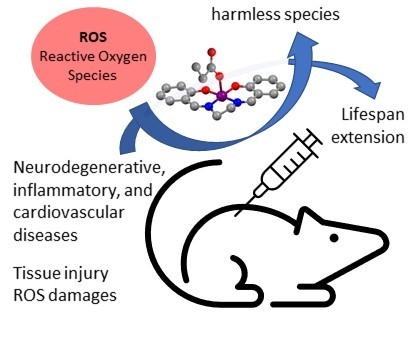
Pursuing the Elixir of Life: In Vivo Antioxidative Effects of Manganosalen Complexes
Abstract
1. Introduction
2. Manganese Superoxide Dismutases, Peroxidases, and Catalases
3. Manganosalen Complexes as Catalytic Antioxidants
4. Therapeutic Effects of Manganosalen Complexes in In Vivo Models
4.1. Neurodegenerative Diseases and Mental Disorders
4.2. Inflammatory Diseases
4.3. Cardiovascular Diseases
4.4. Skin Damage
4.5. Fetal Malformations
4.6. Adrenal and Liver Diseases
4.7. Lifespan Extension
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Melov, S.; Ravenscroft, J.; Malik, S.; Gill, M.S.; Walker, D.W.; Clayton, P.E.; Wallace, D.C.; Malfroy, B.; Doctrow, S.R.; Lithgow, G.J. Extension of life-span with superoxide dismutase/catalase mimetics. Science 2000, 289, 1567–1569. [Google Scholar] [CrossRef] [PubMed]
- Solomon, L.D. The Quest for Human Longevity: Science, Business, and Public Policy; Routledge: New York, NY, USA, 2006. [Google Scholar]
- Bracken, M.B. Why animal studies are often poor predictors of human reactions to exposure. J. R. Soc. Med. 2009, 102, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.; Doctrow, S.R.; Schneider, J.A.; Haberson, J.; Patel, M.; Coskun, P.E.; Huffman, K.; Wallace, D.C.; Malfroy, B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase–catalase mimetics. J. Neurosci. 2001, 21, 8348–8353. [Google Scholar] [CrossRef] [PubMed]
- Keaney, M.; Gems, D. No increase in lifespan in Caenorhabditis elegans upon treatment with the superoxide dismutase mimetic EUK-8. Free Radic. Biol. Med. 2003, 34, 277–282. [Google Scholar] [CrossRef]
- Doctrow, S.R.; Liesa, M.; Melov, S.; Shirihai, O.S.; Tofilon, P. Salen Mn complexes are superoxide dismutase/catalase mimetics that protect the mitochondria. Curr. Inorg. Chem. 2012, 2, 325–334. [Google Scholar] [CrossRef]
- Kostova, I.; Sasao, L. Advances in research of Schiff-base metal complexes as potent antioxidants. Curr. Med. Chem. 2013, 20, 4609–4632. [Google Scholar] [CrossRef]
- Bonetta, R. Potential therapeutic applications of MnSODs and SOD-mimetics. Chem. Eur. J. 2018, 24, 5032–5041. [Google Scholar] [CrossRef]
- Kubota, R.; Asayama, S.; Kawakami, H. Catalytic antioxidants for therapeutic medicine. J. Mat. Chem. B. 2019, 7, 3165–3191. [Google Scholar] [CrossRef]
- Rouco, L.; Maneiro, M. Neuroprotective effects of metalosalen complexes against oxidative stress. Neural. Regen. Res. 2021, 16, 121–122. [Google Scholar] [CrossRef]
- Matthijssens, F.; Back, P.; Braeckman, B.P.; Vanfleteren, J.R. Prooxidant activity of the superoxide dismutase (SOD)-mimetic EUK-8 in proliferating and growth-arrested Escherichia coli cells. Free Radic. Biol. Med. 2008, 45, 708–715. [Google Scholar] [CrossRef]
- Declercq, L.; Sente, I.; Hellemans, L.; Corstjens, H.; Maes, D. Use of the synthetic superoxide dismutase/catalase mimetic EUK-134 to compensate for seasonal antioxidant deficiency by reducing pre-existing lipid peroxides at the human skin surface. Int. J. Cosmet. Sci. 2004, 26, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Batinic-Haberle, I.; Tovmasayan, A.; Roberts, E.R.H.; Vujaskovic, Z.; Leong, K.W.; Spasojevic, I. SOD therapeutics: Latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxid. Redox Signal. 2014, 20, 2372–2415. [Google Scholar] [CrossRef] [PubMed]
- Doctrow, S.R.; Fish, B.; Huffman, K.D.; Lazarova, Z.; Medhora, M.; Williams, J.P.; Moulder, J.E. Salen manganese complexes mitigate radiation injury in normal tissues through modulation of tissue environment, including through redox mechanisms. In Redox-Active Therapeutics (Oxidative Stress in Applied Basic Research and Clinical Practice); Springer: Cham, Switzerland, 2016; pp. 265–285. [Google Scholar] [CrossRef]
- Walke, G.R.; Ranade, D.S.; Bapat, A.M.; Srikanth, R.; Kulkarni, P.P. Mn(III)-salen protect against different ROS species generated by the Aβ16-Cu complex. ChemistrySelect 2016, 1, 3497–3501. [Google Scholar] [CrossRef]
- Baglia, B.A.; Zaragoza, J.P.T.; Goldberg, D.P. Biomimetic reactivity of oxygen-derived manganese and iron porphyrinoid complexes. Chem. Rev. 2017, 117, 13320–13352. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.B.; Massie, A.A.; Jackson, T.A. Manganese-oxygen intermediates in O-O bond activation and hydrogen-atom transfer reactions. Acc. Chem. Res. 2017, 50, 2706–2717. [Google Scholar] [CrossRef]
- Yin, H.Y.; Tang, J.; Zhang, J.L. Introducing metallosalens into biological studies: The renaissance of traditional coordination complexes. Eur. J. Inorg. Chem. 2017, 44, 5085–5093. [Google Scholar] [CrossRef]
- Liberato, A.; Fernández-Trujillo, M.J.; Máñez, A.; Maneiro, M.; Rodríguez-Silva, L.; Basallote, M.G. Pitfalls in the ABTS peroxidase activity test: Interference of photochemical processes. Inorg. Chem. 2018, 57, 14471–14475. [Google Scholar] [CrossRef]
- Signorella, S.; Palopoli, C.; Ledesma, G. Rationally designed mimics of antioxidant manganoenzymes: Role of structural features in the quest for catalysts with catalase and superoxide dismutase activity. Coord. Chem. Rev. 2018, 305, 75–102. [Google Scholar] [CrossRef]
- Erxleben, A. Transition metal salen complexes in bioinorganic and medicinal chemistry. Inorg. Chim. Acta 2018, 472, 40–57. [Google Scholar] [CrossRef]
- Benkafadar, N.; François, F.; Affortit, C.; Casas, F.; Ceccato, J.C.; Menardo, J.; Venail, F.; Malfroy-Camine, B.; Puel, J.L.; Wang, J. ROS-induced activation of DNA damage responses drives senescence-like state in postmitotic cochlear cells: Implication for hearing preservation. Mol. Neurobiol. 2019, 56, 5950–5969. [Google Scholar] [CrossRef]
- Rouco, L.; Liberato, A.; Fernández-Trujillo, M.J.; Máñez, A.; Basallote, M.G.; Alvariño, R.; Alfonso, A.; Botana, L.M.; Maneiro, M. Salen-manganese complexes for controlling ROS damage: Neuroprotective effects, antioxidant activity and kinetic studies. J. Inorg. Biochem. 2020, 203, 110918. [Google Scholar] [CrossRef]
- Villamena, F.A. Chemistry of Reactive Species. Mol. Basis Oxidative 2013, 1–48. [Google Scholar] [CrossRef]
- Lakey, P.S.J.; Berkemeier, T.; Tong, H.; Arangio, A.M.; Lucas, L.; Pöschl, U.; Shiraiwa, M. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Sci. Rep. 2016, 6, 32916. [Google Scholar] [CrossRef] [PubMed]
- Church, D.F.; Pryor, W.A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect 1985, 64, 111–126. [Google Scholar] [CrossRef]
- Narayanan, P.K.; Goodwin, E.H.; Lehnert, B.E. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res. 1997, 57, 3963–3971. [Google Scholar] [PubMed]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef]
- Zarse, K.; Schmeisser, S.; Groth, M.; Priebe, S.; Beuster, G.; Kuhlow, D.; Guthke, R.; Platzer, M.; Kahn, C.R.; Ristow, M. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 2012, 15, 451–465. [Google Scholar] [CrossRef]
- Nyska, A.; Kohen, R. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovosky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2007; pp. 79–186. [Google Scholar]
- Cerutti, P.A. Oxidant stress and carcinogenesis. Eur. J. Clin. Investig. 1991, 21, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Tesler, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidants and human disease: A general introduction. Nutr. Rev. 1997, 55, S44–S49. [Google Scholar] [CrossRef]
- Seedek, M.; Callera, G.; Montezano, A.; Gutsol, A.; Heitz, F.; Szyndralewiez, C.; Page, P.; Kennedy, C.R.J.; Burns, K.D.; Touyz, R.M.; et al. Critical role of Nox-4 based NADPH oxidase in glucose-induced oxidative stress in the kidney: Implications in type 2 diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2010, 299, F1348–F1358. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Idelchik, M.D.S.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Lu, M.H.; Yuan, D.J.; Xu, D.E.; Yao, P.P.; Ji, W.L.; Chen, H.; Liu, W.L.; Yan, C.X.; Xia, Y.Y.; et al. Mitochondrial dysfunction in neural injury. Front. Neurosci. 2019, 13, 30. [Google Scholar] [CrossRef]
- Sharma, P.; Srivastava, P.; Seth, A.; Tripathi, P.N.; Banerjee, A.G.; Shrivastava, S.K. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog. Neurobiol. 2019, 174, 53–89. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.B.; Liu, T.Y.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.J.P.; Shin, D.S.; Getzoff, E.D.; Tainer, J.A. The structural biochemistry of the superoxide dismutases. Biochim. Biophys. Acta 2010, 1804, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Azadmanesh, J.; Borgstahl, G.E.O. A review of the catalytic mechanism of human manganese superoxide dismutase. Antioxidants 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.F.; Teixeira, M.; Valentine, J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef]
- Oszajca, M.; Brindell, M.; Orzeł, Ł.; Dąbrowski, J.M.; Śpiewak, K.; Łabuz, P.; Stochel, G. Mechanistic studies on versatile metal-assisted hydrogen peroxide activation processes for biomedical and environmetal incentives. Coord. Chem. Rev. 2016, 143, 327–328. [Google Scholar] [CrossRef]
- Whittaker, J.W. Non-heme manganese catalase–the ‘other’catalase. Arch. Biochem. Biophys. 2012, 525, 111–120. [Google Scholar] [CrossRef]
- Barynin, V.V.; Whittaker, M.M.; Antonyuk, S.V.; Lamzin, V.S.; Harrison, P.M.; Artymiuk, P.J.; Whittaker, J.W. Crystal structure of manganese catalase from Lactobacillus Plantarum. Structure 2001, 9, 725–738. [Google Scholar] [CrossRef]
- Frausto da Silva, J.J.R.; Williams, R.J.P. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Sundaramoorthy, M.; Gold, M.H.; Poulos, T.L. Ultrahigh (0.93 Å) resolution structure of manganese peroxidase from Phanerochaete chrysosporium: Implications for the catalytic mechanism. J. Inorg. Biochem. 2010, 104, 683–690. [Google Scholar] [CrossRef]
- Imai, H.; Nakagawa. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic. Biol. Med. 2003, 34, 145–169. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Tsikas, D.; Rossi, R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit. Rev. Clin. Lab. Sci. 2009, 46, 241–281. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Seki, T.; Hiroshi, M. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv. Drug Deliv. Rev. 2009, 61, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Venigalla, M.; Sonego, S.; Sharman, M.J.; Much, G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer’s disease. Neurchem. Int. 2016, 95, 63–74. [CrossRef]
- Essa, M.M.; Moghadas, M.; Ba-Omar, T.; Qoronfleh, M.W.; Guillemin, G.J.; Manivasagam, T.; Justin-Thenmozhi, A.; Ray, R.; Bhat, A.; Chidambaram, S.B.; et al. Protective effects of antioxidants in Huntington’s disease: An extensive review. Neurotox. Res. 2019, 3, 739–774. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J.S.; Nunes, S.; Rolo, A.P.; Reis, F.; Palmeira, C.M. Therapeutic options targeting oxidative stress, mitochondrial dysfunction and inflammation to hinder the progression of vascular complications of diabetes. Front. Physiol. 2019, 9, 1857. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Propac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Rosenthal, R.A.; Huffman, K.D.; Fisette, L.W.; Damphousse, C.A.; Callaway, W.B.; Malfroy, B.; Doctrow, S. Orally available Mn porphyrins with superoxide dismutase and catalase activities. J. Biol. Inorg. Chem. 2009, 14, 979–991. [Google Scholar] [CrossRef]
- Coudriet, G.M.; Delmastro-Greenwood, M.M.; Previte, D.M.; Marre, M.L.; O’Connor, E.C.; Novak, E.A.; Vincent, G.; Mollen, K.P.; Lee, S.; Dong, H.H.; et al. Treatment with a Catalytic Superoxide Dismutase (SOD) Mimetic Improves Liver Steatosis, Insulin Sensitivity, and Inflammation in Obesity-Induced Type 2 Diabetes. Antioxidants 2017, 6, 85. [Google Scholar] [CrossRef]
- Shrishrimal, S.; Kosmacek, E.A.; Chatterjee, A.; Tyson, M.J.; Oberley-Deegan, R.E. The SOD Mimic, Mnte-2-PyP, Protects from Chronic Fibrosis and Inflammation in Irradiated Normal Pelvic Tissues. Antioxidants 2017, 6, 87. [Google Scholar] [CrossRef]
- Galli, F.; Azzi, A.; Birringer, M.; Cook-Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Ozer, N.K. Vitamin E: Emerging aspects and new directions. Free Rad. Biol. Med. 2017, 102, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.H.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.L.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Cominacini, L.; Fratta Pasini, A.; Garbin, U.; Pastorino, A.M.; Davoli, A.; Nava, C.; Campagnola, M.; Rossato, P.; Lo Cascio, V. Antioxidant activity of different dihydropyridines. Biochem. Biophys. Res. Commun. 2003, 302, 679–684. [Google Scholar] [CrossRef]
- Pei, K.H.; Ou, J.Y.; Huang, J.Q.; Ou, S.Y. p-coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Breith, E.; Lübbe, E.; Tsumaki, T. Tricyclische orthokondensierte nebenvalenzringe. Justus Liebigs Ann. Chem. 1933, 503, 84–130. [Google Scholar] [CrossRef]
- Katsuki, T. Some recent advances in metallosalen chemistry. Synlett 2003, 3, 281–297. [Google Scholar] [CrossRef]
- Canali, L.; Sherrington, D.C. Utilisation of homogeneous and supported chiral metal(salen) complexes in asymmetric catalysis. Chem. Soc. Rev. 1999, 28, 85–93. [Google Scholar] [CrossRef]
- Shaw, S.; White, J.D. Asymmetric catalysis using chiral salen-metal complexes: Recent advances. Chem. Rev. 2019, 119, 9381–9426. [Google Scholar] [CrossRef] [PubMed]
- Baleizao, C.; Garcia, H. Chiral salen complexes: An overview to recoverable and reusable homogeneous and heterogeneous catalysts. Chem. Rev. 2006, 106, 3987–4043. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, J.C.; Correia, I. Salan vs. salen metal complexes in catalysis and medicinal applications: Virtues and pitfalls. Coord. Chem. Rev. 2019, 388, 227–247. [Google Scholar] [CrossRef]
- Chandra, P.; Ghosh, T.; Choudhary, N.; Mohammad, A.; Mobin, S.M. Recent advancement in oxidation or acceptorless dehytdrogenation of alcohols to valorised products using manganese based catalysts. Coord. Chem. Rev. 2020, 411, 213241. [Google Scholar] [CrossRef]
- Vázquez-Fernández, M.Á.; Bermejo, M.R.; Fernández-García, M.I.; González-Riopedre, G.; Rodríguez-Doutón, M.J.; Maneiro, M. Influence of the geometry around the manganese ion on the peroxidase and catalase activities of Mn(III)-Schiff base complexes. J. Inorg. Biochem. 2011, 105, 1538–1547. [Google Scholar] [CrossRef]
- Vázquez-Fernández, M.A.; Fernández-García, M.I.; González-Riopedre, G.; Maneiro, M.; Rodríguez-Doutón, M.J. Self-assembled biomimetic catalysts: Studies of the catalase and peroxidase activities of Mn(III)-Schiff base complexes. J. Coord. Chem. 2011, 64, 3843–3858. [Google Scholar] [CrossRef]
- González-Riopedre, G.; Bermejo, M.R.; Fernández-García, M.I.; González-Noya, A.M.; Pedrido, R.; Rodríguez-Doutón, M.; Maneiro, M. Alkali-metal-ion-directed self-assembly of redox-active manganese(III) supramolecular boxes. Inorg. Chem. 2015, 54, 2512–2521. [Google Scholar] [CrossRef]
- Bermejo, M.R.; Carballido, R.; Fernandez-García, M.I.; González-Noya, A.M.; González-Riopedre, G.; Maneiro, M.; Rodríguez-Silva, L. Synthesis, characterization, and catalytic studies of Mn(III)-Schiff base-dicyanamide complexes: Checking the rhombicity effect in peroxidase studies. J. Chem. 2017, 2017, 5465890. [Google Scholar] [CrossRef]
- Doctrow, S.R.; Huffman, K.; Bucay Marcus, C.; Tocco, G.; Malfroy, E.; Adinolfi, C.A.; Kruk, H.; Baker, K.; Lazarowych, N.; Mascarenhas, J.; et al. Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: Structure-activity relationship studies. J. Med. Chem. 2002, 45, 4549–4558. [Google Scholar] [CrossRef]
- Vázquez-Fernández, M.Á.; Fernández-García, M.I.; González-Noya, A.M.; Maneiro, M.; Bermejo, M.R.; Rodríguez-Doutón, M.J. Supramolecular networks of Mn(III)-Schiff base complexes assembled by nitrate counterions: X-ray crystal structures of 1D chains and 2D networks. Polyhedron 2012, 31, 379–385. [Google Scholar] [CrossRef]
- Palopoli, C.; Ferreyra, J.; Conte-Daban, A.; Richezzi, M.; Foi, A.; Doctorovich, F.; Anxolabehere-Mallart, E.; Hureau, C.; Signorella, S.R. Insights into second-sphere effects on redox potentials, spectroscopic properties and superoxide dismutase activity of manganese complexes with Schiff-base ligands. ACS Omega 2019, 4, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.I.; Kasiri, S.; Grant, J.D.; Mandal, S.S. Apoptosis and anti-tumour activities of manganese(III)-salen and -salphen complexes. Dalton Trans. 2009, 40, 8525–8531. [Google Scholar] [CrossRef] [PubMed]
- Bahramikia, S.; Yazdanparast, R.; Gheysarzadeh, A. Syntheses and structure-activity relationships of seven manganese-salen derivatives as anti-amyloidogenic and fibril-destabilizing agents against hen egg-white lysozyme aggregation. Chem. Biol. Drug Des. 2012, 80, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Signorella, S.; Hureau, C. Bioinspired functional mimics of the manganese catalases. Coord. Chem. Rev. 2012, 256, 1229–1245. [Google Scholar] [CrossRef]
- Kinnula, V.L.; Crapo, J.D. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care Med. 2003, 167, 1600–1619. [Google Scholar] [CrossRef]
- Watanabe, T.; Owada, S.; Kobayashi, H.P.; Kawakami, H.; Nagaoka, S.; Murakami, E.; Tobe, N.; Ishiuchi, A.; Enomoto, T.; Jinnouchi, Y.; et al. Protective effects of MnM2Py4P and Mn-salen against small bowel ischemia/reperfusion injury in rats using an in vivo and an ex vivo electron paramagnetic resonance technique with a spin probe. Transplant. Proc. 2007, 39, 3002–3006. [Google Scholar] [CrossRef]
- Baudry, M.; Etienne, S.; Bruce, A.; Palucki, M.; Jacobsen, E.; Malfroy, B. Salen-manganese complexes are superoxide dismutase-mimics. Biochem. Biophys. Res. Commun. 1993, 192, 964–968. [Google Scholar] [CrossRef]
- Friedel, F.C.; Lieb, D.; Ivanovic-Burmazovic, I. Compartive studies on manganese-based SOD mimetics, including the phosphate effect, by using global spectral analysis. J. Inorg. Biochem. 2012, 109, 26–32. [Google Scholar] [CrossRef]
- Batinić-Haberle, I.; Rebouças, J.S.; Spasojević, I. Superoxide dismutase mimics: Chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 2010, 13, 877–918. [Google Scholar] [CrossRef]
- Day, B.J. Catalase and glutathione peroxidase mimics. Biochem. Parmacol. 2009, 77, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.K.; Zhuang, J.; Doctrow, S.R.; Malfroy, B.; Smith, M.; Menconi, M.J.; Fink, M.P. Delayed treatment with EUK-8, a novel synthetic superoxide dismutase (SOD) and catalase (CAT) mimetic, ameliorates acute lung injury in endotoxemic pigs. Surg. Forum 1995, 46, 72–73. [Google Scholar]
- Watanabe, Y.; Namba, A.; Umezawa, N.; Kawahata, M.; Yamaguchi, K.; Higuchi, T. Enhanced catalase-like activity of manganese salen complexes in water: Effect of a three-dimensionally fixed auxiliary. Chem. Commun. 2006, 47, 4958–4960. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.I.; Nocera, D.G. Catalase and epoxidation activity of manganese salen complexes bearing two xanthene scaffolds. J. Am. Chem. Soc. 2007, 129, 8192–8198. [Google Scholar] [CrossRef]
- Maneiro, M.; Bermejo, M.R.; Fernández, M.I.; Gómez-Fórneas, E.; González-Noya, A.M.; Tyryshkin, A.M. A new type of manganese-Schiff base complex, catalysts for the disproportionation of hydrogen peroxide as peroxidase mimics. New J. Chem. 2003, 27, 727–733. [Google Scholar] [CrossRef]
- Doctrow, S.R.; Huffman, K.; Marcus, C.B.; Musleh, W.; Bruce, A.; Baudry, M.; Malfroy, B. Salen-manganese complexes: Combined superoxide dismutase/catalase mimics with broad pharmacological efficacy. Adv. Pharmacol. 1996, 38, 247–269. [Google Scholar] [CrossRef]
- Rong, Y.; Doctrow, S.R.; Tocco, G.; Baudry, M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc. Natl. Acad. Sci. USA 1999, 96, 9897–9902. [Google Scholar] [CrossRef]
- Malfroy, B.; Doctrow, S.R.; Orr, P.L.; Tocco, G.; Fedoseyeva, E.V.; Benichou, G. Prevention and suppression of autoimmune encephalomyelitis by EUK-8, a synthetic catalytic scavenger of oxygen-reactive metabolites. Cell Immunol. 1997, 177, 62–68. [Google Scholar] [CrossRef]
- Hinerfeld, D.; Traini, M.D.; Weinberger, R.P.; Cochran, B.; Doctrow, S.R.; Harry, J.; Melov, S. Endogenous mitochondrial oxidative stress: Neurodegeneration, proteomic analysis, specific respiratory chain defects, and efficacious antioxidant therapy in superoxide dismutase 2 null mice. J. Neurochem. 2004, 88, 657–667. [Google Scholar] [CrossRef]
- Melov, S.; Wolf, N.; Strozyk, D.; Doctrow, S.R.; Bush, A.I. Mice transgenic for Alzheimer disease β-amyloid develop lens cataracts that are rescued by antioxidant treatment. Free Radic. Biol. Med. 2005, 38, 258–261. [Google Scholar] [CrossRef]
- Forster, M.J.; Dubey, A.; Dawson, K.M.; Stutts, W.A.; Lal, H.; Sohal, R.S. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc. Natl. Acad. Sci. USA 1996, 93, 4765–4769. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, I.Y.; Bi, X.; Thompson, R.F.; Doctrow, S.R.; Malfroy, B.; Baudry, M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc. Natl. Acad. Sci. USA 2003, 100, 8526–8531. [Google Scholar] [CrossRef] [PubMed]
- Clausen, A.; Doctrow, S.; Baudry, M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol. Aging 2010, 31, 425–433. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Benov, L.; Spasojevic, I.; Fridovich, I. The ortho effect makes manganese (III) meso-tetrakis (N-methylpyridinium-2-yl) porphyrin a powerful and potentially useful superoxide dismutase mimic. J. Biol. Chem. 1998, 273, 24521–24528. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Rong, Y.; Doctrow, S.; Baudry, M.; Malfroy, B.; Xu, Z. Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse amyotrophic lateral sclerosis model. Neurosci. Lett. 2001, 304, 157–160. [Google Scholar] [CrossRef]
- Baker, K.; Marcus, C.B.; Huffman, K.; Kruk, H.; Malfroy, B.; Doctrow, S.R. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: A key role for reactive oxygen species in ischemic brain injury. J. Pharmacol. Exp. Ther. 1998, 284, 215–221. [Google Scholar]
- Peng, J.; Stevenson, F.F.; Doctrow, S.R.; Andersen, J.K. Superoxide Dismutase/Catalase Mimetics Are Neuroprotective against Selective Paraquat-mediated Dopaminergic Neuron Death in the Substantial Nigra. J. Biol. Chem. 2005, 280, 29194–29198. [Google Scholar] [CrossRef]
- Brazier, M.W.; Doctrow, S.R.; Masters, C.L.; Collins, S.J. A manganese-superoxide dismutase/catalase mimetic extends survival in a mouse model of human prion disease. Free Radic. Biol. Med. 2008, 45, 184–192. [Google Scholar] [CrossRef]
- Browne, S.E.; Roberts II, L.J.; Dennery, P.A.; Doctrow, S.R.; Beal, M.F.; Barlow, C.; Levine, R.L. Treatment with a catalytic antioxidant corrects the neurobehavioral defect in ataxia-telangiectasia mice. Free Radic. Biol. Med. 2004, 36, 938–942. [Google Scholar] [CrossRef]
- Raber, J.; Davis, M.J.; Pfankuch, T.; Rosenthal, R.; Doctrow, S.R.; Moulder, J.E. Mitigating effect of EUK-207 on radiation-induced cognitive impairments. Behav. Brain Res. 2017, 320, 457–463. [Google Scholar] [CrossRef]
- Wang, J.; Puel, J.L. Presbycusis: An Update on Cochlear Mechanisms and Therapies. J. Clinic. Med. 2020, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Burgering, B.M.; Medema, R.H. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J. Leukoc. Biol. 2003, 73, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Song, H.; Chen, H.Z.; Rovin, B.H. Activation of the Nrf2/antioxidant response pathway increases IL-8 expression. Eur. J. Immunol. 2005, 35, 3258–3267. [Google Scholar] [CrossRef]
- Muller, J.M.; Rupec, R.A.; Baeuerle, P.A. Study of gene regulation by NF-kappa B and AP-1 in response to reactive oxygen intermediates. Methods 1997, 11, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Langan, A.R.; Khan, M.A.; Yeung, I.W.; Van Dyk, J.; Hill, R.P. Partial volume rat lung irradiation: The protective/mitigating effects of Eukarion-189, a superoxide dismutase–catalase mimetic. Radiother. Oncol. 2006, 79, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lawler, J.M. Amplification of proinflammatory phenotype, damage, and weakness by oxidative stress in the diaphragm muscle of mdx mice. Free Radic. Biol. Med. 2012, 52, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.D.; Greineder, C.F.; Hood, E.D.; Muzykantov, V.R. Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK-134 alleviates acute pulmonary inflammation. J. Control. Release 2014, 177, 34–41. [Google Scholar] [CrossRef]
- Kumar, A.; Shukla, S.; Chauhan, A.K.; Singh, D.; Pandey, H.P.; Singh, C. The manganese-salen compound EUK-134 and N-acetyl cysteine rescue from zinc-and paraquat-induced toxicity in rat polymorphonuclear leukocytes. Chem. Biol. Interact. 2015, 231, 18–26. [Google Scholar] [CrossRef]
- Mahmood, J.; Jelveh, S.; Zaidi, A.; Doctrow, S.R.; Hill, R.P. Mitigation of radiation-induced lung injury with EUK-207 and genistein: Effects in adolescent rats. Radiat. Res. 2013, 179, 125–134. [Google Scholar] [CrossRef]
- Kash, J.C.; Xiao, Y.; Davis, A.S.; Walters, K.A.; Chertow, D.S.; Easterbrook, J.D.; Dunfee, R.L.; Sandouk, A.; Jagger, B.W.; Schwartzman, L.M.; et al. Treatment with the reactive oxygen species scavenger EUK-207 reduces lung damage and increases survival during 1918 influenza virus infection in mice. Free Radic. Biol. Med. 2014, 67, 235–247. [Google Scholar] [CrossRef]
- Kobasa, D.; Jones, S.M.; Shinya, K.; Kash, J.C.; Copps, J.; Ebihara, H.; Hatta, Y.; Kim, J.H.; Halfmann, P.; Hatta, M.; et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 2007, 445, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.K.; Zhuang, J.; Doctrow, S.R.; Malfroy, B.; Benson, P.F.; Menconi, M.J.; Fink, M.P. EUK-8, a synthetic superoxide dismutase and catalase mimetic, ameliorates acute lung injury in endotoxemic swine. J. Pharmacol. Exp. Ther. 1995, 275, 798–806. [Google Scholar] [CrossRef] [PubMed]
- González, P.K.; Zhuang, J.; Doctrow, S.R.; Malfroy, B.; Benson, P.F.; Menconi, M.J.; Fink, M.P. Role of oxidant stress in the adult respiratoy distress syndrome: Evaluation of a novel antioxidant strategy in a porcine model of endotoxin-induced acute lung injury. Shock 1996, 6, S23–S26. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Cheresh, P.; Jablonski, R.P.; Morales-Nebreda, L.; Cheng, Y.; Hogan, E.; Yeldandi, A.; Chi, M.; Piseaux, R.; Ridge, K.; et al. Mitochondrial catalase overexpressed transgenic mice are protected against lung fibrosis in part via preventing alveolar epithelial cell mitochondrial DNA damage. Free Radic. Biol. Med. 2016, 101, 482–490. [Google Scholar] [CrossRef]
- Mahmood, J.; Jelveh, S.; Calveley, V.; Zaidi, A.; Doctrow, S.R.; Hill, R.P. Mitigation of radiation-induced lung injury by genistein and EUK-207. Int. J. Radiat. Biol. 2011, 87, 889–901. [Google Scholar] [CrossRef]
- Mahmood, J.; Jelveh, S.; Zaidi, A.; Doctrow, S.R.; Medhora, M.; Hill, R.P. Targeting the renin–angiotensin system combined with an antioxidant is highly effective in mitigating radiation-induced lung damage. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 722–728. [Google Scholar] [CrossRef]
- Gao, F.; Fish, B.L.; Szabo, A.; Doctrow, S.R.; Kma, L.; Molthen, R.C.; Moulder, J.E.; Jacobs, E.; Medhora, M. Short-term treatment with a SOD/catalase mimetic, EUK-207, mitigates pneumonitis and fibrosis after single-dose total-body or whole-thoracic irradiation. Radiat. Res. 2012, 178, 468–480. [Google Scholar] [CrossRef]
- Mathieu, E.; Bernard, A.-S.; Delsuc, N.; Quévrain, E.; Gazzah, G.; Lai, B.; Chain, F.; Langella, P.; Bachelet, M.; Masliah, J.; et al. A cell-penetrant manganese superoxide dismutase (MnSOD) mimic is able to complement MnSOD and exerts an anti-inflammatory effect on cellular and animal models of inflammatory bowel diseases. Inorg. Chem. 2017, 56, 2545–2555. [Google Scholar] [CrossRef]
- Redl, H.; Gasser, H.; Schlag, G.; Marzi, I. Involvement of oxygen radicals in shock related cell injury. Br. Med. Bull. 1993, 49, 556–565. [Google Scholar] [CrossRef]
- van Empel, V.P.; Bertrand, A.T.; van Oort, R.J.; van der Nagel, R.; Engelen, M.; van Rijen, H.V.; Doevendans, P.A.; Crijns, H.J.; Ackerman, S.L.; Sluiter, W.; et al. EUK-8, a superoxide dismutase and catalase mimetic, reduces cardiac oxidative stress and ameliorates pressure overload-induced heart failure in the harlequin mouse mutant. J. Am. Coll. Cardiol. 2006, 48, 824–832. [Google Scholar] [CrossRef]
- Srinivasan, V.; Doctrow, S.; Singh, V.K.; Whitnall, M.H. Evaluation of EUK-189, a synthetic superoxide dismutase/catalase mimetic as a radiation countermeasure. Immunopharm. Immunot. 2008, 30, 271–290. [Google Scholar] [CrossRef] [PubMed]
- García-Quintans, N.; Prieto, I.; Sánchez-Ramos, C.; Luque, A.; Arza, E.; Olmos, Y.; Monsalve, M. Regulation of endothelial dynamics by PGC-1α relies on ROS control of VEGF-A signaling. Free Radic. Biol. Med. 2016, 93, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Himori, K.; Abe, M.; Tatebayashi, D.; Lee, J.; Westerblad, H.; Lanner, J.T.; Yamada, T. Superoxide dismutase/catalase mimetic EUK-134 prevents diaphragm muscle weakness in monocrotalin-induced pulmonary hypertension. PLoS ONE 2017, 12, e0169146. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.; Packwood, W.; Xie, A.; Liang, S.; Qi, Y.; Ruggeri, Z.; Lopez, J.; Davidson, B.P.; Lindner, J.R. Assessment of novel antioxidant therapy in atherosclerosis by contrast ultrasound molecular imaging. J. Am. Soc. Echocardiog. 2018, 31, 1252–1259. [Google Scholar] [CrossRef]
- Nishigori, C.; Hattori, Y.; Arima, Y.; Miyachi, Y. Photoaging and oxidative stress. Exp. Dermatol. 2003, 12, 18–21. [Google Scholar] [CrossRef]
- Tocco, G.; Illigens, B.M.W.; Malfroy, B.; Benichou, G. Prolongation of alloskin graft survival by catalytic scavengers of reactive oxygen species. Cell. Immunol. 2006, 241, 59–65. [Google Scholar] [CrossRef]
- Kvale, E.O.; Patel, N.S.A.; Chatterjee, P.K.; Sharpe, M.A.; Thiemermann, C. The SOD mimetic EUK-134 reduces oxidative stress-mediated renal dysfunction in the rat in vivo. Br. J. Pharmacol. 2002, 135, 69. [Google Scholar]
- Decraene, D.; Smaers, K.; Gan, D.; Mammone, T.; Matsui, M.; Maes, D.; Declercq, L.; Garmyn, M. A synthetic superoxide dismutase/catalase mimetic (EUK-134) inhibits membrane damage-induced activation of mitogen-activated protein kinase pathways and reduces p53 accumulation in ultraviolet B-exposes primary human keratinocytes. J. Investig. Dermatol. 2004, 122, 484–491. [Google Scholar] [CrossRef]
- Doctrow, S.R.; Lopez, A.; Schock, A.M.; Duncan, N.E.; Jourdan, M.M.; Olasz, E.B.; Moulder, J.E.; Fish, B.L.; Mäder, M.; Lazar, J.; et al. A synthetic superoxide dismutase/catalase mimetic EUK-207 mitigates radiation dermatitis and promotes wound healing in irradiated rat skin. J. Investig. Dermatol. 2013, 133, 1088–1096. [Google Scholar] [CrossRef]
- Jelveh, S.; Kaspler, P.; Bhogal, N.; Mahmood, J.; Lindsay, P.E.; Okunieff, P.; Doctrow, S.R.; Bristow, R.G.; Hill, R.P. Investigations of antioxidant-mediated protection and mitigation of radiation-induced DNA damage and lipid peroxidation in murine skin. Int. J. Radiat. Biol. 2013, 89, 618–627. [Google Scholar] [CrossRef]
- Laforgia, N.; Di Mauro, A.; Guarnieri, G.F.; Varvara, D.; De Cosmo, L.; Panza, R.; Capozza, M.; Baldassarre, M.E.; Resta, N. The role of oxidative stress in the pathomechanism of congenital malformations. Oxid. Med. Cell Longev. 2018, 2018, 7404082. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Huang, X.; Xiao, D.; Zhang, L. Direct effect of chronic hypoxia in suppressing large conductance Ca2+-activated K+ channel activity in ovine uterine arteries via increasing oxidative stress. J. Physiol. 2016, 594, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Dehart, D.B.; Sulik, K.K. Protection from ethanol-induced limb malformations by the superoxide dismutase/catalase mimetic, EUK-134. FASEB J. 2004, 18, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Doctrow, S.R.; Xu, L.; Oberley, L.W.; Beecher, B.; Morrison, J.; Kregel, K.C. Redox modulation of the liver with chronic antioxidant enzyme mimetic treatment prevents age-related oxidative damage associated with environmental stress. FASEB J. 2004, 18, 1547–1549. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Yazdanparast, R.; Molaei, M. Amelioration of diet-induced nonalcoholic steatohepatitis in rats by Mn-salen complexes via reduction of oxidative stress. J. Biomed. Sci. 2012, 19, 26. [Google Scholar] [CrossRef]
- Meftah, S.; Yazdanparast, R.; Molaei, M. Ameliorative Action of Mn-Salen Derivatives on CCl4-Induced Destructive Effects and Lipofuscin-Like Pigment Formation in Rats’ Liver and Brain: Post-Treatment of Young Rats with EUKs. CellBio 2014, 3, 96. [Google Scholar] [CrossRef][Green Version]
- Magder, S.; Parthenis, D.G.; Ghouleh, I.A. Preservation of Renal Blood Flow by the Antioxidant EUK-134 in LPS-Treated Pigs. Int. J. Mol. Sci. 2015, 16, 6801–6817. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Kirkwood, T.B.L.; Kowald, A. The free-radical theory of ageing—Older, wiser and still alive: Modelling positional effects of the primary targets of ROS reveals new support. Bioessays 2012, 34, 692–700. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein oxidation and aging. Science 1992, 257, 1220–1224. [Google Scholar] [CrossRef]
- Gladyshev, V.N. The free radical theory of aging is dead. Long live the damage theory! Antioxid. Redox Signal. 2014, 20, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, J.M.; Hekimi, S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009, 5, e1000361. [Google Scholar] [CrossRef] [PubMed]
- Keaney, M.; Matthijssens, F.; Sharpe, M.; Vanfleteren, J.; Gems, D. Superoxide dismutase mimetics elevate superoxide dismutase activity in vivo but do not retard aging in the nematode Caenorhabditis elegans. Free Radic. Biol. Med. 2004, 37, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Sampayo, J.N.; Olsen, A.; Lithgow, G.J. Oxidative stress in Caenorhabditis elegans: Protective effects of superoxide dismutase/catalase mimetics. Aging Cell. 2003, 2, 319–326. [Google Scholar] [CrossRef]
- Magwere, T.; West, M.; Riyahi, K.; Murphy, M.P.; Smith, R.A.; Partridge, L. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech. Ageing Dev. 2005, 127, 356–370. [Google Scholar] [CrossRef]
- Bayne, A.-C.V.; Sohal, R.S. Effects of superoxide dismutase/catalase mimetics on life span and oxidative stress resistance in the housefly, Musca domestica. Free Radic. Biol. Med. 2002, 32, 1229–1234. [Google Scholar] [CrossRef]
- Watanabe, D.; Aibara, C.; Wada, M. Treatment with EUK-134 improves sarcoplasmic reticulum Ca2+ release but not myofibrillar Ca2+ sensitivity after fatiguing contraction of rat fast-twitch muscle. Am. J. Physiol. Reg. I. 2019, 316, R543–R551. [Google Scholar] [CrossRef]
- Arndt, A.; Borella, M.I.; Espósito, B.P. Toxicity of manganese metallodrugs toward Danio rerio. Chemosphere 2014, 96, 46–50. [Google Scholar] [CrossRef]
- Grodstein, F.; O’Brien, J.; Kang, J.H.; Dushkes, R.; Cook, N.R.; Okereke, O.; Sesso, H.D. Long-term multivitamin supplementation and cognitive function in men: A randomized trial. Ann. Intern. Med. 2013, 159, 806–814. [Google Scholar] [CrossRef]
- Oertel, W.; Schulz, J.B. Current and experimental treatments of Parkinson disease: A guide for neuroscientists. J. Neurochem. 2016, 139, 325–337. [Google Scholar] [CrossRef]
- Shen, L.; Hong-Fang, J. Is antioxidant supplement beneficial? New avenue to explore. Trends Food Sci. Tech. 2017, 68, 51–55. [Google Scholar] [CrossRef]
- Moghaddam, N.S.A.; Oskouie, M.N.; Butler, A.E.; Petit, P.X.; Barreto, G.E.; Sahebkar, A. Hormetic effects of curcumin: What is the evidence? J. Cell. Physiol. 2019, 234, 10060–10071. [Google Scholar] [CrossRef]
- Posandino, A.M.; Cossu, A.; Giordo, R.; Zinellu, A.; Sotgia, S.; Vardeu, A.; Hoa, P.T.; Van Nguyen, L.H.; Carru, C.; Pintus, G. Revesratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem. Toxicol. 2015, 78, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone II, C.B.; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical applications and potential for image-guided drug delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef] [PubMed]







| Antioxidant Model | Disease | Animal Model | Dose | Outcomes | Ref. |
|---|---|---|---|---|---|
| EUK-8 | Spongiform neurodegenerative disorder | SOD2 nullizygous mice | 30 mg/kg | Rescue of the neurodegenerative disorder | [4] |
| EUK-134 | |||||
| EUK-189 | |||||
| EUK-207 | Age-related cochlear cell degeneration | SAMP8/SAMR1 mice | 0.2 mg/kg/day | Prevention of age-related hearing loss | [22] |
| EUK-8 | Ischemia/reperfusion injury | Sprague-Dawley rats | 1 mg/kg, 2 times | Protective effects | [91] |
| EUK-134 | Kainite-induced neuropathology | Sprague-Dawley rats | 10 mg/kg, 2 times | Prevention of excitotoxic neuronal injury | [101] |
| EUK-8 | Autoimmune encephalomyelitis | Guinea pig MBP | 100 mg/kg | Prevention and suppression of the disease | [102] |
| EUK-8 | Neurodegeneration | SOD2 nullizygous mice | 30 mg/kg | Rescue of neuronal cell death | [103] |
| EUK-189 | Alzheimer disease | Tg2576 mice | 30 mg/kg, 3 t/w | Amelioration of cataracts in the lenses | [104] |
| EUK-189 | Loss of learning and memory function | C57BL/6N Sim mice | 9 nmol/day-0.09 µM/day | Reversion of cognitive deficits | [106] |
| EUK-207 | |||||
| EUK-189 | Age-related cognitive deficits | C57BL/6N Sim mice | 15–16 µg/Kg/day | Reduc. 3 of the age-related cognitive impairment | [107] |
| EUK-207 | |||||
| EUK-8 | Amyotrophic lateral sclerosis | SOD1-G93A mice | 33 mg/kg, 3 t/w | Prolongation of survival | [109] |
| EUK-134 | |||||
| EUK-8 | Ischemic brain injury | Sprague-Dawley rats | 30 mg/kg, 3 t/w | Reduction of brain infarct size | [110] |
| EUK-134 | |||||
| EUK-134 | Dopaminergic neurons death by neurotoxic | C57BL/6 mice | 15 mM 1 day prior to the toxic | Attenuated the loss of nigral dopamine neurons | [111] |
| EUK-139 | |||||
| EUK-189 | Human prion disease | Balb/c mice | 30 mg/kg, 3 t/w 1 | Modest prolong. 2 survival. Reduc. 3 in oxidative damage to proteins. | [112] |
| EUK-189 | Ataxia-telangiectasia | Mice lacking ATM gene | 1.2 mg/kg/day | Correction of the neurobehaviroral abnormality | [113] |
| EUK-207 | Radiation-induce cognitive impairments | C57Bl6/J mice | 0.2 mg/kg/day | Mitigation of the cognitive injury | [114] |
| Antioxidant Model | Disease | Animal Model | Dose | Outcomes | Ref. |
|---|---|---|---|---|---|
| EUK-189 | Radiation-induced lung injury | Sprague–Dawley rats | 30 mg/kg | Reduction micronucleous formation in lung fibroblasts | [119] |
| EUK-134 | Proinflammatory damage in the diaphragm muscle | Mdx mice | 30 mg/kg/day | Reduction of muscle damage | [120] |
| EUK-134 | Acute pulmonary inflammation | C57BL/6 mice | 200 µL aliquots of 3.2 mg total lipid at 2000 CPM/µL | Alleviate acute pulmonary inflammation | [121] |
| EUK-134 | Paraquat-induced inflammation | Wistar rats | 10 mg/kg | Protection from the toxic effects | [122] |
| EUK-207 | Radiation-induced lung injury | Fisher rats | 8 mg/kg | Mitigation of lung injury | [123] |
| EUK-207 | 1918 influenza virus | BALB/c mice | 30 μg/day | Reduction of severity lung injury | [124] |
| EUK-8 | Acute lung injury | Pigs | 10 mg/kg bolus and 3 mg/kg.h, n = 6 | Alleviate acute lung injury | [96,126,127] |
| EUK-207 | Radiation-induced lung damage | Sprague–Dawley rats | 8 mg/kg/day | Limitation of pulmonary fibrosis | [129,130] |
| EUK-207 | Radiation-induced lung injury | Sprague–Dawley rats | 8 mg/kg/day | Mitigation of the radiation effects | [131] |
| Antioxidant Model | Disease | Animal Model | Dose | Outcomes | Ref. |
|---|---|---|---|---|---|
| EUK-8 | Pressure overload-induced heart failure | B6CBA mice hemizygous or homozygous for the X-linked Hq mutation | 25 mg/kg/day | Prevention myocardial damage. Attenuation cardiac hypertrophy and fibrosis | [134] |
| EUK-189 | Gamma irradiation | C3H/HeN and CD2F1 mice | 70 mg/kg | Increase of 30-day survival | [135] |
| EUK-134 | Pulmonary hypertension | Wistar rats | 3 mg/kg/day | Prevention of diaphragm muscle weakness | [137] |
| EUK-207 | Atherosclerosis | C57B1/6 mice | 1 mg/kg/day | Reduction for endothelial-associated events | [138] |
| Antioxidant Model | Disease | Animal Model | Dose | Outcomes | Ref. |
|---|---|---|---|---|---|
| EUK-8 | Rejection of allogeneic skin grafts | BALB/c and C57BL/6 mice | 25 mg/kg/day | Attenuation on graft rejection | [140] |
| EUK-134 | |||||
| EUK-189 | |||||
| EUK-134 | UV-induced skin damage | Humans volunteers | Topical 0.01–0.1%, 3 µL cm−2 | Protection of skin surface from accumulating oxidative damage | [12] |
| EUK-207 | Radiation dermatitis | WAG/RijCmcr mice | 1.8 mg/kg/day | Promotion wound healing in irradiated skin | [143] |
| EUK-207 | Radiation-induced DNA damage or lipid peroxidation | C3H/HeJ mice | 30 mg/kg | Protection before irradiation. Mitigation of lipid peroxidation | [144] |
| Antioxidant Model | Animal Model | Dose | Outcomes | Ref. |
|---|---|---|---|---|
| EUK-8 | Caenorhabditis elegans nematode | 0.05 mM | Increase in mean-life span of 44% | [1] |
| EUK-134 | ||||
| EUK-8 | Caenorhabditis elegans nematode | 0.05–5 mM | No increase in lifespan | [5] |
| EUK-8 | Caenorhabditis elegans nematode | 0.25–0.5 mM | Increase lifespan in presence of superoxide generators | [157] |
| EUK-134 | ||||
| EUK-8 | Caenorhabditis elegans nematode | 0.05–1 mM | Increase lifespan in presence of superoxide generators | [158] |
| EUK-134 | ||||
| EUK-8 | Drosophila melanogaster Fruit fly | 0.025–0.5 mM | No extension lifespan in normal animals | [159] |
| EUK-134 | ||||
| EUK-8 | Musca domestica fly | 0.025–0.5 mM. | No extension lifespan | [160] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouco, L.; González-Noya, A.M.; Pedrido, R.; Maneiro, M. Pursuing the Elixir of Life: In Vivo Antioxidative Effects of Manganosalen Complexes. Antioxidants 2020, 9, 727. https://doi.org/10.3390/antiox9080727
Rouco L, González-Noya AM, Pedrido R, Maneiro M. Pursuing the Elixir of Life: In Vivo Antioxidative Effects of Manganosalen Complexes. Antioxidants. 2020; 9(8):727. https://doi.org/10.3390/antiox9080727
Chicago/Turabian StyleRouco, Lara, Ana M. González-Noya, Rosa Pedrido, and Marcelino Maneiro. 2020. "Pursuing the Elixir of Life: In Vivo Antioxidative Effects of Manganosalen Complexes" Antioxidants 9, no. 8: 727. https://doi.org/10.3390/antiox9080727
APA StyleRouco, L., González-Noya, A. M., Pedrido, R., & Maneiro, M. (2020). Pursuing the Elixir of Life: In Vivo Antioxidative Effects of Manganosalen Complexes. Antioxidants, 9(8), 727. https://doi.org/10.3390/antiox9080727