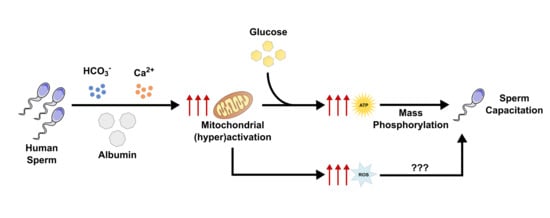
Mitochondrial Activation and Reactive Oxygen-Species Overproduction during Sperm Capacitation are Independent of Glucose Stimuli
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ethical Approval
2.3. Human Sperm Samples
2.4. Experimental Groups
2.5. Sperm Viability and Total Motility Analysis
2.6. Proton Nuclear Magnetic Resonance (1H-NMR)
2.7. Sperm Capacitation Analysis
2.8. JC-1 Assay for Mitochondrial Membrane Potential
2.9. Detection of Intracellular Reactive Oxygen Species (ROS)
2.10. Evaluation of Oxidative Stress-Related Damage
2.11. Statistical Analysis
3. Results
3.1. Glucose is Essential for Human Sperm Viability Maintenance and Capacitation
3.2. Glucose Exposure Increases Human Sperm Total Motility
3.3. The Main Metabolites Produced during Human Sperm Capacitation Are Lactate, Acetate, and Malate
3.4. Mitochondrial Activation during Human Sperm Capacitation Does not Require Glucose Presence
3.5. Human Sperm Capacitation Leads to Increased Endogenous ROS Production without Inducing Oxidative Stress-Related Damage
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puga Molina, L.C.; Luque, G.M.; Balestrini, P.A.; Marin-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular Basis of Human Sperm Capacitation. Front. Cell Dev. Biol. 2018, 6, 72. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Bioenergetics of mammalian sperm capacitation. BioMed Res. Int. 2014, 2014, 902953. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, R.L.; Carrageta, D.F.; Sousa, M.; Alves, M.G.; Oliveira, P.F. pH and male fertility: Making sense on pH homeodynamics throughout the male reproductive tract. Cell. Mol. Life Sci. 2019, 76, 3783–3800. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.R. Observations on the Penetration of the Sperm into the Mammalian Egg. Aust. J. Sci. Res. Ser. B 1951, 4, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C. Fertilizing Capacity of Spermatozoa Deposited into the Fallopian Tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, P.C.; Edwards, R.G. Birth after the reimplantation of a human embryo. Lancet 1978, 2, 366. [Google Scholar] [CrossRef]
- Vyklicka, L.; Lishko, P.V. Dissecting the signaling pathways involved in the function of sperm flagellum. Curr. Opin. Cell Biol. 2020, 63, 154–161. [Google Scholar] [CrossRef]
- Williams, A.C.; Ford, W.C. The role of glucose in supporting motility and capacitation in human spermatozoa. J. Androl. 2001, 22, 680–695. [Google Scholar]
- Kirichok, Y.; Lishko, P.V. Rediscovering sperm ion channels with the patch-clamp technique. Mol. Hum. Reprod. 2011, 17, 478–499. [Google Scholar] [CrossRef]
- Goodson, S.G.; Qiu, Y.; Sutton, K.A.; Xie, G.; Jia, W.; O’Brien, D.A. Metabolic substrates exhibit differential effects on functional parameters of mouse sperm capacitation. Biol. Reprod. 2012, 87, 75. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, J.M.; Shi, L.Z.; Tam, J.; Chandsawangbhuwana, C.; Durrant, B.; Botvinick, E.L.; Berns, M.W. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real-time automated tracking and trapping. J. Cell. Physiol. 2008, 217, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Obert, G.; Deffosez, A.; Formstecher, P.; Marchetti, P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum. Reprod. 2002, 17, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Gallon, F.; Marchetti, C.; Jouy, N.; Marchetti, P. The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil. Steril. 2006, 86, 1526–1530. [Google Scholar] [CrossRef]
- Ferramosca, A.; Provenzano, S.P.; Coppola, L.; Zara, V. Mitochondrial respiratory efficiency is positively correlated with human sperm motility. Urology 2012, 79, 809–814. [Google Scholar] [CrossRef]
- Stendardi, A.; Focarelli, R.; Piomboni, P.; Palumberi, D.; Serafini, F.; Ferramosca, A.; Zara, V. Evaluation of mitochondrial respiratory efficiency during in vitro capacitation of human spermatozoa. Int. J. Androl. 2011, 34, 247–255. [Google Scholar] [CrossRef]
- Hicks, J.J.; Martinez-Manautou, J.; Pedron, N.; Rosado, A. Metabolic changes in human spermatozoa related to capacitation. Fertil. Steril. 1972, 23, 172–179. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Ferramosca, A.; Pinto Provenzano, S.; Montagna, D.D.; Coppola, L.; Zara, V. Oxidative stress negatively affects human sperm mitochondrial respiration. Urology 2013, 82, 78–83. [Google Scholar] [CrossRef]
- Rivlin, J.; Mendel, J.; Rubinstein, S.; Etkovitz, N.; Breitbart, H. Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol. Reprod. 2004, 70, 518–522. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Jeyendran, R.S.; Caroppo, E.; Rouen, A.; Anderson, A.; Puscheck, E. Selecting the most competent sperm for assisted reproductive technologies. Fertil. Steril. 2019, 111, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.; Carrageta, D.F.; Alves, M.G.; Rocha, A.; Agarwal, A.; Barros, A.; Oliveira, P.F. Sperm selection strategies and their impact on assisted reproductive technology outcomes. Andrologia 2020, e13725. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Alves, M.G.; Dias, T.R.; Lopes, G.; Cavaco, J.E.; Socorro, S.; Oliveira, P.F. High-energy diets may induce a pre-diabetic state altering testicular glycolytic metabolic profile and male reproductive parameters. Andrology 2013, 1, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Moreira, B.P.; Silva, J.F.; Jarak, I.; de Lourdes Pereira, M.; Oliveira, P.F.; Alves, M.G. Technical-grade chlordane compromises rat Sertoli cells proliferation, viability and metabolic activity. Toxicol. In Vitro 2020, 63, 104673. [Google Scholar] [CrossRef] [PubMed]
- Calle-Guisado, V.; Gonzalez-Fernandez, L.; Martin-Hidalgo, D.; Garcia-Marin, L.J.; Bragado, M.J. Metformin inhibits human spermatozoa motility and signalling pathways mediated by protein kinase A and tyrosine phosphorylation without affecting mitochondrial function. Reprod. Fertil. Dev. 2019, 31, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Dias, T.R.; Bernardino, R.L.; Alves, M.G.; Silva, J.; Barros, A.; Sousa, M.; Casal, S.; Silva, B.M.; Oliveira, P.F. L-Theanine promotes cultured human Sertoli cells proliferation and modulates glucose metabolism. Eur. J. Nutr. 2019, 58, 2961–2970. [Google Scholar] [CrossRef]
- Carrageta, D.F.; Dias, T.R.; Jarak, I.; Alves, M.G.; Oliveira, P.F.; Van der Walt, M.M.; Terre’Blanche, G.; Monteiro, M.P.; Silva, B.M. 8-(3-phenylpropyl)-1,3,7-triethylxanthine is a synthetic caffeine substitute with stronger metabolic modulator activity. Toxicol. In Vitro 2018, 53, 114–120. [Google Scholar] [CrossRef]
- Buckett, W.M.; Lewis-Jones, D.I. Fructose concentrations in seminal plasma from men with nonobstructive azoospermia. Arch. Androl. 2002, 48, 23–27. [Google Scholar] [CrossRef][Green Version]
- Hoppe, P.C. Glucose requirement for mouse sperm capacitation in vitro. Biol. Reprod. 1976, 15, 39–45. [Google Scholar] [CrossRef]
- Fraser, L.R.; Quinn, P.J. A glycolytic product is obligatory for initiation of the sperm acrosome reaction and whiplash motility required for fertilization in the mouse. J. Reprod. Fertil. 1981, 61, 25–35. [Google Scholar] [CrossRef]
- Povoa, H., Jr.; Bastos, J.J.; Silva, M.E.; Ariza, A.; Moraes, M.I.; Rodrigues, R.B.; Silva, M.S. Glucose in human semen. Biomed. Biochim. Acta 1986, 45, 685–686. [Google Scholar] [PubMed]
- Weed, J.C.; Carrera, A.E. Glucose content of cervical mucus. Fertil. Steril. 1970, 21, 866–872. [Google Scholar] [CrossRef]
- Ehrstrom, S.; Yu, A.; Rylander, E. Glucose in vaginal secretions before and after oral glucose tolerance testing in women with and without recurrent vulvovaginal candidiasis. Obstet. Gynecol. 2006, 108, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Paiva, C.; Baptista, M.; Sousa, A.P.; Ramalho-Santos, J. Exogenous glucose improves long-standing human sperm motility, viability, and mitochondrial function. Fertil. Steril. 2011, 96, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K.; Rajesh, P.B. Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod. Biol. Endocrinol. 2004, 2, 75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valsa, J.; Skandhan, K.P.; Sumangala, B.; Jaya, V. Time bound changes (in 24 h) in human sperm motility and level of calcium and magnesium in seminal plasma. Alex. J. Med. 2019, 52, 235–241. [Google Scholar] [CrossRef][Green Version]
- Portela, J.M.; Tavares, R.S.; Mota, P.C.; Ramalho-Santos, J.; Amaral, S. High glucose concentrations per se do not adversely affect human sperm function in vitro. Reproduction 2015, 150, 77–84. [Google Scholar] [CrossRef]
- Calvert, S.J.; Reynolds, S.; Paley, M.N.; Walters, S.J.; Pacey, A.A. Probing human sperm metabolism using 13C-magnetic resonance spectroscopy. Mol. Hum. Reprod. 2019, 25, 30–41. [Google Scholar] [CrossRef]
- Reynolds, S.; Ismail, N.F.B.; Calvert, S.J.; Pacey, A.A.; Paley, M.N.J. Evidence for Rapid Oxidative Phosphorylation and Lactate Fermentation in Motile Human Sperm by Hyperpolarized (13)C Magnetic Resonance Spectroscopy. Sci. Rep. 2017, 7, 4322. [Google Scholar] [CrossRef]
- Bobyleva-Guarriero, V.; Lardy, H.A. The role of malate in exercise-induced enhancement of mitochondrial respiration. Arch. Biochem. Biophys. 1986, 245, 470–476. [Google Scholar] [CrossRef]
- Gerez de Burgos, N.M.; Gallina, F.; Burgos, C.; Blanco, A. Effect of L-malate on pyruvate dehydrogenase activity of spermatozoa. Arch. Biochem. Biophys. 1994, 308, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Storey, B.T.; Kayne, F.J. Energy metabolism of spermatozoa. VII. Interactions between lactate, pyruvate and malate as oxidative substrates for rabbit sperm mitochondria. Biol. Reprod. 1978, 18, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Focarelli, R.; Piomboni, P.; Coppola, L.; Zara, V. Oxygen uptake by mitochondria in demembranated human spermatozoa: A reliable tool for the evaluation of sperm respiratory efficiency. Int. J. Androl. 2008, 31, 337–345. [Google Scholar] [CrossRef]
- Ruiz-Pesini, E.; Díez-Sánchez, C.; López-Pérez, M.J.; Enríquez, J.A. The role of the mitochondrion in sperm function: Is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr. Top. Dev. Biol. 2007, 77, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Vorup-Jensen, T.; Hjort, T.; Abraham-Peskir, J.V.; Guttmann, P.; Jensenius, J.C.; Uggerhoj, E.; Medenwaldt, R. X-ray microscopy of human spermatozoa shows change of mitochondrial morphology after capacitation. Hum. Reprod. 1999, 14, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Boell, E.J. Oxygen consumption of mouse sperm and its relationship to capacitation. J. Exp. Zool. 1985, 234, 105–116. [Google Scholar] [CrossRef]
- Fraser, L.R.; Lane, M.R. Capacitation- and fertilization-related alterations in mouse sperm oxygen consumption. J. Reprod. Fertil. 1987, 81, 385–393. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.K.; Chen, L.M. The physiology of bicarbonate transporters in mammalian reproduction. Biol. Reprod. 2012, 86, 99. [Google Scholar] [CrossRef]
- Harper, M.E.; Bevilacqua, L.; Hagopian, K.; Weindruch, R.; Ramsey, J.J. Ageing, oxidative stress, and mitochondrial uncoupling. Acta Physiol. Scand. 2004, 182, 321–331. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Dias, T.R.; Martin-Hidalgo, D.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Endogenous and Exogenous Antioxidants as a Tool to Ameliorate Male Infertility Induced by Reactive Oxygen Species. Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.H.; Sharma, R.K.; Thornton, J.; Mascha, E.; Abdel-Hafez, M.A.; Thomas, A.J., Jr.; Agarwal, A. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum. Reprod. 2004, 19, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Martinez, M.; Parekh, N. Are antioxidants a viable treatment option for male infertility? Andrologia 2020, e13644. [Google Scholar] [CrossRef] [PubMed]
- Governini, L.; Ponchia, R.; Artini, P.G.; Casarosa, E.; Marzi, I.; Capaldo, A.; Luddi, A.; Piomboni, P. Respiratory Mitochondrial Efficiency and DNA Oxidation in Human Sperm after In Vitro Myo-Inositol Treatment. J. Clin. Med. 2020, 9, 1638. [Google Scholar] [CrossRef] [PubMed]
- Martin-Hidalgo, D.; Bragado, M.J.; Batista, A.R.; Oliveira, P.F.; Alves, M.G. Antioxidants and Male Fertility: From Molecular Studies to Clinical Evidence. Antioxidants 2019, 8, 89. [Google Scholar] [CrossRef]
- Kawahito, S.; Kitahata, H.; Oshita, S. Problems associated with glucose toxicity: Role of hyperglycemia-induced oxidative stress. World J. Gastroenterol. 2009, 15, 4137–4142. [Google Scholar] [CrossRef]
- Griveau, J.F.; Renard, P.; Le Lannou, D. An in vitro promoting role for hydrogen peroxide in human sperm capacitation. Int. J. Androl. 1994, 17, 300–307. [Google Scholar] [CrossRef]
- Herrero, M.B.; de Lamirande, E.; Gagnon, C. Nitric oxide regulates human sperm capacitation and protein-tyrosine phosphorylation in vitro. Biol. Reprod. 1999, 61, 575–581. [Google Scholar] [CrossRef]
- Balercia, G.; Moretti, S.; Vignini, A.; Magagnini, M.; Mantero, F.; Boscaro, M.; Ricciardo-Lamonica, G.; Mazzanti, L. Role of nitric oxide concentrations on human sperm motility. J. Androl. 2004, 25, 245–249. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrageta, D.F.; Guerra-Carvalho, B.; Sousa, M.; Barros, A.; Oliveira, P.F.; Monteiro, M.P.; Alves, M.G. Mitochondrial Activation and Reactive Oxygen-Species Overproduction during Sperm Capacitation are Independent of Glucose Stimuli. Antioxidants 2020, 9, 750. https://doi.org/10.3390/antiox9080750
Carrageta DF, Guerra-Carvalho B, Sousa M, Barros A, Oliveira PF, Monteiro MP, Alves MG. Mitochondrial Activation and Reactive Oxygen-Species Overproduction during Sperm Capacitation are Independent of Glucose Stimuli. Antioxidants. 2020; 9(8):750. https://doi.org/10.3390/antiox9080750
Chicago/Turabian StyleCarrageta, David F., Bárbara Guerra-Carvalho, Mário Sousa, Alberto Barros, Pedro F. Oliveira, Mariana P. Monteiro, and Marco G. Alves. 2020. "Mitochondrial Activation and Reactive Oxygen-Species Overproduction during Sperm Capacitation are Independent of Glucose Stimuli" Antioxidants 9, no. 8: 750. https://doi.org/10.3390/antiox9080750
APA StyleCarrageta, D. F., Guerra-Carvalho, B., Sousa, M., Barros, A., Oliveira, P. F., Monteiro, M. P., & Alves, M. G. (2020). Mitochondrial Activation and Reactive Oxygen-Species Overproduction during Sperm Capacitation are Independent of Glucose Stimuli. Antioxidants, 9(8), 750. https://doi.org/10.3390/antiox9080750