J. Biomed. Health Inform. 2019, 23, 1304–1315. [Google Scholar] [CrossRef]
- Kumar, A.; Tiwari, A.; Sharma, A. Changing paradigm from one target one ligand towards multi-target directed ligand design for key drug targets of alzheimer disease: An important role of in silico methods in multi-target directed ligands design. Curr. Neuropharmacol. 2018, 16, 726–739. [Google Scholar] [CrossRef]
- Langbein, K.; Hesse, J.; Gussew, A.; Milleit, B.; Lavoie, S.; Amminger, G.P.; Gaser, C.; Wagner, G.; Reichenbach, J.R.; Hipler, U.-C.; et al. Disturbed glutathione antioxidative defense is associated with structural brain changes in neuroleptic-naïve first-episode psychosis patients. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 103–110. [Google Scholar] [CrossRef]
- Casaril, A.M.; Domingues, M.; de Andrade Lourenço, D.; Birmann, P.T.; Padilha, N.; Vieira, B.; Begnini, K.; Seixas, F.K.; Collares, T.; Lenardão, E.J.; et al. Depression- and anxiogenic-like behaviors induced by lipopolysaccharide in mice are reversed by a selenium-containing indolyl compound: Behavioral, neurochemical and computational insights involving the serotonergic system. J. Psychiatr. Res. 2019, 115, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, P.; Malik, N.; Khatkar, A. Exploration of umbelliferone based derivatives as potent MAO inhibitors: Dry vs. wet lab evaluation. Curr. Top. Med. Chem. 2019, 18, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Muraro, C.; Dalla Tiezza, M.; Pavan, C.; Ribaudo, G.; Zagotto, G.; Orian, L. Major depressive disorder and oxidative stress: In silico investigation of fluoxetine activity against ROS. Appl. Sci. 2019, 9, 3631. [Google Scholar] [CrossRef] [Green Version]
- Bortoli, M.; Dalla Tiezza, M.; Muraro, C.; Pavan, C.; Ribaudo, G.; Rodighiero, A.; Tubaro, C.; Zagotto, G.; Orian, L. Psychiatric disorders and oxidative injury: Antioxidant effects of zolpidem therapy disclosed in silico. Comput. Struct. Biotechnol. J. 2019, 17, 311–318. [Google Scholar] [CrossRef]
- Galano, A.; Raúl Alvarez-Idaboy, J. Computational strategies for predicting free radical scavengers’ protection against oxidative stress: Where are we and what might follow? Int. J. Quantum Chem. 2019, 119, e25665. [Google Scholar] [CrossRef] [Green Version]
- Avram, S.; Borcan, F.; Borcan, L.-C.; Milac, A.L.; Mihailescu, D. QSAR approaches applied to antidepressants induced neurogenesis—In vivo and in silico applications. Mini-Rev. Med. Chem. 2015, 16, 230–240. [Google Scholar] [CrossRef]
- Mernea, M.; Borcan, L.-C.; Borcan, F.; Avram, S. Antipsychotics as psychosis drugs and neuroprotective promoters evaluated by chemical QSAR—In silico and in vivo studies. Lett. Drug Des. Discov. 2016, 13, 269–275. [Google Scholar] [CrossRef]
- Silva, D.R.; Barigye, S.J.; Santos-Garcia, L.; Fontes Ferreira da Cunha, E. Molecular modelling of potential candidates for the treatment of depression. Mol. Inform. 2019, 38, 1900024. [Google Scholar] [CrossRef]
- Zanatta, G.; Nunes, G.; Bezerra, E.M.; da Costa, R.F.; Martins, A.; Caetano, E.W.S.; Freire, V.N.; Gottfried, C. Antipsychotic haloperidol binding to the human dopamine D3 receptor: Beyond docking through QM/MM refinement toward the design of improved schizophrenia medicines. ACS Chem. Neurosci. 2014, 5, 1041–1054. [Google Scholar] [CrossRef]
- Sasahara, K.; Mashima, A.; Yoshida, T.; Chuman, H. Molecular dynamics and density functional studies on the metabolic selectivity of antipsychotic thioridazine by cytochrome P450 2D6: Connection with crystallographic and metabolic results. Bioorg. Med. Chem. 2015, 23, 5459–5465. [Google Scholar] [CrossRef]
- Zanatta, G.; Della Flora Nunes, G.; Bezerra, E.M.; da Costa, R.F.; Martins, A.; Caetano, E.W.S.; Freire, V.N.; Gottfried, C. Two binding geometries for risperidone in dopamine D3 receptors: Insights on the fast-off mechanism through docking, quantum biochemistry, and molecular dynamics simulations. ACS Chem. Neurosci. 2016, 7, 1331–1347. [Google Scholar] [CrossRef]
- Ekhteiari Salmas, R.; Serhat Is, Y.; Durdagi, S.; Stein, M.; Yurtsever, M. A QM protein–ligand investigation of antipsychotic drugs with the dopamine D2 Receptor (D2R). J. Biomol. Struct. Dyn. 2018, 36, 2668–2677. [Google Scholar] [CrossRef]
- Abraham, C.S.; Muthu, S.; Prasana, J.C.; Armaković, S.J.; Armaković, S.; Fathima Rizwana, B.; Ben Geoffrey, A.S. Spectroscopic profiling (FT-IR, FT-Raman, NMR and UV-Vis), autoxidation mechanism (H-BDE) and molecular docking investigation of 3-(4-chlorophenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amine by DFT/TD-DFT and molecular dynamics: A potential SSRI drug. Comput. Biol. Chem. 2018, 77, 131–145. [Google Scholar] [CrossRef]
- Sagdinc, S.G.; Azkeskin, C.; Eşme, A. Theoretical and spectroscopic studies of a tricyclic antidepressant, imipramine hydrochloride. J. Mol. Struct. 2018, 1161, 169–184. [Google Scholar] [CrossRef]
- Kuruvilla, T.K.; Prasana, J.C.; Muthu, S.; George, J. Vibrational spectroscopic (FT-IR, FT-Raman) and quantum mechanical study of 4-(2-chlorophenyl)-2-ethyl-9-methyl-6H-thieno[3,2-f] [1,2,4]triazolo[4,3-a][1,4] diazepine. J. Mol. Struct. 2018, 1157, 519–529. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 2006, 125, 194101. [Google Scholar] [CrossRef] [Green Version]
- Galano, A.; Alvarez-Idaboy, J.R. A computational methodology for accurate predictions of rate constants in solution: Application to the assessment of primary antioxidant activity. J. Comput. Chem. 2013, 34, 2430–2445. [Google Scholar] [CrossRef]
- Galano, A. On the direct scavenging activity of melatonin towards hydroxyl and a series of peroxyl radicals. Phys. Chem. Chem. Phys. 2011, 13, 7178. [Google Scholar] [CrossRef]
- Galano, A. Mechanism and kinetics of the hydroxyl and hydroperoxyl radical scavenging activity of N-acetylcysteine amide. Theor. Chem. Acc. 2011, 130, 51–60. [Google Scholar] [CrossRef]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to Isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Mennucci, B.; Cancès, E.; Tomasi, J. Evaluation of solvent effects in isotropic and anisotropic dielectrics and in ionic solutions with a unified integral equation method: Theoretical bases, computational implementation, and numerical applications. J. Phys. Chem. B 1997, 101, 10506–10517. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cancès, E. The IEF version of the PCM solvation method: An overview of a new method addressed to study molecular solutes at the QM ab initio level. J. Mol. Struct. 1999, 464, 211–226. [Google Scholar] [CrossRef]
- Reina, M.; Castañeda-Arriaga, R.; Perez-Gonzalez, A.; Guzman-Lopez, E.G.; Tan, D.-X.; Reiter, R.J.; Galano, A. A computer-assisted systematic search for melatonin derivatives with high potential as antioxidants. Melatonin Res. 2018, 1, 27–58. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Li, D.-X.; Pan, Q.; Yu, H.-X.; Chen, C.-L.; Liu, B.-L. Mechanism research on antioxidant activity of drugs containing sulfhydryl groups. Med. Biopharm. 2016, 897–906. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Deng, G.; Guo, R.; Chen, D.-F.; Fu, Z.-M. DFT studies on the antioxidant activity of naringenin and its derivatives: Effects of the substituents at C3. Int. J. Mol. Sci. 2019, 20, 1450. [Google Scholar] [CrossRef] [Green Version]
- Jabbari, M.; Mir, H.; Kanaani, A.; Ajloo, D. Kinetic solvent effects on the reaction between flavonoid naringenin and 2,2-diphenyl-1-picrylhydrazyl radical in different aqueous solutions of ethanol: An experimental and theoretical study. J. Mol. Liq. 2014, 196, 381–391. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, J.; Lu, Y.; Qian, L.-L.; Yang, Z.-Y.; Han, R.-M.; Zhang, J.-P.; Skibsted, L.H. Copper(II) coordination and translocation in luteolin and effect on radical scavenging. J. Phys. Chem. B 2020, 124, 380–388. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Bensasson, R.V.; Sowlati-Hashjin, S.; Zoete, V.; Dauzonne, D.; Matta, C.F. Physicochemical properties of exogenous molecules correlated with their biological efficacy as protectors against carcinogenesis and inflammation. Int. Rev. Phys. Chem. 2013, 32, 393–434. [Google Scholar] [CrossRef]
- Dangles, O.; Dufour, C.; Tonnelé, C.; Trouillas, P. The physical chemistry of polyphenols. In Recent Advances in Polyphenol Research; John Wiley & Sons, Ltd.: Chichester, UK, 2016; Volume 5, pp. 1–35. ISBN 9781118883303. [Google Scholar]
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and flavonols’ antiradical structure–activity relationship—A quantum chemical study. Antioxidants 2020, 9, 461. [Google Scholar] [CrossRef]
- Ribaudo, G.; Bortoli, M.; Ongaro, A.; Oselladore, E.; Gianoncelli, A.; Zagotto, G.; Orian, L. Fluoxetine scaffold to design tandem molecular antioxidants and green catalysts. RSC Adv. 2020, 10, 18583–18593. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why nature chose selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Orian, L.; Mauri, P.; Roveri, A.; Toppo, S.; Benazzi, L.; Bosello-Travain, V.; De Palma, A.; Maiorino, M.; Miotto, G.; Zaccarin, M.; et al. Selenocysteine oxidation in glutathione peroxidase catalysis: An MS-supported quantum mechanics study. Free Radic. Biol. Med. 2015, 87, 1–14. [Google Scholar] [CrossRef]
- Bortoli, M.; Torsello, M.; Bickelhaupt, F.M.; Orian, L. Role of the chalcogen (S, Se, Te) in the oxidation mechanism of the glutathione peroxidase active site. ChemPhysChem 2017, 18, 2990–2998. [Google Scholar] [CrossRef]
- Orian, L.; Cozza, G.; Maiorino, M.; Toppo, S.; Ursini, F. The mechanism of glutathione peroxidases. In Glutathione; Flohé, L., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 53–66. [Google Scholar]
- Dalla Tiezza, M.; Bickelhaupt, F.M.; Flohé, L.; Maiorino, M.; Ursini, F.; Orian, L. A dual attack on the peroxide bond. The common principle of peroxidatic cysteine or selenocysteine residues. Redox Biol. 2020, 34, 101540. [Google Scholar] [CrossRef]
- Orian, L.; Toppo, S. Organochalcogen peroxidase mimetics as potential drugs: A long story of a promise still unfulfilled. Free Radic. Biol. Med. 2014, 66, 65–74. [Google Scholar] [CrossRef]
- Dalla Tiezza, M.; Ribaudo, G.; Orian, L. Organodiselenides: Organic catalysis and drug design learning from glutathione peroxidase. Curr. Org. Chem. 2019, 23, 1381–1402. [Google Scholar] [CrossRef] [Green Version]
- Wolters, L.P.; Orian, L. Peroxidase activity of organic selenides: Mechanistic insights from quantum chemistry. Curr. Org. Chem. 2016, 20, 189–197. [Google Scholar] [CrossRef]
- Bortoli, M.; Wolters, L.P.; Orian, L.; Bickelhaupt, F.M. Addition-elimination or nucleophilic substitution? Understanding the energy profiles for the reaction of chalcogenolates with dichalcogenides. J. Chem. Theory Comput. 2016, 12, 2752–2761. [Google Scholar] [CrossRef]
- Ribaudo, G.; Bellanda, M.; Menegazzo, I.; Wolters, L.P.; Bortoli, M.; Ferrer-Sueta, G.; Zagotto, G.; Orian, L. Mechanistic insight into the oxidation of organic phenylselenides by H2O2. Chem. Eur. J. 2017, 23, 2405–2422. [Google Scholar] [CrossRef] [PubMed]
- Bortoli, M.; Zaccaria, F.; Dalla Tiezza, M.; Bruschi, M.; Fonseca Guerra, C.; Bickelhaupt, F.M.; Orian, L. Oxidation of organic diselenides and ditellurides by H2O2 for bioinspired catalyst design. Phys. Chem. Chem. Phys. 2018, 20, 20874–20885. [Google Scholar] [CrossRef] [PubMed]
- Bortoli, M.; Bruschi, M.; Swart, M.; Orian, L. Sequential oxidations of phenylchalcogenides by H2O2: Insights into the redox behavior of selenium via DFT analysis. New J. Chem. 2020, 44, 6724–6731. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribaudo, G.; Bortoli, M.; Pavan, C.; Zagotto, G.; Orian, L. Antioxidant Potential of Psychotropic Drugs: From Clinical Evidence to In Vitro and In Vivo Assessment and toward a New Challenge for in Silico Molecular Design. Antioxidants 2020, 9, 714. https://doi.org/10.3390/antiox9080714
Ribaudo G, Bortoli M, Pavan C, Zagotto G, Orian L. Antioxidant Potential of Psychotropic Drugs: From Clinical Evidence to In Vitro and In Vivo Assessment and toward a New Challenge for in Silico Molecular Design. Antioxidants. 2020; 9(8):714. https://doi.org/10.3390/antiox9080714
Chicago/Turabian StyleRibaudo, Giovanni, Marco Bortoli, Chiara Pavan, Giuseppe Zagotto, and Laura Orian. 2020. "Antioxidant Potential of Psychotropic Drugs: From Clinical Evidence to In Vitro and In Vivo Assessment and toward a New Challenge for in Silico Molecular Design" Antioxidants 9, no. 8: 714. https://doi.org/10.3390/antiox9080714
APA StyleRibaudo, G., Bortoli, M., Pavan, C., Zagotto, G., & Orian, L. (2020). Antioxidant Potential of Psychotropic Drugs: From Clinical Evidence to In Vitro and In Vivo Assessment and toward a New Challenge for in Silico Molecular Design. Antioxidants, 9(8), 714. https://doi.org/10.3390/antiox9080714







