Characterization of Novel Synthetic Polyphenols: Validation of Antioxidant and Vasculoprotective Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. Preparation of Polyhydroxylated Amides (1 to 10)
2.2.1. N-(2,4-Dihydroxyphenyl)-2-(2′,5′-dihydroxyphenyl)acetamide (1)
2.2.2. 2-(2′,4′-Dihydroxyphenyl)-N-(2,5-dihydroxyphenyl)acetamide (2)
2.2.3. 2-(2′,5′-Dihydroxyphenyl)-N-(4-hydroxyphenyl)acetamide (3)
2.2.4. N-(2,5-Dihydroxyphenyl)-2-(2′,5′-dihydroxyphenyl)acetamide (4)
2.2.5. N-(2,4-Dihydroxyphenyl)-2-(2′,4′-dihydroxyphenyl)acetamide (5)
2.2.6. 2-(2′,5′-Dihydroxyphenyl)-N-(3,4-dihydroxyphenyl)acetamide (6)
2.2.7. 2-(3′,4′-Dihydroxyphenyl)-N-(4-hydroxyphenyl)acetamide (7)
2.2.8. 2-(2′,4′-Dihydroxyphenyl)-N-(4-hydroxyphenyl)acetamide (8)
2.2.9. 2-(2′,5′-Dihydroxyphenyl)-N,N-bis(4-hydroxyphenyl)acetamide (9)
2.2.10. N-(2′,5′-Dihydroxybenzyl)-2-(2″,5″-dihydroxyphenyl)-N-(4-hydroxyphenyl) acetamide (10)
2.3. Synthesis of Polyhydroxylated Ureas (11 to 18)
2.3.1. N-(2,4-Dihydroxyphenyl)-N′-(2′,5′-dihydroxyphenyl)urea (11)
2.3.2. N-(2,5-Dihydroxyphenyl)-N′-(4′-hydroxyphenyl)urea (12)
2.3.3. N-(2,5-Dihydroxyphenyl)- N′-(3′,4′-dihydroxyphenyl)urea (13)
2.3.4. N-(3,4-Dihydroxyphenyl)-N′-(4′-hydroxyphenyl)urea (14)
2.3.5. N,N′-bis(4-Hydroxyphenyl)urea (15)
2.3.6. N,N′-bis(2,5-Dihydroxyphenyl)urea (16)
2.3.7. N,N-bis(4-Hydroxyphenyl)-N′-(4′-hydroxyphenyl)urea (17)
2.3.8. N-(2′,5′-Dihydroxybenzyl)- N′-(2″,5″-dihydroxiphenyl)-N-(4-hydroxyphenyl)urea (18)
2.4. Antioxidant Activity
2.4.1. Oxygen Radical Absorbance Capacity (ORAC) Experiment
2.4.2. ABTS Experiment
2.4.3. DPPH Experiment
2.5. In Vitro Cellular Assays
Cell Viability
2.6. Reduced Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase Activity Assay in Vascular Systems
2.7. In Vivo Antioxidant Capacity in Stressed Yeasts
2.7.1. Yeast Strain and Induction of Oxidative Stress
2.7.2. Measurement of Antioxidant Capacity
3. Results and Discussion
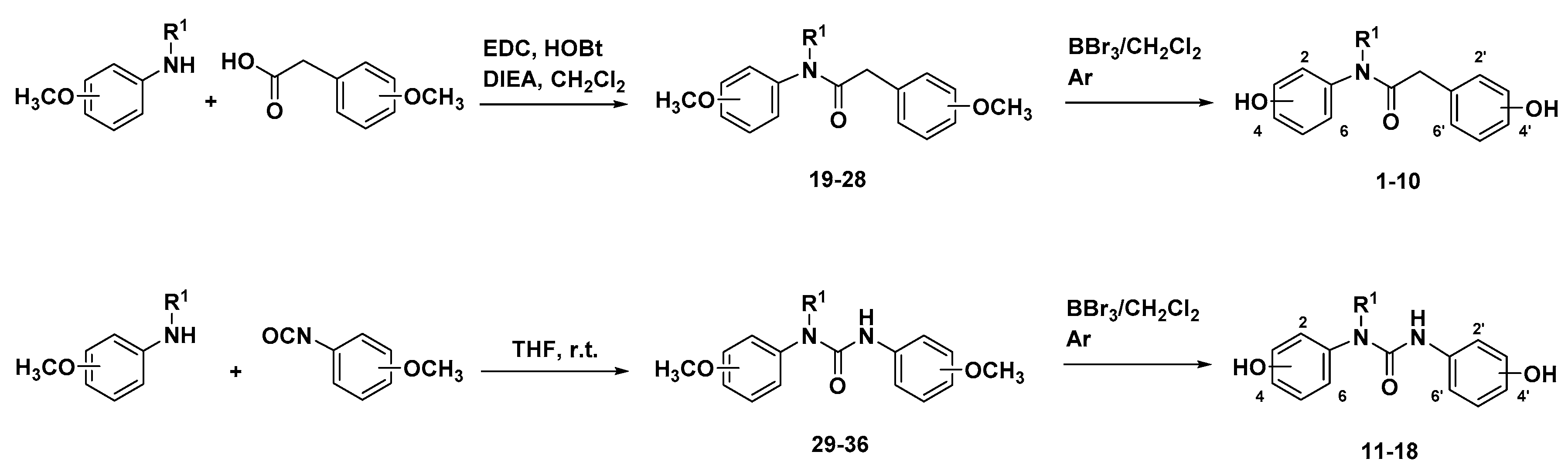
3.1. Preparation of Designed Polyphenols
3.2. Antioxidant Characterization of the New Polyphenols Collection
3.3. Biological Evaluation of Synthetic Polyphenols
3.3.1. Cell Viability
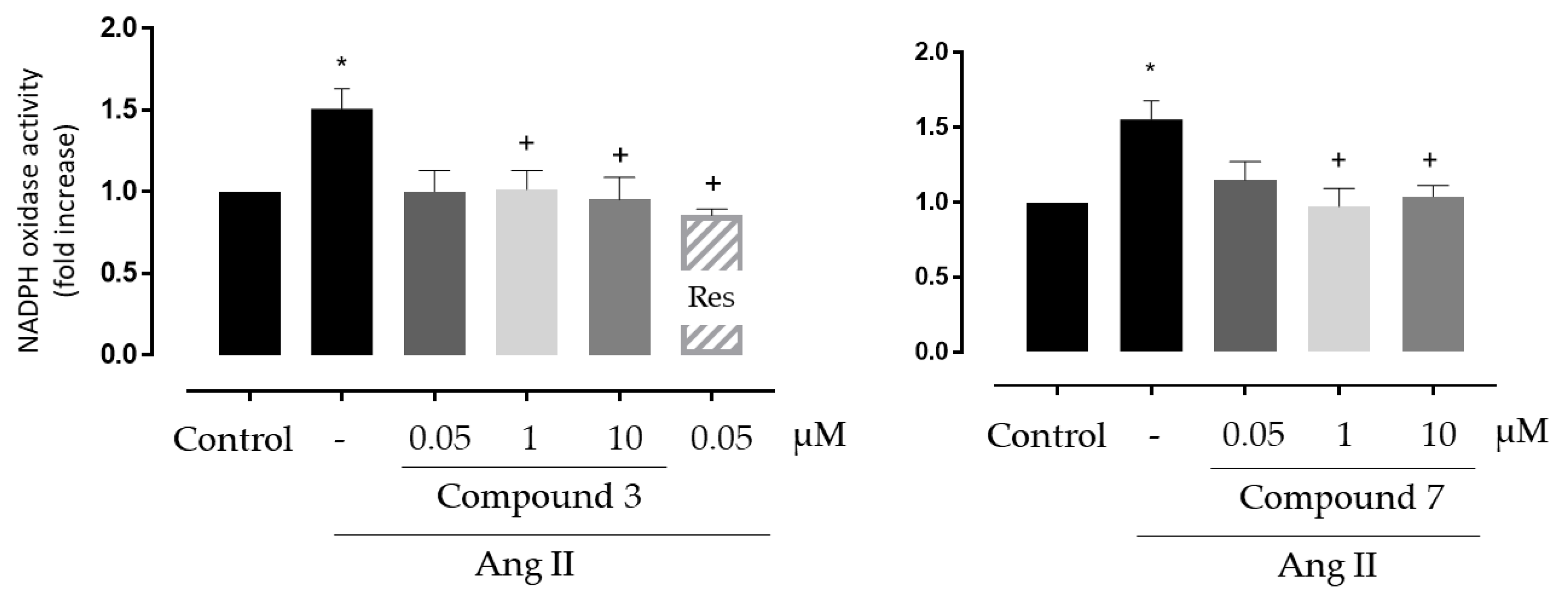
3.3.2. Antioxidant Activity in Vascular Systems
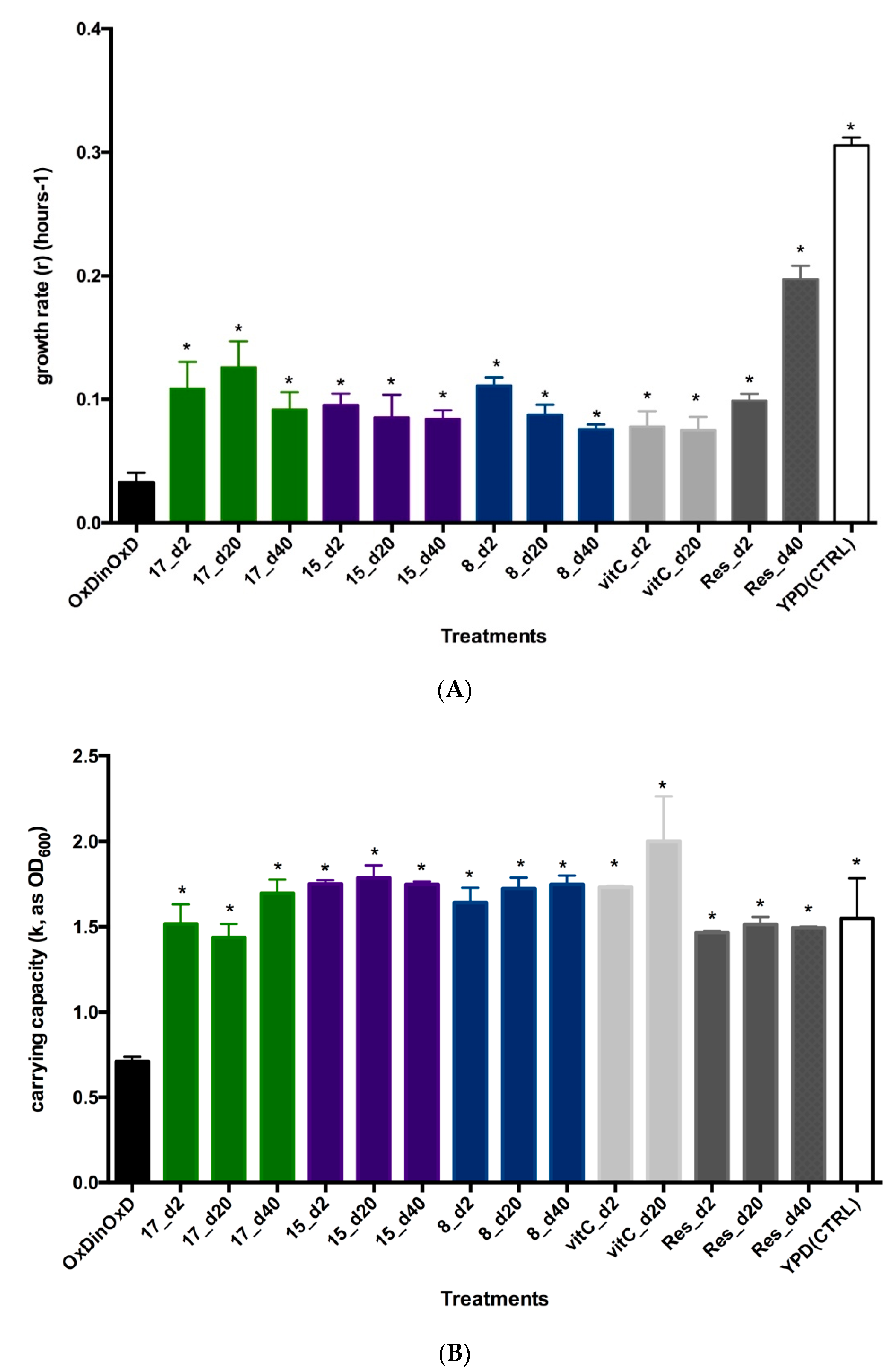
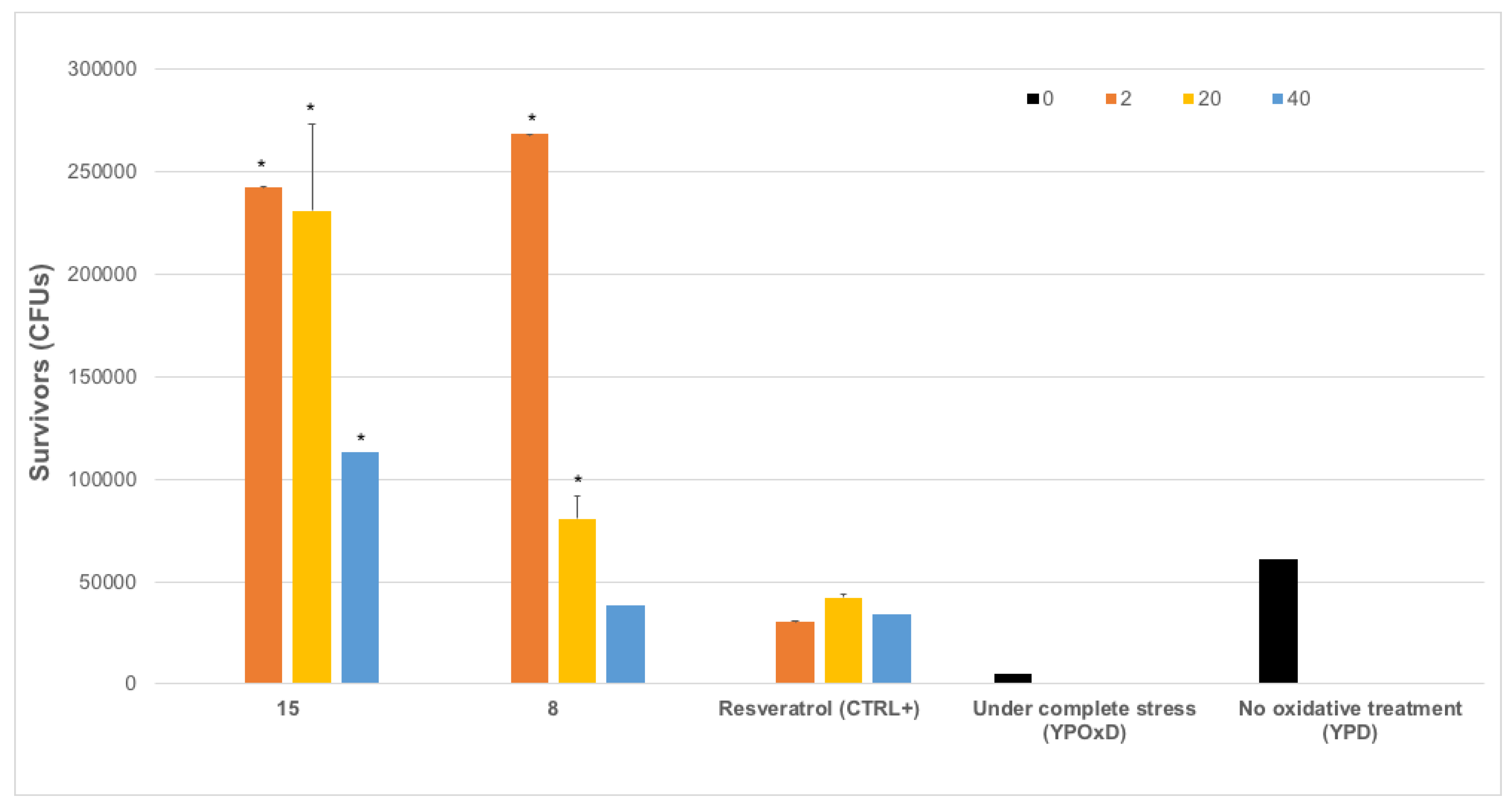
3.3.3. In Vivo Antioxidant Activity of Selected Compounds in Yeasts
4. Final Remarks and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicoletti, M. Nutraceuticals and botanicals: Overview and perspectives. Int. J. Food Sci. Nutr. 2012, 63, 2–6. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef]
- Sharma, R.; Padwad, Y. Perspectives of the potential implications of polyphenols in influencing the interrelationship between oxi-inflammatory stress, cellular senescence and immunosenescence during aging. Trends Food Sci. Technol. 2020, 98, 41–52. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2019, 11, 53. [Google Scholar] [CrossRef] [Green Version]
- Moss, J.W.E.; Williams, J.O.; Ramji, D.P. Nutraceuticals as therapeutic agents for atherosclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Rasines-Perea, Z.; Teissedre, P.-L. Grape polyphenols’ effects in human cardiovascular diseases and diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on natural polyphenols as anticancer agents for skin cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Dyck, G.; Raj, P.; Zieroth, S.; Dyck, J.; Ezekowitz, J. The effects of resveratrol in patients with cardiovascular disease and heart failure: A narrative review. Int. J. Mol. Sci. 2019, 20, 904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martillanes, S.; Rocha-Pimienta, J.; Cabrera-Bañegil, M.; Martín-Vertedor, D.; Delgado-Adámez, J. Application of phenolic compounds for food preservation: Food additive and active packaging. In Phenolic Compounds—Biological Activity; InTech: London, UK, 2017. [Google Scholar]
- Michalak, I.; Chojnacka, K.; Saeid, A. Plant growth biostimulants, dietary feed supplements and cosmetics formulated with supercritical CO2 algal extracts. Molecules 2017, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.; Balsera, B.; Mulet, J.; Sala, S.; Sala, F.; de la Torre-Martínez, R.; Fernández-Carvajal, A.; Ferrer-Montiel, A.; Moreno-Fernández, S.; Miguel, M.; et al. 1,3-diphenylpropan-1-ones as allosteric modulators of α7 nACh receptors with analgesic and antioxidant properties. Future Med. Chem. 2016, 8, 731–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez De Vega, M.J.; Fernandez-Mendivil, C.; De La Torre Martínez, R.; González-Rodríguez, S.; Mullet, J.; Sala, F.; Sala, S.; Criado, M.; Moreno-Fernández, S.; Miguel, M.; et al. 1- (2′, 5′-Dihydroxyphenyl)-3- (2-fluoro-4-hydroxyphenyl)-1-propanone (RGM079): A positive allosteric modulator of α7 nicotinic receptors with analgesic and neuroprotective activity. ACS Chem. Neurosci. 2019, 10, 3900–3909. [Google Scholar] [CrossRef] [PubMed]
- Bonache, M.A.; Moreno-Fernández, S.; Miguel, M.; Sabater-Muñoz, B.; González-Muñiz, R. Small library of triazolyl polyphenols correlating antioxidant activity and stability with number and position of hydroxyl groups. ACS Comb. Sci. 2018, 20, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Franz, R.A.; Applegath, F.; Morriss, F.V.; Baiocchi, F.; Bolze, C. A New synthesis of ureas. III. the reaction of aromatic amines with carbon monoxide and sulfur. J. Org. Chem. 1961, 26, 3309–3312. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Garcés-Rimón, M.; López-Expósito, I.; López-Fandiño, R.; Miguel, M. Egg white hydrolysates with in vitro biological multiactivities to control complications associated with the metabolic syndrome. Eur. Food Res. Technol. 2016, 242, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oki, T.; Nagai, S.; Yoshinaga, M.; Nishiba, Y.; Suda, I. Contribution of BETA.-carotene to radical scavenging capacity varies among orange-fleshed sweet potato cultivars. Food Sci. Technol. Res. 2006, 12, 156–160. [Google Scholar] [CrossRef]
- Mattenberger, F.; Sabater-Muñoz, B.; Hallsworth, J.E.; Fares, M.A. Glycerol stress in Saccharomyces cerevisiae: Cellular responses and evolved adaptations. Environ. Microbiol. 2017, 19, 990–1007. [Google Scholar] [CrossRef] [PubMed]
- Mattenberger, F.; Sabater-Muñoz, B.; Toft, C.; Fares, M.A. The phenotypic plasticity of duplicated genes in Saccharomyces cerevisiae and the origin of adaptations. G3 Genes Genomes Genet. 2017, 7, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprouffske, K.; Wagner, A. Growthcurver: An R package for obtaining interpretable metrics from microbial growth curves. BMC Bioinform. 2016, 17, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT–Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Touyz, R.M.; Briones, A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res. 2011, 34, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Aguado, A.; Fischer, T.; Rodríguez, C.; Manea, A.; Martínez-González, J.; Touyz, R.M.; Hernanz, R.; Alonso, M.J.; Dixon, D.A.; Briones, A.M.; et al. Hu antigen R is required for NOX-1 but not NOX-4 regulation by inflammatory stimuli in vascular smooth muscle cells. Hypertens. Res. 2016, 34, 253–265. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [Green Version]
- Korczyński, M.; Witkowska, Z.; Opaliński, S.; Świniarska, M.; Dobrzański, Z. Algae extract as a potential feed additive. In Marine Algae Extracts; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Martorell, P.; Forment, J.V.; De Llanos, R.; Montón, F.; Llopis, S.; González, N.; Genovés, S.; Cienfuegos, E.; Monzó, H.; Ramón, D. Use of Saccharomyces cerevisiae and Caenorhabditis elegans as model organisms to study the effect of cocoa polyphenols in the resistance to oxidative stress. J. Agric. Food Chem. 2011, 59, 2077–2085. [Google Scholar] [CrossRef]
- De La Torre-Ruiz, M.; Pujol, N.; Sundaran, V. Coping with oxidative stress. The yeast model. Curr. Drug Targets 2015, 16, 2–12. [Google Scholar] [CrossRef]
- Jamieson, D.J. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast 1998, 14, 1511–1527. [Google Scholar] [CrossRef]
- Lushchak, V.I. Budding yeast Saccharomyces cerevisiae as a model to study oxidative modification of proteins in eukaryotes. Acta Biochim. Pol. 2006, 53, 679–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saffi, J.; Sonego, L.; Varela, Q.D.; Salvador, M. Antioxidant activity of L-ascorbic acid in wild-type and superoxide dismutase deficient strains of Saccharomyces cerevisiae. Redox Rep. 2006, 11, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Paczka, A.; Moloń, M.; Bartosz, G. Dimethyl sulfoxide induces oxidative stress in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2013, 13, 820–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoce, O.A.; Antoce, V.; Takahashi, K.; Yoshizako, F. Quantitative study of yeast growth in the presence of added ethanol and methanol using a calorimetric approach. Biosci. Biotechnol. Biochem. 1997, 61, 664–669. [Google Scholar] [CrossRef] [Green Version]
- Piechowiak, T.; Balawejder, M. Onion skin extract as a protective agent against oxidative stress in Saccharomyces cerevisiae induced by cadmium. J. Food Biochem. 2019, 43, e12872. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A.; Kot, A.M. Effect of selenium on growth and antioxidative system of yeast cells. Mol. Biol. Rep. 2019, 46, 1797–1808. [Google Scholar] [CrossRef] [Green Version]
- Poljak, A.; Dawes, I.W.; Ingelse, B.A.; Duncan, M.W.; Smythe, G.A.; Grant, C.M. Oxidative damage to proteins in yeast cells exposed to adaptive levels of H2O2. Redox Rep. 2003, 8, 371–377. [Google Scholar] [CrossRef]
- Sha, W.; Martins, A.M.; Laubenbacher, R.; Mendes, P.; Shulaev, V. The genome-wide early temporal response of Saccharomyces cerevisiae to oxidative stress induced by cumene hydroperoxide. PLoS ONE 2013, 8, e74939. [Google Scholar] [CrossRef]
- Cao, L.; Tang, Y.; Quan, Z.; Zhang, Z.; Oliver, S.G.; Zhang, N. Chronological lifespan in yeast is dependent on the accumulation of storage carbohydrates mediated by Yak 1, Mck 1 and Rim 15 kinases. PLoS Genet. 2016, 12, e1006458. [Google Scholar] [CrossRef]
- Kim, I.-S.; Kim, Y.-S.; Kim, Y.-H.; Park, A.-K.; Kim, H.-W.; Lee, J.-H.; Yoon, H.-S. Potential application of the Oryza sativa monodehydroascorbate reductase gene (OsMDHAR) to improve the stress tolerance and fermentative capacity of Saccharomyces cerevisiae. PLoS ONE 2016, 11, e0158841. [Google Scholar]


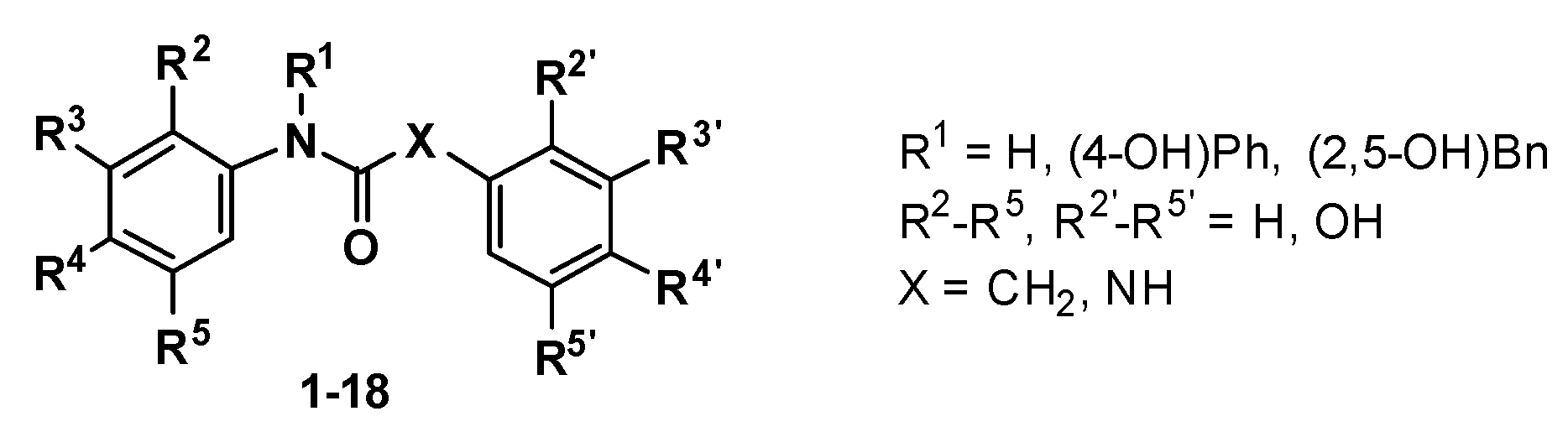
| Compound | R1 | R2 | R3 | R4 | R5 | R2′ | R3′ | R4′ | R5′ | X | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H | OH | H | OH | H | OH | H | H | OH | CH2 | 41 |
| 2 | H | OH | H | H | OH | OH | H | OH | H | CH2 | 44 |
| 3 | H | H | H | OH | H | OH | H | H | OH | CH2 | 79 |
| 4 | H | OH | H | H | OH | OH | H | H | OH | CH2 | 79 |
| 5 | H | OH | H | OH | H | OH | H | OH | H | CH2 | 94 |
| 6 | H | H | OH | OH | H | OH | H | H | OH | CH2 | 56 |
| 7 | H | H | H | OH | H | H | OH | OH | H | CH2 | 75 |
| 8 | H | H | H | OH | H | OH | H | OH | H | CH2 | 80 |
| 9 | (4-OH)Ph | H | H | OH | H | OH | H | H | OH | CH2 | 72 |
| 10 | (2,5-OH)Bn | H | H | OH | H | OH | H | H | OH | CH2 | 81 |
| 11 | H | OH | H | OH | H | OH | H | H | OH | NH | 95 |
| 12 | H | OH | H | H | OH | H | H | OH | H | NH | 56 |
| 13 | H | OH | H | H | OH | H | OH | OH | H | NH | 68 |
| 14 | H | H | OH | OH | H | H | H | OH | H | NH | 60 |
| 15 | H | H | H | OH | H | H | H | OH | H | NH | 84 |
| 16 | H | OH | H | H | OH | OH | H | H | OH | NH | 20 |
| 17 | (4-OH)Ph | H | H | OH | H | H | H | OH | H | NH | 68 |
| 18 | (2,5-OH)Bn | H | H | OH | H | OH | H | H | OH | NH | 10 |

| Compound | R1 | R2 | R3 | R4 | R5 | R2′ | R3′ | R4′ | R5′ | X | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 19 | H | OMe | H | OMe | H | OMe | H | H | OMe | CH2 | 81 |
| 20 | H | OMe | H | H | OMe | OMe | H | OMe | H | CH2 | 77 |
| 21 | H | H | H | OMe | H | OMe | H | H | OMe | CH2 | 50 |
| 22 | H | OMe | H | H | OMe | OMe | H | H | OMe | CH2 | 65 |
| 23 | H | OMe | H | OMe | H | OMe | H | OMe | H | CH2 | 74 |
| 24 | H | H | OMe | OMe | H | OMe | H | H | OMe | CH2 | 73 |
| 25 | H | H | H | OMe | H | H | OMe | OMe | H | CH2 | 81 |
| 26 | H | H | H | OMe | H | OMe | H | OMe | H | CH2 | 76 |
| 27 | (4-OMe)Ph | H | H | OMe | H | OMe | H | H | OMe | CH2 | 28 |
| 28 | (2,5-OMe)Bn | H | H | OMe | H | OMe | H | H | OMe | CH2 | 74 |
| 29 | H | OMe | H | OMe | H | OMe | H | H | OMe | NH | 55 |
| 30 | H | OMe | H | H | OMe | H | H | OMe | H | NH | 85 |
| 31 | H | OMe | H | H | OMe | H | OMe | OMe | H | NH | 69 |
| 32 | H | H | OMe | OMe | H | H | H | OMe | H | NH | 42 |
| 33 | H | H | H | OMe | H | H | H | OMe | H | NH | 80 |
| 34 | H | OMe | H | H | OMe | OMe | H | H | OMe | NH | 84 |
| 35 | (4-OMe)Ph | H | H | OMe | H | H | H | OMe | H | NH | 63 |
| 36 | (2,5-OMe)Bn | H | H | OMe | H | OMe | H | H | OMe | NH | 17 |
| Compound | Chemical Structure | ORAC a | Aqueous Stability b(%) |
|---|---|---|---|
| 1 |  | 11.0 ± 0.4 | 2 |
| 2 | 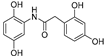 | 8.1 ± 0.1 | - |
| 3 | 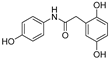 | 19.2 ± 0.2 | 77 |
| 4 | 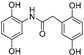 | 14.4 ± 0.2 | 0 |
| 5 | 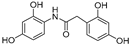 | 5.2 ± 0.4 | - |
| 6 | 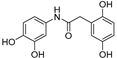 | 9.4 ± 0.4 | 0 |
| 7 | 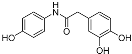 | 19.2 ± 0.4 | 90 |
| 8 | 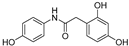 | 19.3 ± 0.5 | 98 |
| 9 | 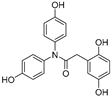 | 29.5 ± 0.5 | 0 |
| 10 | 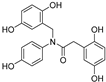 | 27.6 ± 0.5 | 0 |
| 11 | 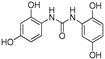 | 6.6 ± 0.1 | - |
| 12 | 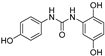 | 12.5 ± 0.7 | 0 |
| 13 | 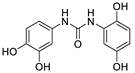 | 7.8 ± 0.3 | - |
| 14 | 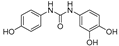 | 9.6 ± 0.1 | 0 |
| 15 |  | 8.9 ± 0.4 | 90 |
| 16 |  | 11.9 ± 0.4 | 0 |
| 17 | 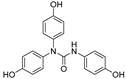 | 19.4 ± 0.7 | 93 |
| 18 |  | 15.3 ± 0.7 | 0 |
| Resveratrol |  | 8.1 ± 1.17 | - |
| Comp | Chemical Structure | ORAC a | ABTS a | DPPH PSA IC50 (µM) b | |
|---|---|---|---|---|---|
| 10 min | 2 h | ||||
| 3 | 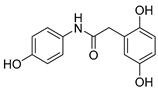 | 19.2 ± 0.2 | 3.27 ± 0.1 | 8.30 | 6.77 |
| 7 | 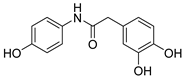 | 19.2 ± 0. | 8.12 ± 0.09 | 24.10 | 23.30 |
| 8 | 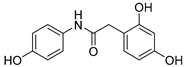 | 19.3 ± 0.5 | 5.00 ± 0.09 | 150.90 | 64.20 |
| 15 | 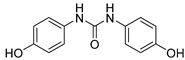 | 8.9 ± 0.4 | 1.17 ± 0.01 | ND c | ND c |
| 17 | 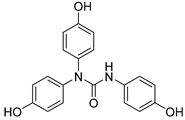 | 19.4 ± 0.7 | 3.57 ± 0.00 | 17.92 | 9.60 |
| Resveratrol | 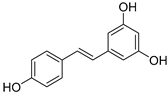 | 8.08 ± 1.2 | 2.13 ± 0.02 | 66.22 | 19.48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez de Vega, M.J.; Moreno-Fernández, S.; Pontes-Quero, G.M.; González-Amor, M.; Vázquez-Lasa, B.; Sabater-Muñoz, B.; Briones, A.M.; Aguilar, M.R.; Miguel, M.; González-Muñiz, R. Characterization of Novel Synthetic Polyphenols: Validation of Antioxidant and Vasculoprotective Activities. Antioxidants 2020, 9, 787. https://doi.org/10.3390/antiox9090787
Pérez de Vega MJ, Moreno-Fernández S, Pontes-Quero GM, González-Amor M, Vázquez-Lasa B, Sabater-Muñoz B, Briones AM, Aguilar MR, Miguel M, González-Muñiz R. Characterization of Novel Synthetic Polyphenols: Validation of Antioxidant and Vasculoprotective Activities. Antioxidants. 2020; 9(9):787. https://doi.org/10.3390/antiox9090787
Chicago/Turabian StylePérez de Vega, María Jesús, Silvia Moreno-Fernández, Gloria María Pontes-Quero, María González-Amor, Blanca Vázquez-Lasa, Beatriz Sabater-Muñoz, Ana M. Briones, María R. Aguilar, Marta Miguel, and Rosario González-Muñiz. 2020. "Characterization of Novel Synthetic Polyphenols: Validation of Antioxidant and Vasculoprotective Activities" Antioxidants 9, no. 9: 787. https://doi.org/10.3390/antiox9090787
APA StylePérez de Vega, M. J., Moreno-Fernández, S., Pontes-Quero, G. M., González-Amor, M., Vázquez-Lasa, B., Sabater-Muñoz, B., Briones, A. M., Aguilar, M. R., Miguel, M., & González-Muñiz, R. (2020). Characterization of Novel Synthetic Polyphenols: Validation of Antioxidant and Vasculoprotective Activities. Antioxidants, 9(9), 787. https://doi.org/10.3390/antiox9090787









