Macrocycles and Supramolecules as Antioxidants: Excellent Scaffolds for Development of Potential Therapeutic Agents
Abstract
:1. Introduction
2. Supramolecular Scaffolds Used for the Development of Antioxidants
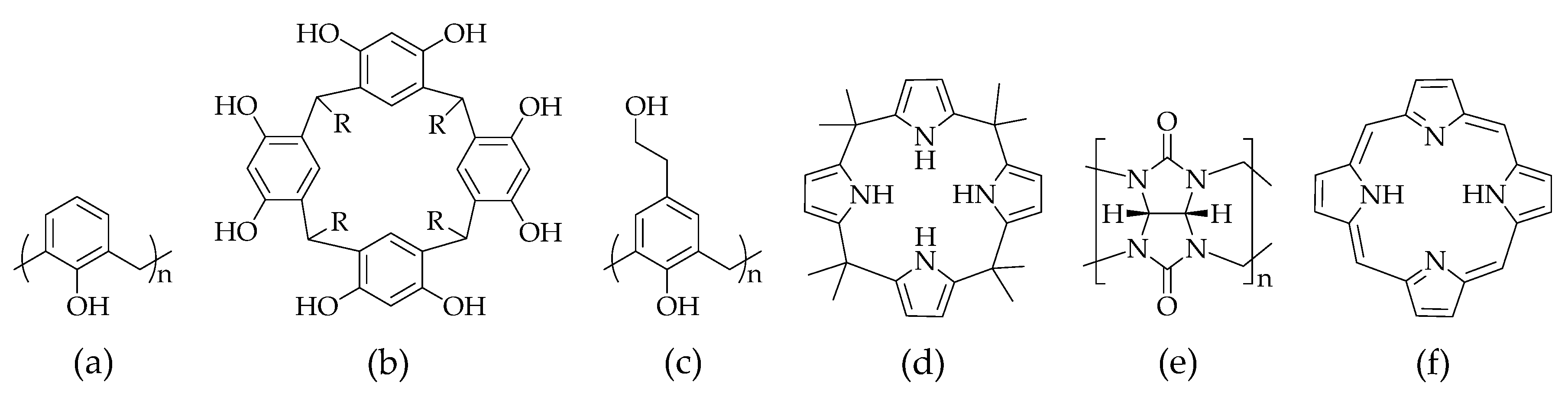
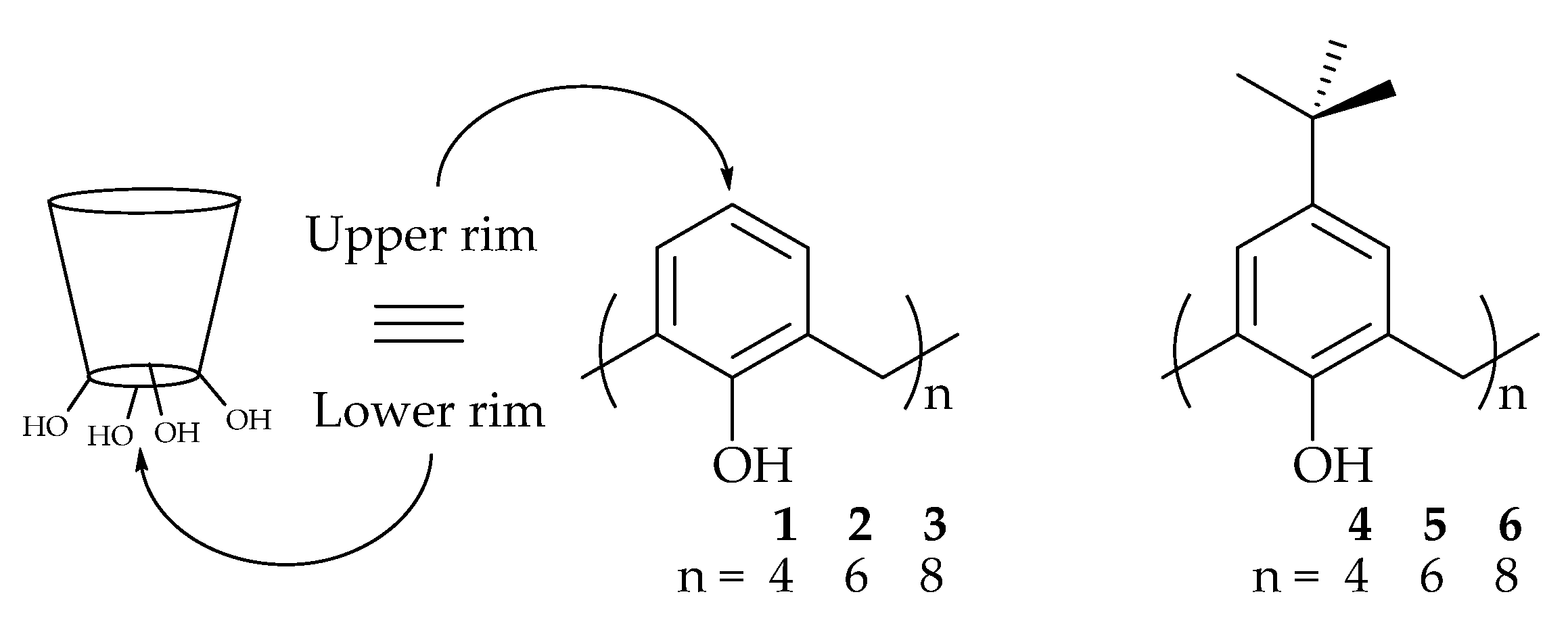
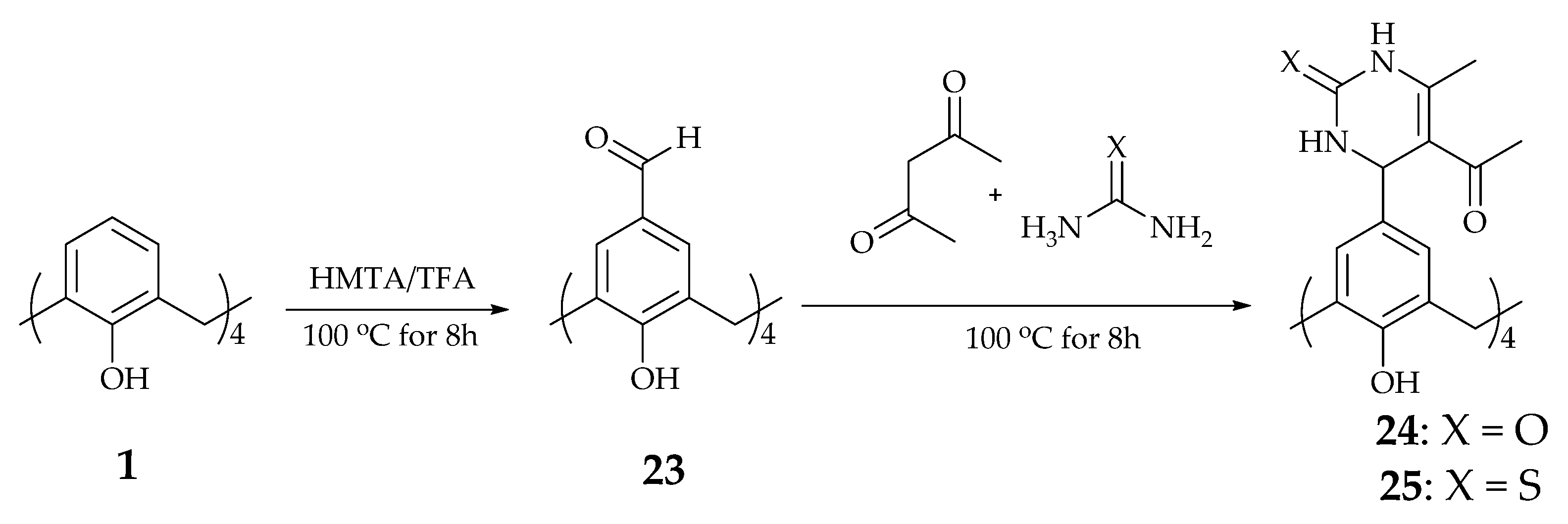
2.1. Calix[n]arene
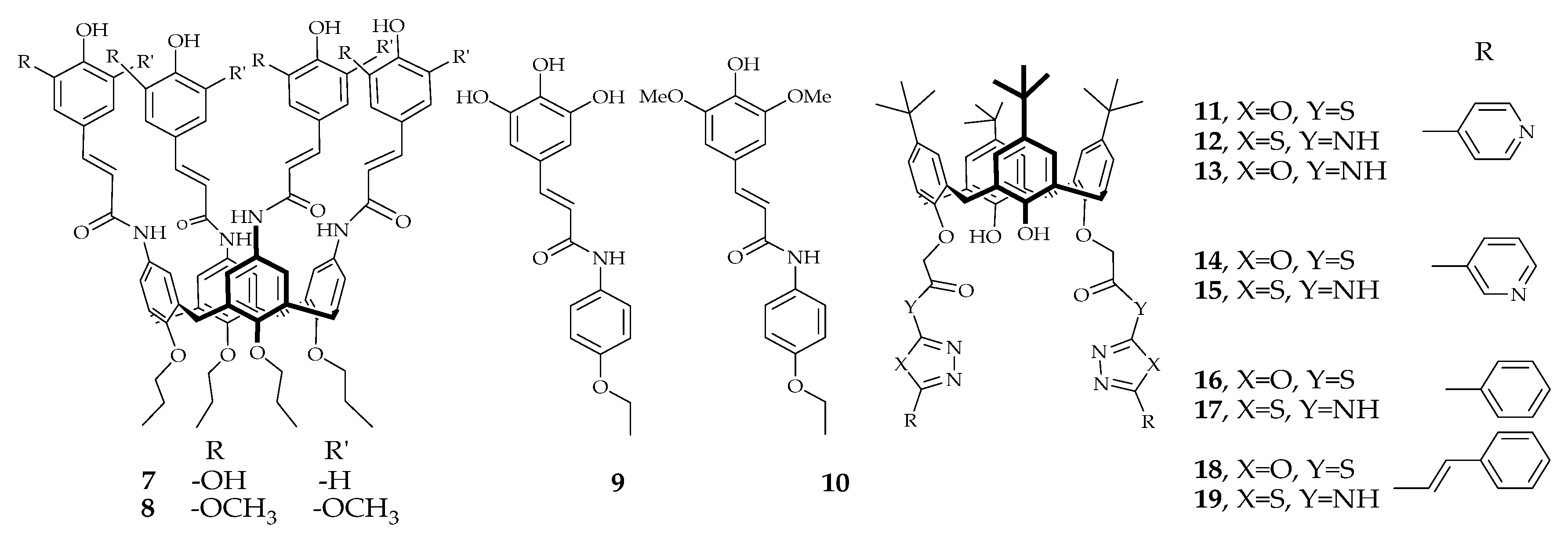
2.2. Resorcinarene (calix[4]resorcinarene)
2.3. Calixtyrosol
2.4. Calix[4]pyrrole
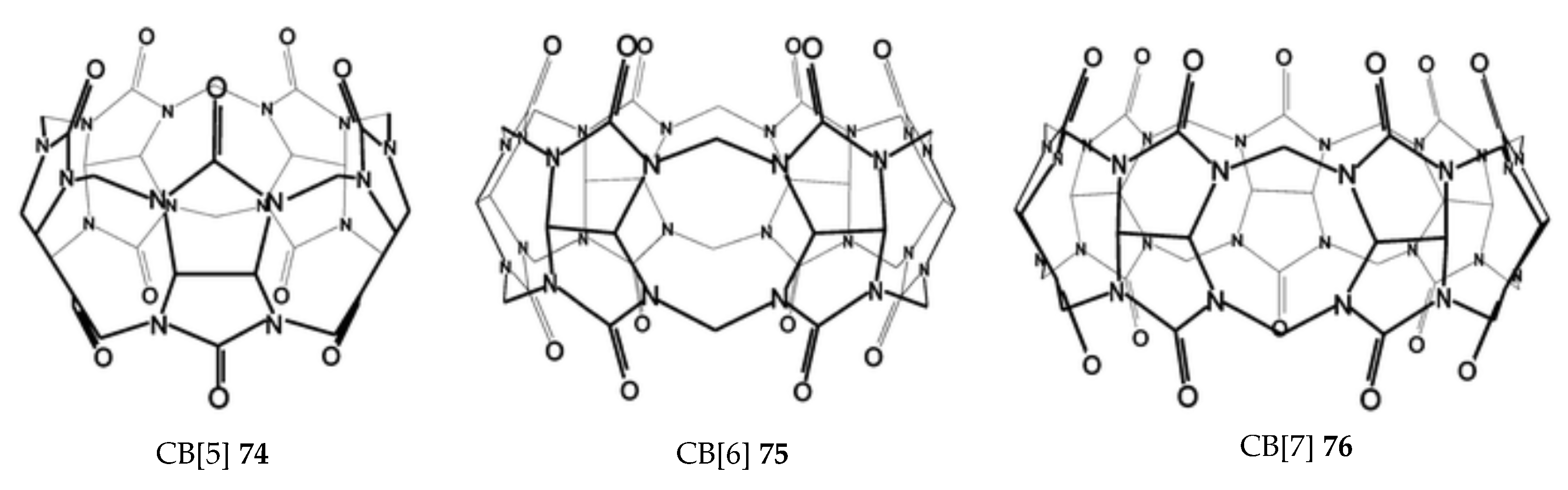
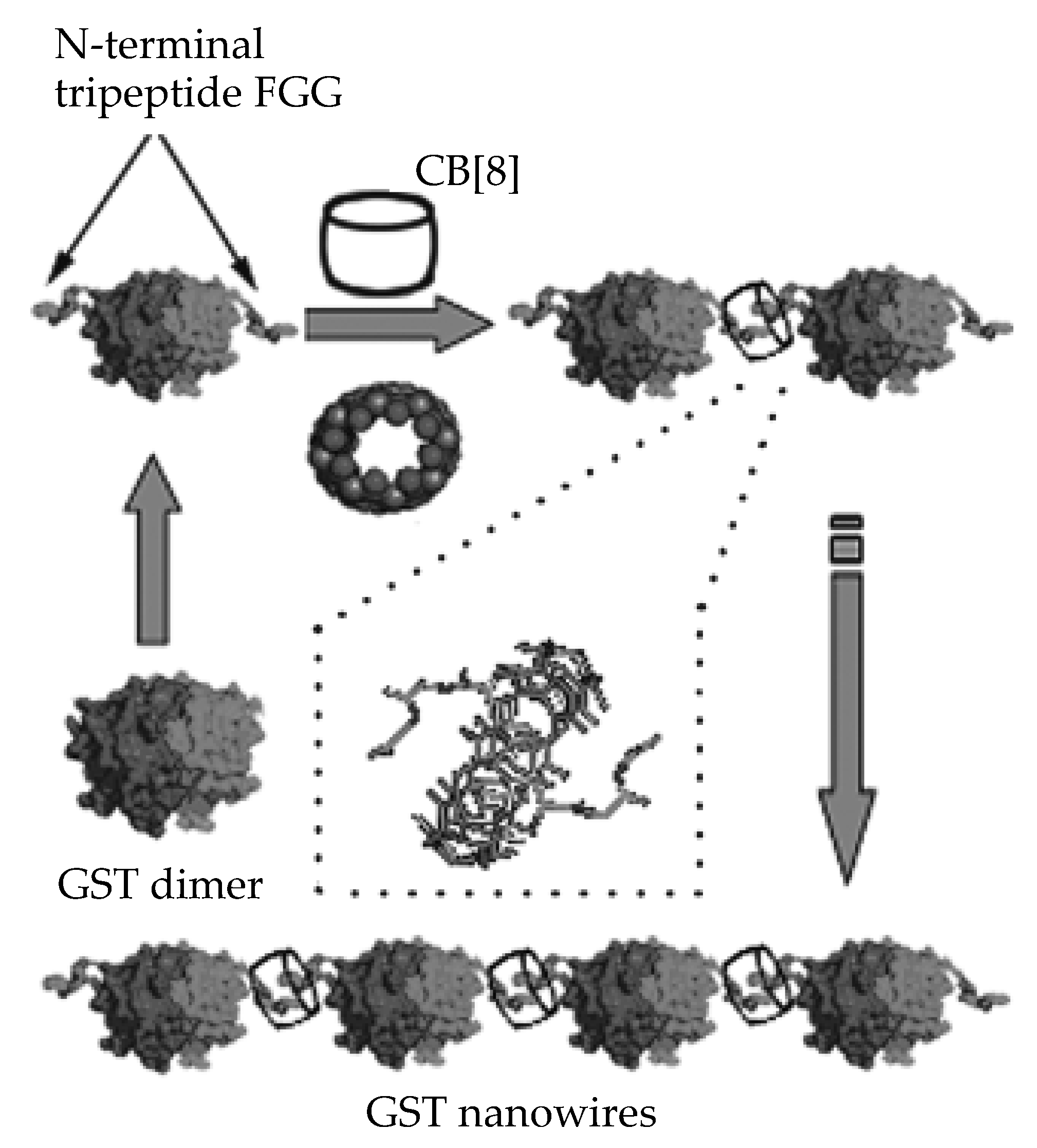
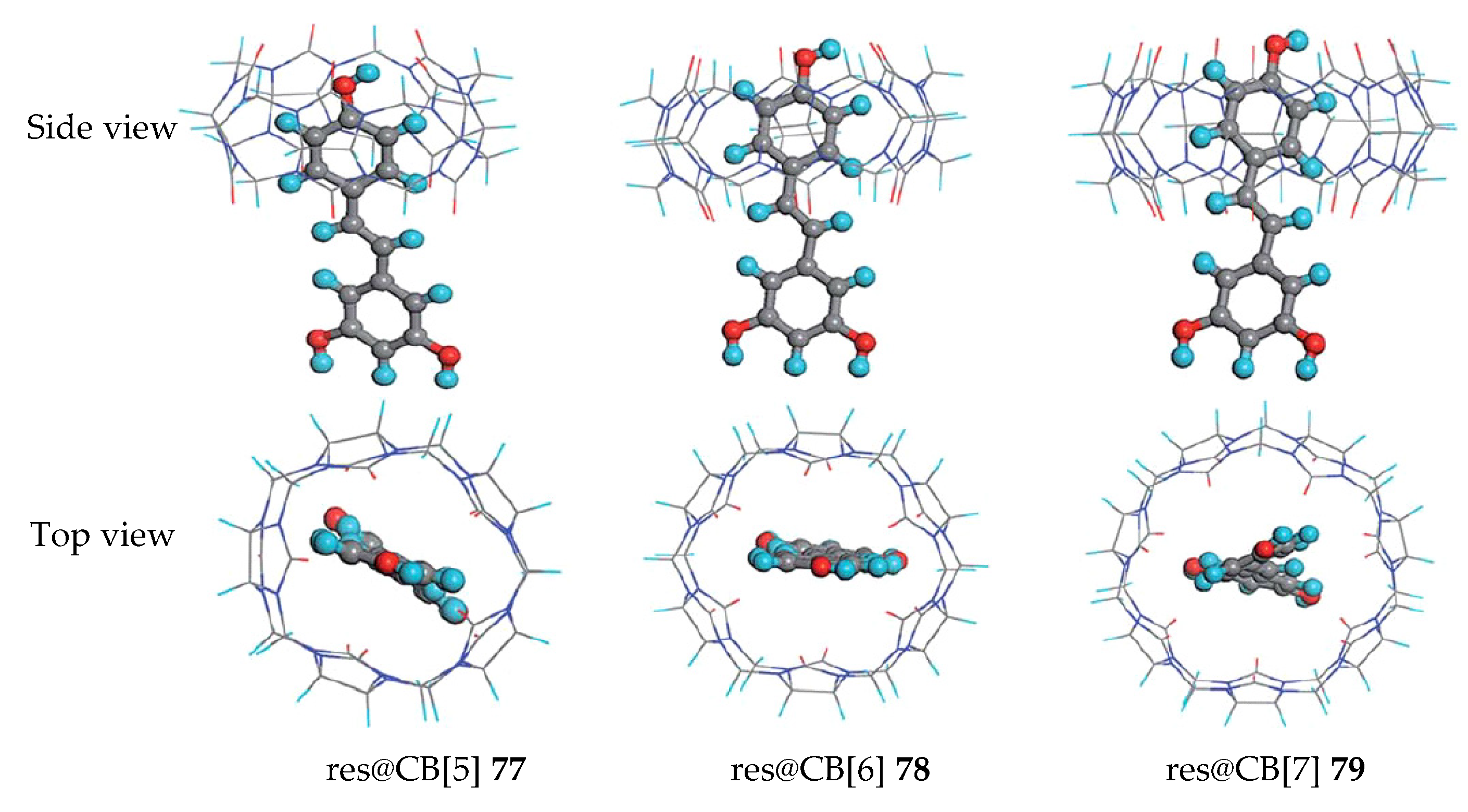
2.5. Cucurbituril (CB)
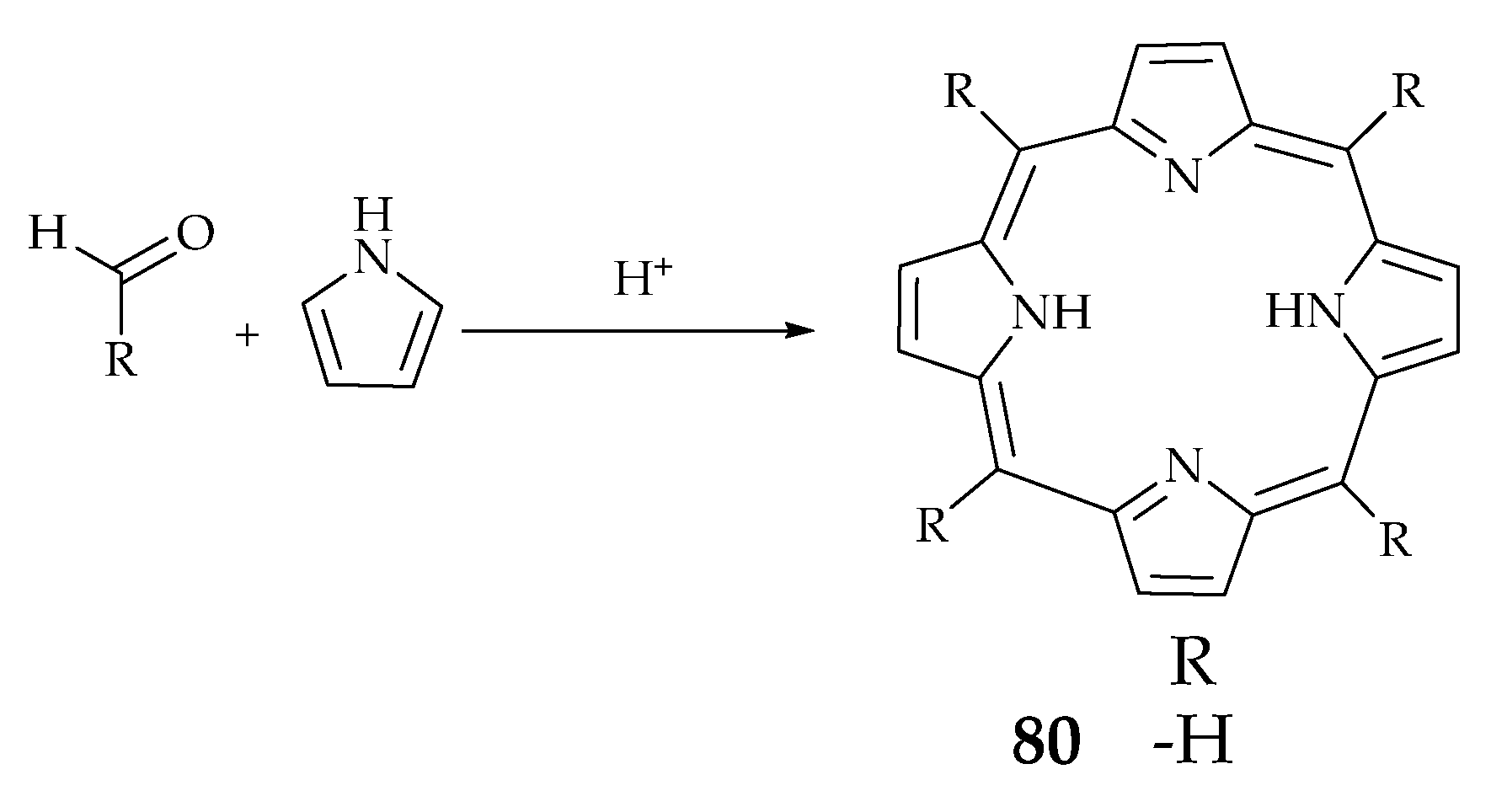
2.6. Porphyrin
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.; Dayem, A.A.; Cho, S. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandra, C.J.A.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Lin, Y.; Hausenloy, D.J. Mitochondria in acute myocardial infarction and cardioprotection. EBioMedicine 2020, 57, 102884. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, J.; Rayner, B.S.; Lowe, H.C.; Witting, P.K. Oxidative stress in myocardial ischaemia reperfusion injury: A renewed focus on a long-standing area of heart research. Redox Rep. 2005, 10, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef]
- Elahi, M.M.; Kong, Y.X.; Matata, B.M. Oxidative stress as a mediator of cardiovascular disease. Oxid. Med. Cell Longev. 2009, 2, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interview Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Yang, J.; Jiang, Y.; Wen, L.; Wang, Z.; Yang, B. The antioxidant activity and neuroprotective mechanism of isoliquiritigenin. Free Radic. Biol. Med. 2020, 152, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Warraich, U.; Hussain, F.; Kayani, H.U.R. Aging-Oxidative stress, antioxidants and computational modeling. Heliyon 2020, 6, e04107. [Google Scholar] [CrossRef] [PubMed]
- Čolak, E.; Ignjatović, S.; Radosavljević, A.; Žorić, L. The association of enzymatic and non-enzymatic antioxidant defense parameters with inflammatory markers in patients with exudative form of age-related macular degeneration. J. Clin. Biochem. Nutr. 2017, 60, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Patlevič, P.; Vašková, J.; Švorc, P., Jr.; Vaško, L.; Švorc, P. Reactive oxygen species and antioxidant defense in human gastrointestinal diseases. Integr. Med. Res. 2016, 5, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Hamley, I.W. Small Bioactive Peptides for Biomaterials Design and Therapeutics. Chem. Rev. 2017, 117, 14015–14041. [Google Scholar] [CrossRef] [Green Version]
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential applications of antioxidants—A review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Panieri, E.; Buha, A.; Telkoparan-Akillilar, P.; Cevik, D.; Kouretas, D.; Veskoukis, A.; Skaperda, Z.; Tsatsakis, A.; Wallace, D.; Suzen, S. Luciano Saso 1Potential Applications of NRF2 Modulators in Cancer Therapy. Antioxidants 2020, 9, 193. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Ingold, K.U.; Pratt, D.A. Advances in Radical-Trapping Antioxidant Chemistry in the 21st Century: A Kinetics and Mechanisms Perspective. Chem. Rev. 2014, 114, 9022–9046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017, 11, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, B.; Li, X.; Lu, W.; Yang, J.; Hu, Y.; Huang, P.; Wen, S. Inhibition of cancer growth in vitro and in vivo by a novel ROS-modulating agent with ability to eliminate stem-like cancer cells. Cell Death Dis. 2017, 8, e2887. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.H.; Babic, G.; Durst, T.; Wright, J.S.; Flueraru, M.; Chichirau, A.; Chepelev, L.L. Development of novel antioxidants: Design, synthesis, and reactivity. J. Org. Chem. 2003, 68, 7023–7032. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; García-Carmona, F. Cyclodextrins and Antioxidants. Crit. Rev. Food Sci. Nutr. 2014, 54, 251–276. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Zhu, H.; Wang, S.; Xing, J. Inclusion Complexes of Lycopene and β-Cyclodextrin: Preparation, Characterization, Stability and Antioxidant Activity. Antioxidants 2019, 8, 314. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Zhao, L.; Yang, S.; Huang, Y.; Chi, S.; Ruan, Q.; Zheng, P.; Hu, R.; Zhao, Y. Preparation, characterization, solubilization and antioxidant activity of polyamine modified β-cyclodextrins with baicalein inclusion complexes. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 203–213. [Google Scholar] [CrossRef]
- Xu, Z.; Lian, X.; Li, M.; Zhang, X.; Wang, Y.; Tao, Z.; Zhang, Q. Effects of inclusion of chrysin in cucurbit [8] uril on its stability, solubility and antioxidant potential. Chem. Res. Chin. Univ. 2017, 33, 736–741. [Google Scholar] [CrossRef]
- Chao, J.; Wang, X.; Xu, M.; Zuo, Y. Characterization and enhanced antioxidant activity of the inclusion complexes of baicalin with p-sulfonatocalix [n] arenes. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 361–370. [Google Scholar] [CrossRef]
- Gutsche, C.D. Calixarenes an Introduction, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Vicens, J.; Boehmer, V. Calixarenes: A Versatile Class of Macrocyclic Compounds; Springer: Dordrecht, The Netherlands, 1991. [Google Scholar]
- Nimse, S.B.; Kim, T. Biological Applications of Functionalized Calixarenes. Chem. Soc. Rev. 2013, 42, 366–386. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Song, K.; Jung, C.; Eoum, W.; Kim, T. Molecular recognition of neutral substrates by new tetraaminocalix [4] arene derivative. Bull. Korean Chem. Soc. 2009, 30, 1247–1251. [Google Scholar]
- Nimse, S.B.; Song, K.; Kim, J.; Kim, H.; Nguyen, V.; Eoum, W.; Jung, C.; Ta, V.; Kim, T. Aminocalix [4] arene: The effect of pH on the dynamics of gate and portals on the hydrophobic cavity. Tetrahedron Lett. 2010, 51, 6156–6160. [Google Scholar] [CrossRef]
- Nimse, S.B.; Kim, J.; Song, K.; Kim, J.; Lee, J.T.; Nguyen, V.; Ta, V.; Kim, T. Selective recognition of the ditopic trimethylammonium cations by water-soluble aminocalix [4] arene. Tetrahedron Lett. 2011, 52, 3751–3755. [Google Scholar] [CrossRef]
- Seiffarth, K.; Schulz, M.; Goermar, G.; Bachmann, J. Calix [n] arenes—New light stabilizers for polyolefins. Polym. Degrad. Stab. 1989, 24, 73–80. [Google Scholar] [CrossRef]
- Feng, W.; Yuan, L.H.; Zheng, S.Y.; Huang, G.L.; Qiao, J.L.; Zhou, Y. The effect of p-tert-butylcalix [n] arene on γ-radiation degradation of polypropylene. Rad. Phys. Chem. 2000, 57, 425–429. [Google Scholar] [CrossRef]
- Gorghiu, L.M.; Dumitrescu, C.; Olteanu, R.L.; Bumbac, M.; Jipa, S. Comparative Study of Some Calix[n] arenes and other Phenolic Compounds Influence on the thermal Stability of LDPE. Rom. Rep. 2004, 56, 466–472. [Google Scholar]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 5666. [Google Scholar] [CrossRef] [Green Version]
- Wrigstedt, P.; Kylli, P.; Pitkänen, L.; Nousiainen, P.; Tenkanen, M.; Sipilä, J. Synthesis and Antioxidant Activity of Hydroxycinnamic Acid Xylan Esters. J. Agric. Food Chem. 2010, 58, 6937–6943. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D.; Mazumder, A.; Mazumder, R. Synthesis of Cinnamanilide Derivatives and their Antioxidant and Antimicrobial Activity. J. Chem. 2015, 2015, 208910. [Google Scholar] [CrossRef] [Green Version]
- Consoli, G.M.L.; Galante, E.; Daquino, C.; Granata, G.; Cunsolo, F.; Geraci, C. Hydroxycinnamic acid clustered by a calixarene platform: Radical scavenging and antioxidant activity. Tetrahedron Lett. 2006, 47, 6611–6614. [Google Scholar] [CrossRef]
- Patel, M.B.; Modi, N.R.; Raval, J.P.; Menon, S.K. Calix [4] arene based 1,3,4-oxadiazole and thiadiazole derivatives: Design, synthesis, and biological evaluation. Org. Biomol. Chem. 2012, 10, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- James, E.; Eggers, P.K.; Harvey, A.R.; Dunlop, S.A.; Fitzgerald, M.; Stubbs, K.A.; Raston, C.L. Antioxidant phospholipid calix [4] arene mimics as micellular delivery systems. Org. Biomol. Chem. 2013, 11, 6108–6112. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Singh, H.; Suating, P.; Kim, H.S.; Sunwoo, K.; Shim, I.; Gibb, B.C.; Kim, J.S. Revisiting Fluorescent Calixarenes: From Molecular Sensors to Smart Materials. Chem. Rev. 2019, 119, 9657–9721. [Google Scholar] [CrossRef]
- Kumar, R.; Lee, Y.O.; Bhalla, V.; Kumar, M.; Kim, J.S. Recent developments of thiacalixarene based molecular motifs. Chem. Soc. Rev. 2014, 43, 4824–4870. [Google Scholar] [CrossRef]
- Pur, F.N.; Dilmaghani, K.A. New Antiradical Clusters Synthesized Using the First Green Biginelli Reactions of Calix [4] arene. Pharm. Chem. J. 2016, 50, 80–82. [Google Scholar] [CrossRef]
- Stephens, E.K.; Tauran, Y.; Coleman, A.W.; Fitzgerald, M. Structural requirements for anti-oxidant activity of calix [n] arenes and their associated anti-bacterial activity. Chem. Commun. 2015, 51, 851–854. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Ruan, Z.; Ni, J.; Chen, J.; Shu, H.; Wang, Y.; Liu, Y. Improvement of antioxidative activity of resveratrol by calix [4] arene-like tetramer: A theoretical study. Comput. Theor. Chem. 2019, 1148, 1–7. [Google Scholar] [CrossRef]
- Ni, J.; Lu, L.; Liu, Y. Antiradical and Antioxidative Activity of Azocalix [4] arene Derivatives: Combined Experimental and Theoretical Study. Molecules 2019, 24, 485. [Google Scholar] [CrossRef] [Green Version]
- Nimse, S.B.; Song, K.; Kim, J.; Ta, V.; Nguyen, V.; Kim, T. Synthesis and Modification of Novel Iminecalix [4] arene Derivatives. Bull. Korean Chem. Soc. 2011, 32, 1143–1145. [Google Scholar] [CrossRef] [Green Version]
- Nimse, S.B.; Kim, J.; Ta, V.; Kim, H.; Song, K.; Jung, C.; Nguyen, V.; Kim, T. New water-soluble iminecalix [4] arene with a deep hydrophobic cavity. Tetrahedron Lett. 2009, 52, 7346–7350. [Google Scholar] [CrossRef]
- Silva, C.M.; da Silva, D.L.; Magalhães, T.F.F.; Alves, R.B.; de Resende-Stoianoff, M.A.; Martins, F.T.; Fátima, A. Iminecalix [4] arenes: Microwave-assisted synthesis, X-ray crystal structures, and anticandidal activity. Arabian J. Chem. 2019, 12, 4365–4376. [Google Scholar] [CrossRef] [Green Version]
- Oguz, M.; Kalay, E.; Akocak, S.; Nocentini, A.; Lolak, N.; Boga, M.; Yilmaz, M.; Supuran, C.T. Synthesis of calix [4] azacrown substituted sulphonamides with antioxidant, acetylcholinesterase, butyrylcholinesterase, tyrosinase and carbonic anhydrase inhibitory action. J. Enz. Inhib. Med. Chem. 2020, 35, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, Y.; Tanaka, Y.; Toi, H.; Ogoshi, H. Polar host–guest interaction. Binding of nonionic polar compounds with a resorcinol-aldehyde cyclooligomer as a lipophilic polar host. J. Am. Chem. Soc. 1988, 110, 634–635. [Google Scholar] [CrossRef]
- Gropp, C.; Quigley, B.L.; Diederich, F. Molecular Recognition with Resorcin [4] arene Cavitands: Switching, Halogen-Bonded Capsules, and Enantioselective Complexation. J. Am. Chem. Soc. 2018, 140, 2705–2717. [Google Scholar] [CrossRef]
- MacGillivray, L.R.; Atwood, J.L. A chiral spherical molecular assembly held together by 60 hydrogen bonds. Nature 1997, 389, 469–472. [Google Scholar] [CrossRef]
- Shivanyuk, A.; Rebek, J. Assembly of Resorcinarene Capsules in Wet Solvents. J. Am. Chem. Soc. 2003, 125, 3432–3433. [Google Scholar] [CrossRef]
- Zhang, Q.; Catti, L.; Tiefenbacher, K. Catalysis inside the Hexameric Resorcinarene Capsule. Acc. Chem. Res. 2018, 51, 2107–2114. [Google Scholar] [CrossRef] [Green Version]
- Avram, L.; Cohen, Y.; Rebek, J. Recent advances in hydrogen-bonded hexameric encapsulation complexes. Chem. Commun. 2011, 47, 5368–5375. [Google Scholar] [CrossRef]
- Payne, D.T.; Webre, W.A.; Matsushita, Y.; Zhu, N.; Futera, Z.; Labuta, J.; Jevasuwan, W.; Fukata, N.; Fossey, J.S.; D’Souza, F.; et al. Multimodal switching of a redox-active macrocycle. Nat. Commun. 2019, 10, 1007. [Google Scholar] [CrossRef] [PubMed]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuni, A.M.; Barenholz, Y. Site-activity relationship of nitroxide radical’s antioxidative effect. Free Radic. Biol. Med. 2003, 34, 177–185. [Google Scholar] [CrossRef]
- Damiani, E.; Castagna, R.; Astolfi, P.; Greci, L. Aromatic and aliphatic mono- and bis-nitroxides: A study on their radical scavenging abilities. Free Radic. Res. 2005, 39, 325–336. [Google Scholar] [CrossRef]
- Vovk, A.I.; Shivanyuk, A.M.; Bugas, R.V.; Muzychka, O.V.; Melnyk, A.K. Antioxidant and antiradical activities of resorcinarene tetranitroxides. Bioorg. Med. Chem. Lett. 2009, 19, 1314–1317. [Google Scholar] [CrossRef] [PubMed]
- Ngurah, B.I.G.M. Synthetic C-Methoxyphenyl Calix [4] Resorcinarene and Its Antioxidant Activity. J. Appl. Chem. Sci. 2018, 5, 403–408. [Google Scholar] [CrossRef]
- Oliveira, C.B.S.; Meurer, Y.S.R.; Oliveira, M.G.; Medeiros, W.M.T.Q.; Silva, F.O.N.; Brito, A.C.F.; Pontes, D.L.; Andrade-Neto, V.F. Comparative Study on the Antioxidant and Anti-Toxoplasma Activities of Vanillin and Its Resorcinarene Derivative. Molecules 2014, 19, 5898–5912. [Google Scholar] [CrossRef] [Green Version]
- Abosadiya, H.M.; Hasbullah, S.A.; Mackeen, M.M.; Low, S.C.; Ibrahim, N.; Koketsu, M.; Yamin, B.M. Synthesis, Characterization, X-ray Structure and Biological Activities of C-5-Bromo-2-hydroxyphenylcalix [4]-2-methyl Resorcinarene. Molecules 2013, 18, 13369–13384. [Google Scholar] [CrossRef]
- Pur, N.F. Calixdrugs: Calixarene-based clusters of established therapeutic drug agents. Mol. Divers. 2016, 20, 781–787. [Google Scholar]
- Pur, N.F.; Dilmaghani, K.A. Calixtyrosol: A Novel Calixarene Based Potent Radical Scavenger. Iranian J. Pharma. Res. 2015, 14, 1181–1187. [Google Scholar]
- Pinalli, R.; Pedrini, A.; Dalcanale, A. Biochemical sensing with macrocyclic receptors. Chem. Soc. Rev. 2018, 47, 7006–7026. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Sessler, J.L. Calix [4] pyrroles: Versatile molecular containers with ion transport, recognition, and molecular switching functions. Chem. Soc. Rev. 2015, 44, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Anzenbacher, P.; Sessler, J.L. Calixpyrroles II. Coord. Chem. Rev. 2001, 222, 57–102. [Google Scholar] [CrossRef]
- Kongor, A.; Panchal, M.; Mehta, V.; Bhatt, K.; Bhagat, D.; Tipre, D.; Jain, V.K. Basketing nanopalladium into calix [4] pyrrole as an efficient catalyst for Mizoroki-Heck reaction. Arab. J. Chem. 2017, 10, 1125–1135. [Google Scholar] [CrossRef] [Green Version]
- Kongor, A.; Panchal, M.; Athar, M.; Makwana, B.; Sindhav, G.; Jha, P.C.; Jain, V. Synthesis and modeling of calix [4] pyrrole wrapped Au nanoprobe for specific detection of Pb(II): Antioxidant and radical scavenging efficiencies. J. Photochem. Photobiol. A Chem. 2018, 364, 801–810. [Google Scholar] [CrossRef]
- Freeman, W.A.; Mock, W.L.; Shih, N.Y. Cucurbituril. J. Am. Chem. Soc. 1981, 103, 7367–7368. [Google Scholar] [CrossRef]
- Kim, J.; Jung, I.-S.; Kim, S.-Y.; Lee, E.; Kang, J.-K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. New Cucurbituril Homologues: Syntheses, Isolation, Characterization, and X-ray Crystal Structures of Cucurbit [n] uril (n = 5, 7, and 8). J. Am. Chem. Soc. 2000, 122, 540–541. [Google Scholar] [CrossRef]
- Day, A.I.; Blanch, R.J.; Arnold, A.P.; Lorenzo, S.; Lewis, G.R.; Dance, I. A Cucurbituril-Based Gyroscane: A New Supramolecular Form. Angew. Chem., Int. Ed. 2002, 41, 275–277. [Google Scholar] [CrossRef]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; Barrio, J.; Scherman, O.A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.; Kim, K.; Ogoshi, T.; Yao, W.; Gibb, B.C. The aqueous supramolecular chemistry of cucurbit [n] urils, pillar [n] arenes and deep-cavity cavitands. Chem. Soc. Rev. 2017, 46, 2479–2496. [Google Scholar] [CrossRef] [Green Version]
- Day, A.; Arnold, A.P.; Blanch, R.J.; Snushall, B. Controlling Factors in the Synthesis of Cucurbituril and Its Homologues. J. Org. Chem. 2001, 66, 8094–8100. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Samal, S.; Selvapalam, N.; Kim, H.; Kim, K. Cucurbituril Homologues and Derivatives: New Opportunities in Supramolecular Chemistry. Acc. Chem. Res. 2003, 36, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. The Cucurbit [n] uril Family. Angew. Chem. 2005, 117, 4922–4949. [Google Scholar] [CrossRef]
- Hou, C.; Li, J.; Zhao, L.; Zhang, W.; Luo, Q.; Dong, Z.; Xu, J.; Liu, J. Construction of Protein Nanowires through Cucurbit [8] uril-based Highly Specific Host–Guest Interactions: An Approach to the Assembly of Functional Proteins. Angew. Chem. Int. Ed. Engl. 2013, 52, 5590–5593. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, S.; Zhang, H.; Li, F.; Zhang, S. Theoretical study of complexation of resveratrol with cyclodextrins and cucurbiturils: Structure and antioxidative activity. RSC Adv. 2015, 5, 14114–14122. [Google Scholar] [CrossRef]
- Kubota, R.; Takabe, T.; Arima, K.; Taniguchi, H.; Asayama, S.; Kawakami, H. New class of artificial enzyme composed of Mn-porphyrin, imidazole, and cucurbit [10] uril toward use as a therapeutic antioxidant. J. Mater. Chem. B 2018, 6, 7050–7059. [Google Scholar] [CrossRef]
- Tian, J.; Huang, B.; Nawaz, M.H.; Zhang, W. Recent advances of multi-dimensional porphyrin-based functional materials in photodynamic therapy. Coord. Chem. Rev. 2020, 420, 213410. [Google Scholar] [CrossRef]
- Lee, H.; Hong, K.I.; Jang, W.D. Design and applications of molecular probes containing porphyrin derivatives. Coord. Chem. Rev. 2018, 354, 46–73. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, A.; Bhupathiraju, N.V.S.D.K.; Arianna, G.; Tiwari, K.; Drain, C.M. Glycosylated Porphyrins, Phthalocyanines, and Other Porphyrinoids for Diagnostics and Therapeutics. Chem. Rev. 2015, 115, 10261–10306. [Google Scholar] [CrossRef]
- Mackensen, G.B.; Patel, M.; Sheng, H.; Calvi, C.L.; Batinic’-Haberle, I.; Day, B.J.; Liang, L.P.; Fridovich, I.; Crapo, J.D.; Pearlstein, R.D.; et al. Neuroprotection from Delayed Postischemic Administration of a Metalloporphyrin Catalytic Antioxidant. J. Neurosci. 2001, 21, 4582–4592. [Google Scholar] [CrossRef] [Green Version]
- Asayama, S.; Mori, T.; Nagaoka, S.; Kawakami, H. Chemical modification of manganese porphyrins with biomolecules for new functional antioxidants. J. Biomater. Sci. Polym. Ed. 2003, 14, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Fadda, A.A.; El-Mekawy, R.E.; El-Shafei, A.; Freeman, H.S.; Hinks, D.; El-Fedawy, M. Design, Synthesis, and Pharmacological Screening of Novel Porphyrin Derivatives. J. Chem. 2013, 340230. [Google Scholar] [CrossRef] [Green Version]
- Fadda, A.A.; El-Mekawy, R.E.; El-Shafei, A.I.; Freeman, H. Synthesis and pharmacological screening of novel meso-substituted porphyrin analogs. Arch. Pharm. (Weinheim) 2013, 346, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Tyurin, V.Y.; Zhang, J.; Moiseeva, A.A.; Milaeva, E.R.; Belykh, D.V.; Buravlev, E.V.; Rocheva, T.K.; Chukicheva, I.Y.; Kuchin, A.V. Comparative study of redox characteristics and antioxidant activity of porphyrins with 2,6-dialkylphenol groups. Dokl. Chem. 2013, 450, 152–155. [Google Scholar] [CrossRef]
- Kubota, R.; Imamura, S.; Shimizu, T.; Asayama, S.; Kawakami, H. Synthesis of Water-Soluble Dinuclear Mn-Porphyrin with Multiple Antioxidative Activities. ACS Med. Chem. Lett. 2014, 5, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins—From superoxide dismutation to H2O2-driven pathways. Redox Biol. 2015, 5, 43–65. [Google Scholar] [CrossRef] [Green Version]
- Tesakova, M.V.; Parfenyuk, V.I. The Electrochemical Evaluation of the Antioxidant Activity of Substituted Tetraphenylporphyrins. Russ. J. Electrochem. 2017, 53, 1281–1285. [Google Scholar] [CrossRef]
- Abu-Melha, S. Efficient synthesis of meso-substituted porphyrins and molecular docking as potential new antioxidant and cytotoxicity agents. Arch. Pharm. Chem. Life Sci. 2018, 352, 1800221. [Google Scholar] [CrossRef]
- Shaaban, S.; Negm, A.; Sobh, M.A.; Wessjohann, L.A. Expeditious Entry to Functionalized Pseudo-peptidic Organoselenide Redox Modulators via Sequential Ugi/SN Methodology. Anti-Cancer Agents Med. Chem. 2016, 16, 621–632. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A.; Zhao, W.; Li, C.; Chen, X.; Wang, Y.; Zhu, W.; Zhong, Q. Influence of metal-porphyrins on the photocatalysis of graphitic carbon nitride. Dyes Pigment. 2018, 153, 241–247. [Google Scholar] [CrossRef]
- Shabangu, S.M.; Babu, B.; Soy, R.C.; Managa, M.; Sekhosana, K.E.; Nyokong, T. Photodynamic antimicrobial chemotherapy of asymmetric porphyrin-silver conjugates towards photoinactivation of Staphylococcus aureus. J. Coord. Chem. 2020, 73, 593–608. [Google Scholar] [CrossRef]
- Munechika, R.; Biju, V.; Takano, Y.; Harashima, H.; Yamada, Y. The optimization of cancer photodynamic therapy by utilization of a pi-extended porphyrin-type photosensitizer in combination with MITO-Porter. Chem. Commun. 2020, 56, 1145–1148. [Google Scholar]
- Zhang, Q.; He, J.; Yu, W.; Li, Y.; Liu, Z.; Zhou, B.; Liu, Y. A promising anticancer drug: A photosensitizer based on the porphyrin skeleton. RSC Med. Chem. 2020, 11, 427–437. [Google Scholar] [CrossRef]
- Ahmed, A.; Omar, W.A.E.; El-Asmy, H.A.; Abou-Zeid, L.; Fadda, A.A. Docking studies, antitumor and antioxidant evaluation of newly synthesized porphyrin and metalloporphyrin derivatives. Dyes Pigment. 2020, 108728. [Google Scholar] [CrossRef]


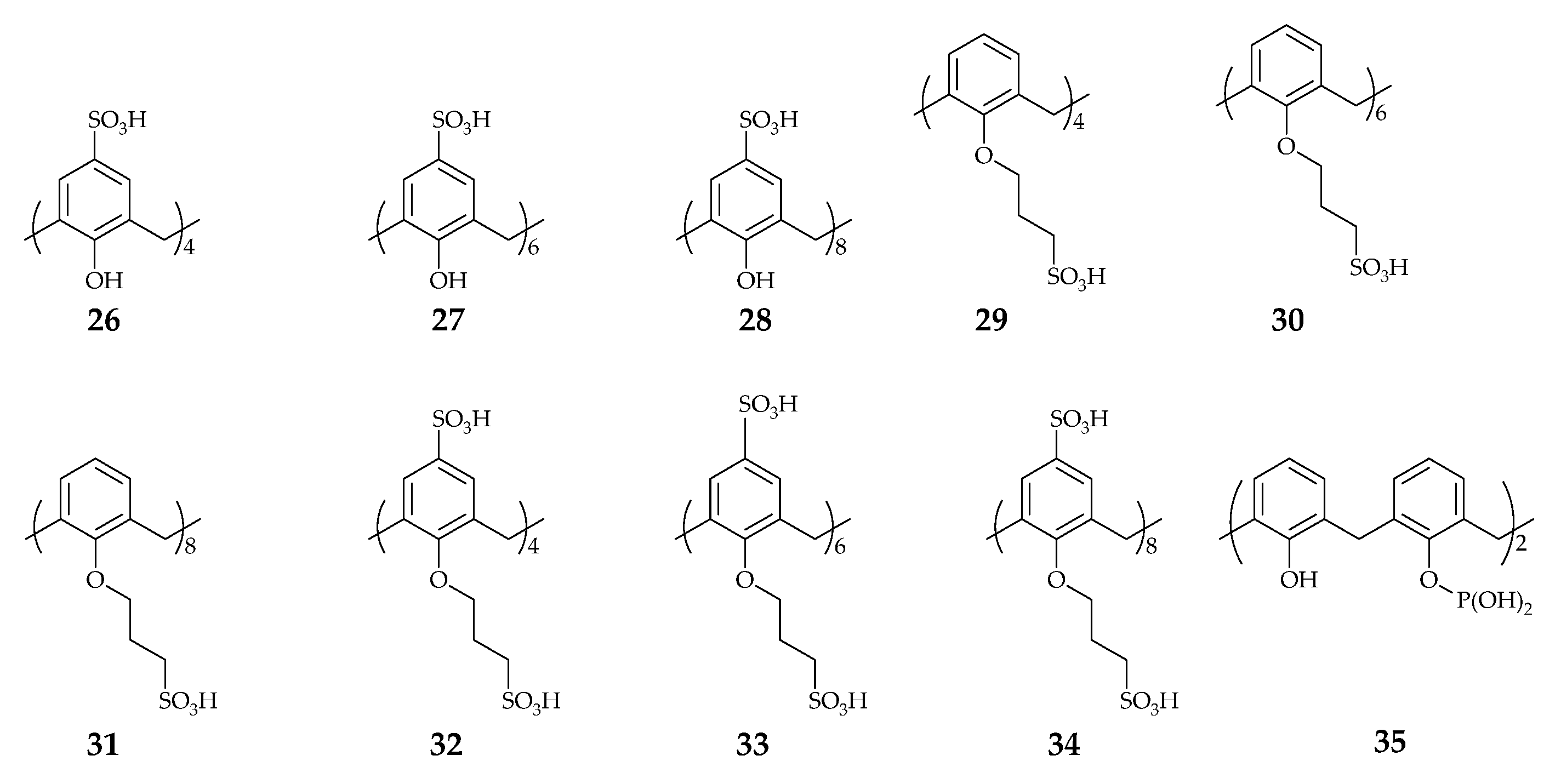
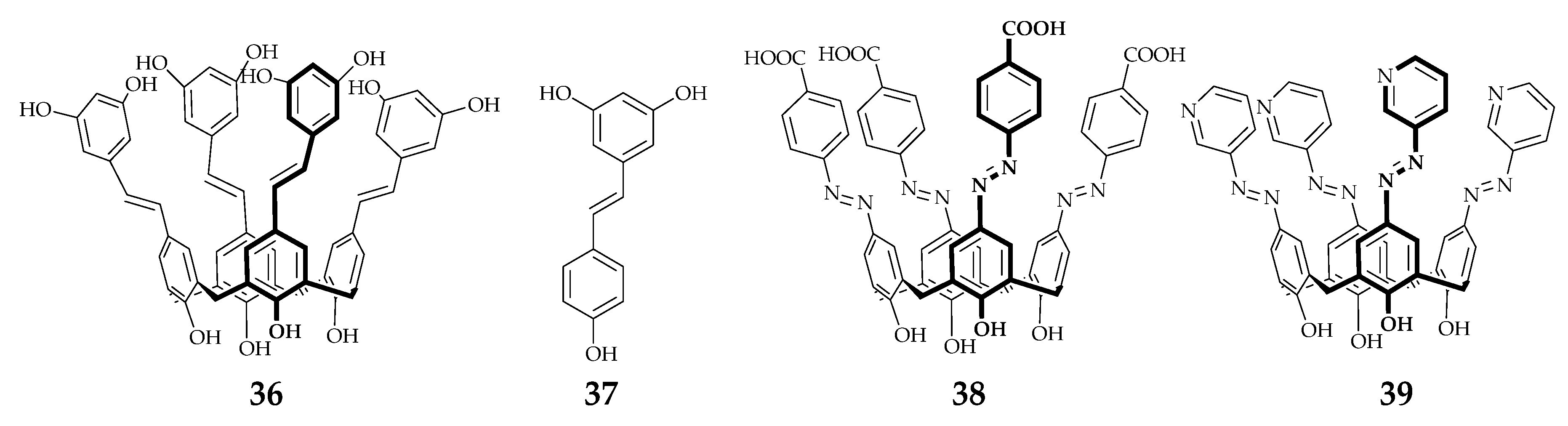
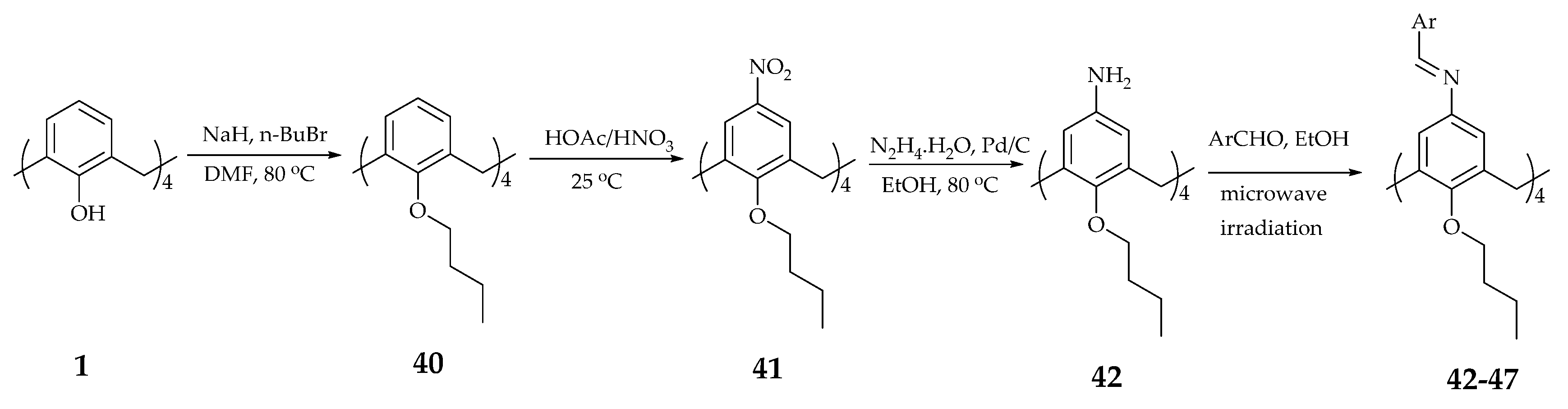





| Supramolecular Scaffold | Compound | DPPH Radical Scavenging (%) | kinh/kp (M−1 S−1) a | kSOD Activity (M−1 s−1) | Ref. |
|---|---|---|---|---|---|
| Calix[n]arene | 7 | - | 7.25 × 105 | - | [44] |
| 8 | - | 5.75 × 105 | - | ||
| 18 | 82.6 | - | - | [45] | |
| 38 | b 71.7 | - | [52] | ||
| 39 | b 63.2 | - | |||
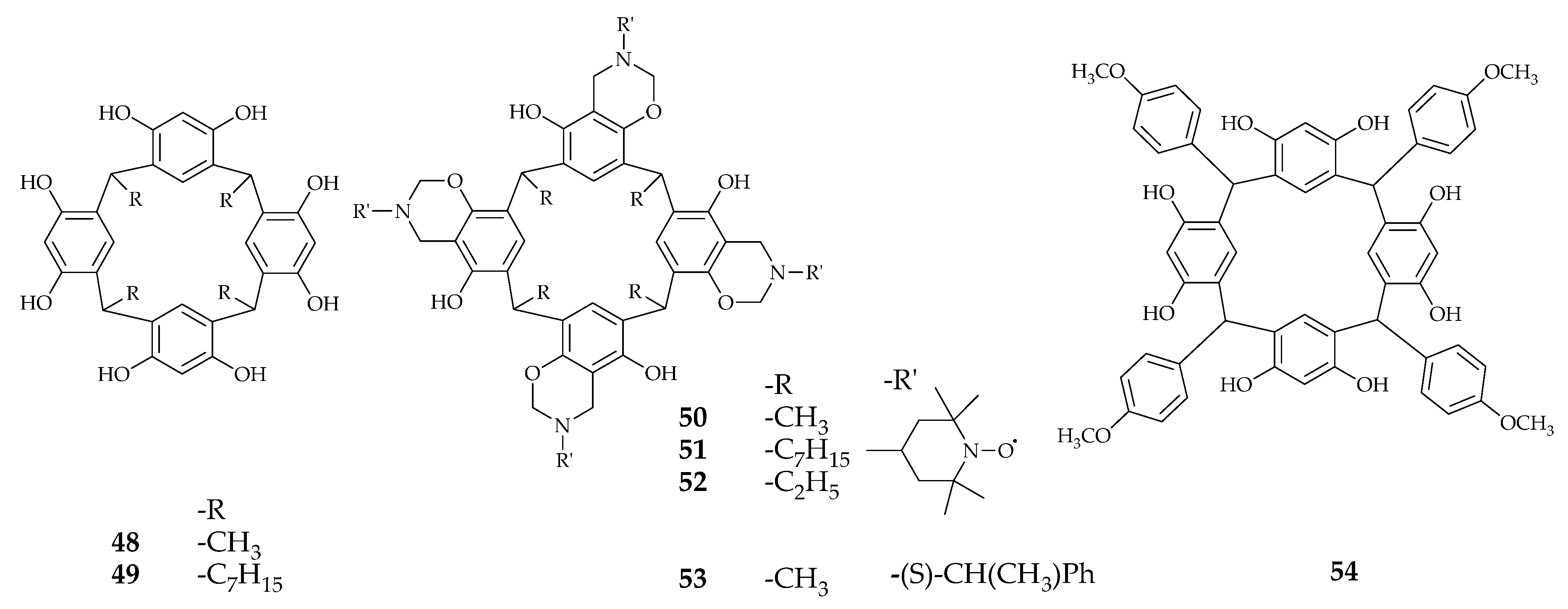
| Resorcinarene | 50 | c 0.19 ± 0.04 | - | - | [67] |
| 52 | c 0.18 ± 0.01 | - | - | ||
| 54 | 86.3 | - | - | ||
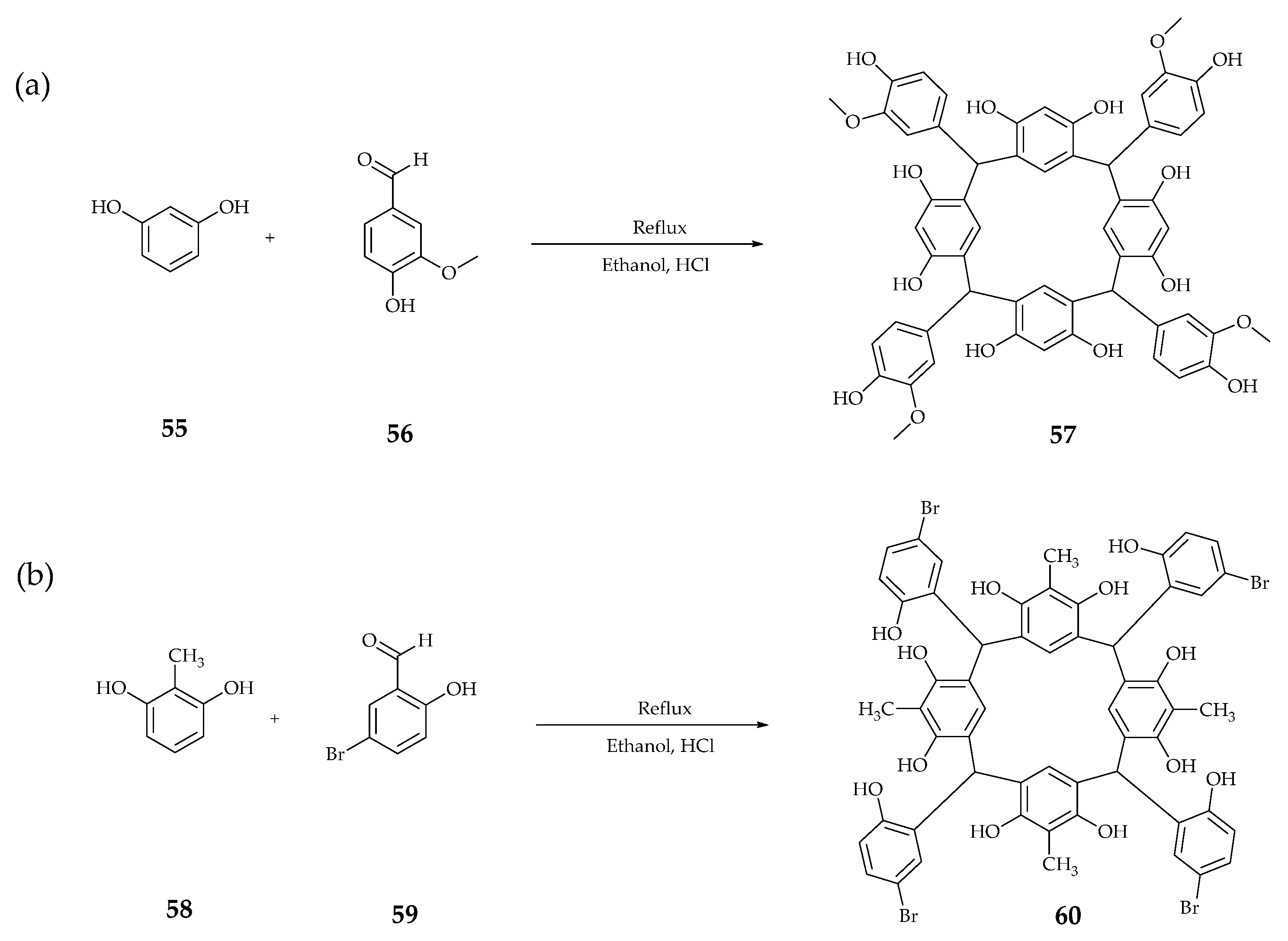
| 57 | 84.9 | - | - | [70] | |
| 60 | 85.0 | - | - | ||
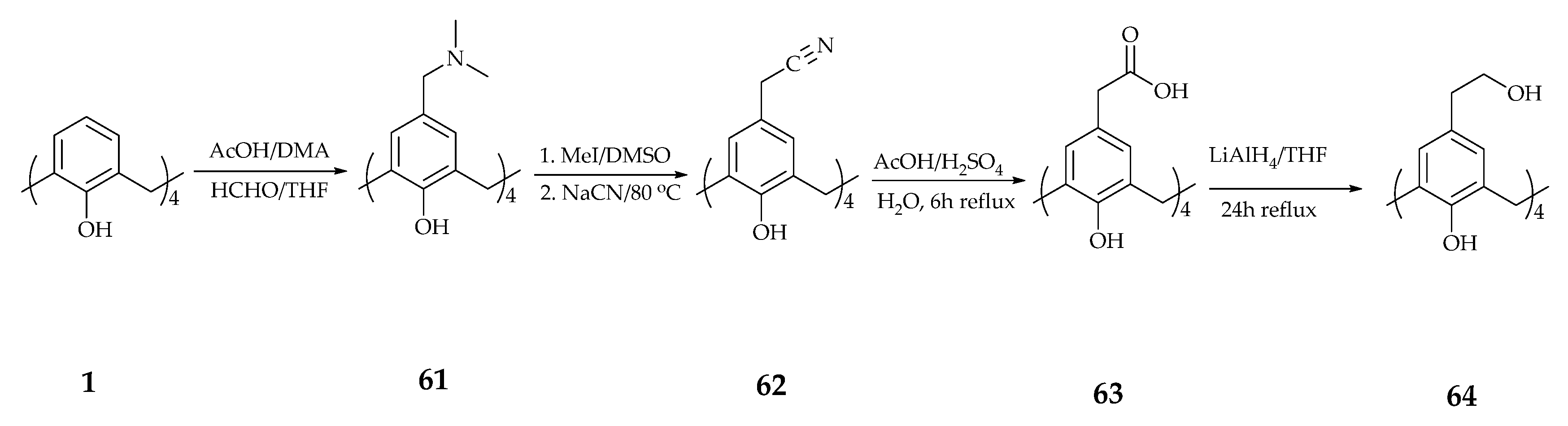
| Calixtyrosol | 64 | Significant | - | - | [72] |
| Calixpyrrole | 70 | 76.4% | - | - | [77] |
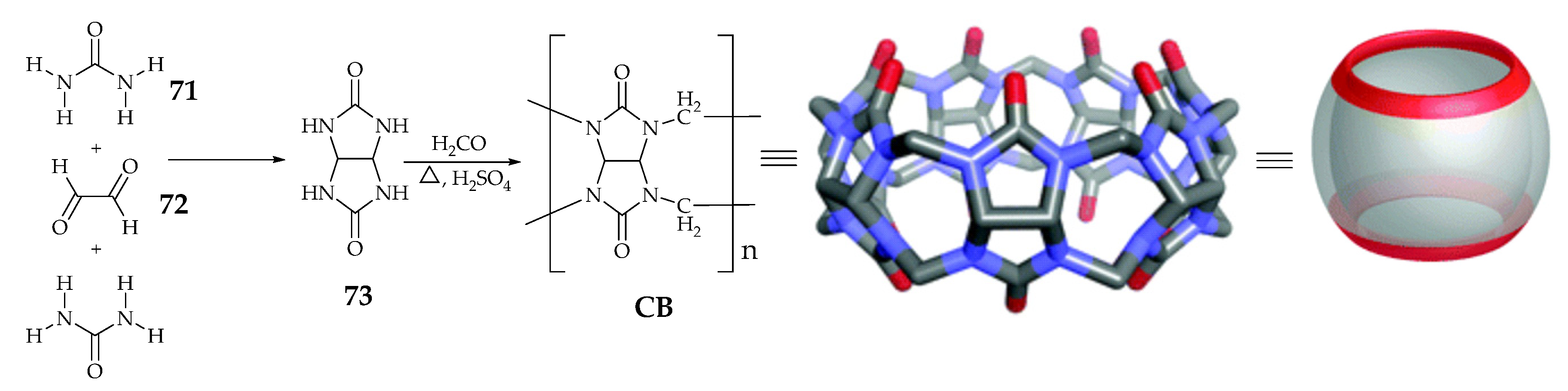
| Cucurbit[n]uril | 77–79 | d Significant | - | - | [87] |
| MndMIm4P@CB[10];Im | - | - | 1 × 107 | [88] | |
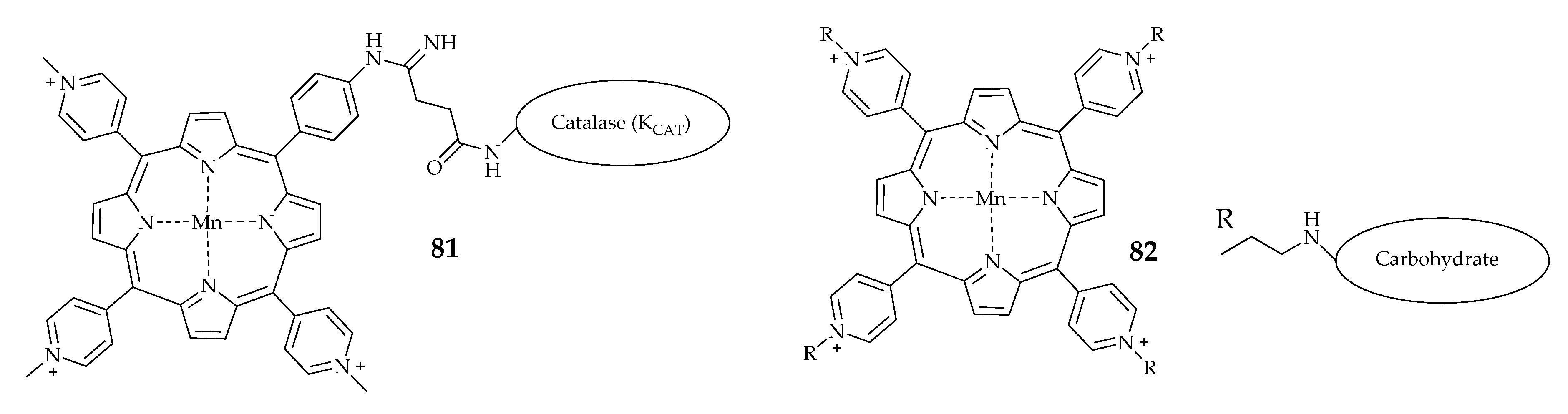
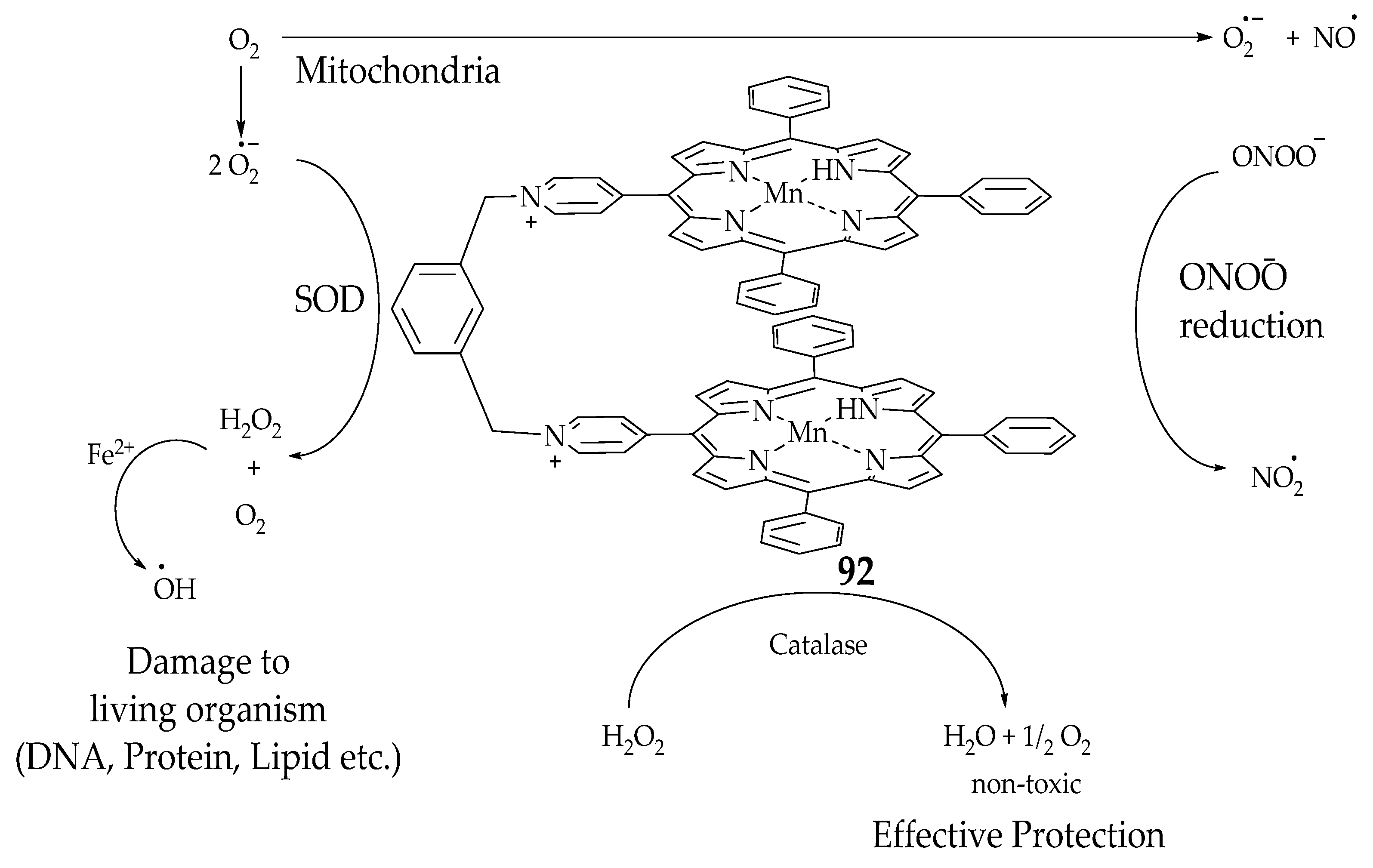
| Porphyrin | 80 | - | - | 5.4 × 105 | [92] |
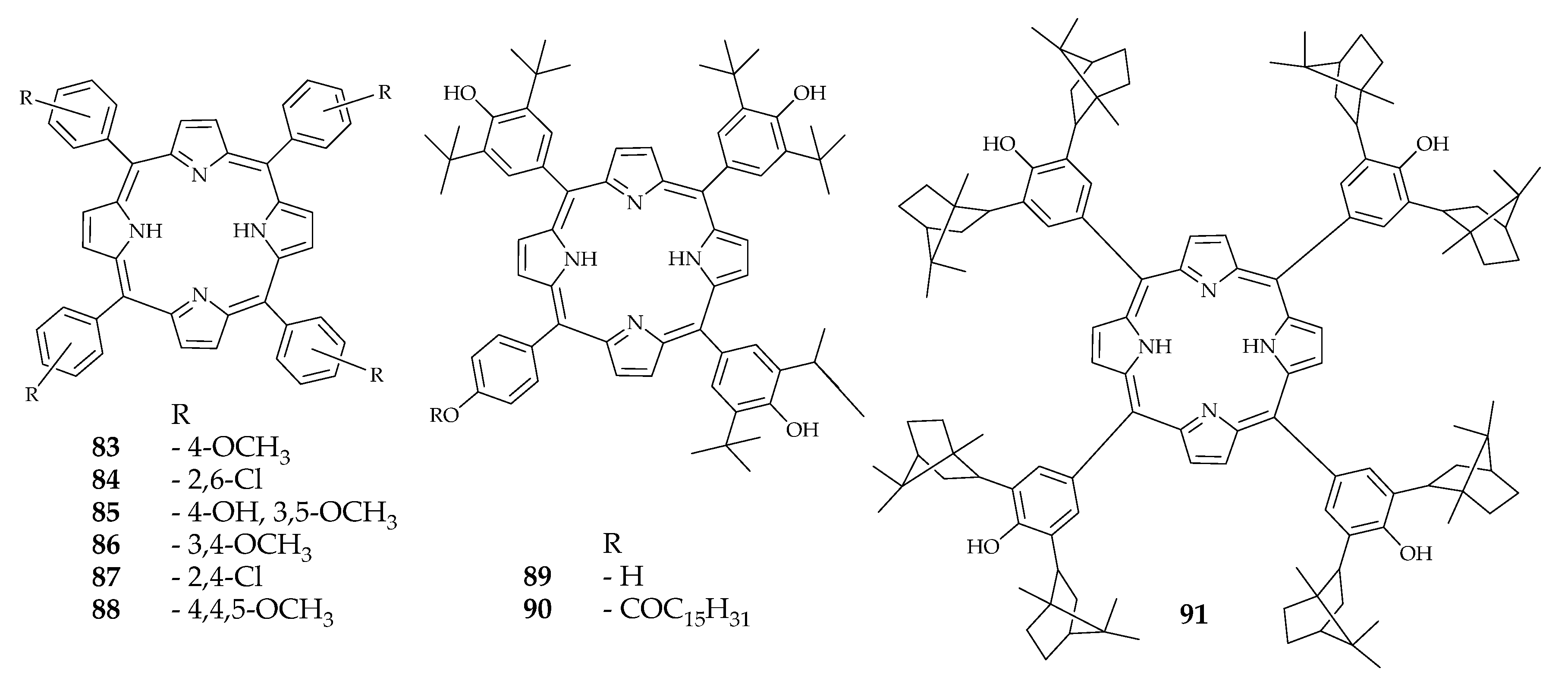
| 86 | 74.3 | - | - | [95] | |
| 88 | 79.5 | - | - | ||
| 90 | 77.36 | - | - | [96] | |
| 113 | 83.40 | - | - | [106] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-S.; Song, I.-h.; Shinde, P.B.; Nimse, S.B. Macrocycles and Supramolecules as Antioxidants: Excellent Scaffolds for Development of Potential Therapeutic Agents. Antioxidants 2020, 9, 859. https://doi.org/10.3390/antiox9090859
Lee J-S, Song I-h, Shinde PB, Nimse SB. Macrocycles and Supramolecules as Antioxidants: Excellent Scaffolds for Development of Potential Therapeutic Agents. Antioxidants. 2020; 9(9):859. https://doi.org/10.3390/antiox9090859
Chicago/Turabian StyleLee, Jung-Seop, In-ho Song, Pramod B. Shinde, and Satish Balasaheb Nimse. 2020. "Macrocycles and Supramolecules as Antioxidants: Excellent Scaffolds for Development of Potential Therapeutic Agents" Antioxidants 9, no. 9: 859. https://doi.org/10.3390/antiox9090859
APA StyleLee, J.-S., Song, I.-h., Shinde, P. B., & Nimse, S. B. (2020). Macrocycles and Supramolecules as Antioxidants: Excellent Scaffolds for Development of Potential Therapeutic Agents. Antioxidants, 9(9), 859. https://doi.org/10.3390/antiox9090859








