Regulatory Functions of L-Carnitine, Acetyl, and Propionyl L-Carnitine in a PCOS Mouse Model: Focus on Antioxidant/Antiglycative Molecular Pathways in the Ovarian Microenvironment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
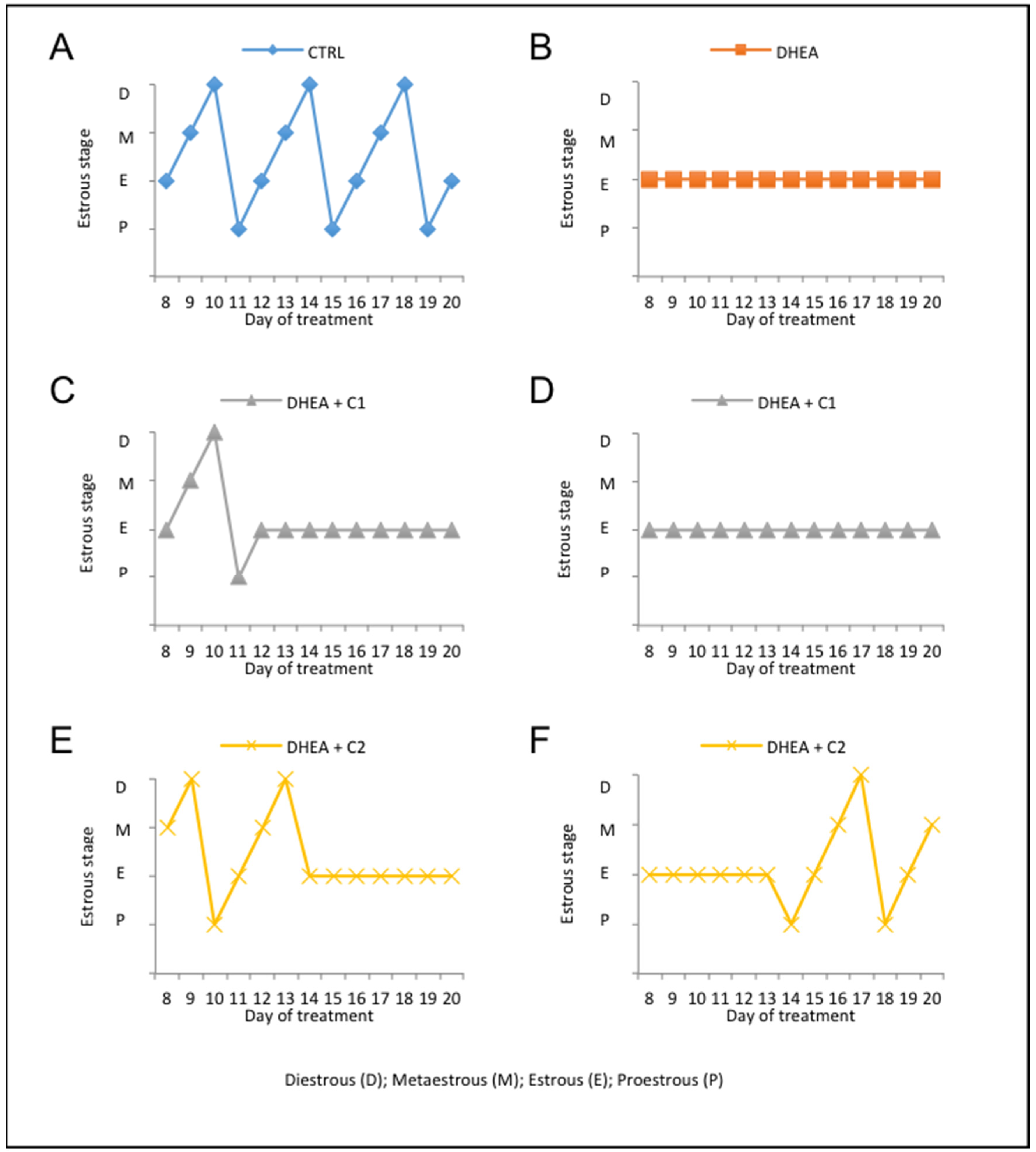
2.2. Estrous Cycle Determination
2.3. Hormone Assay
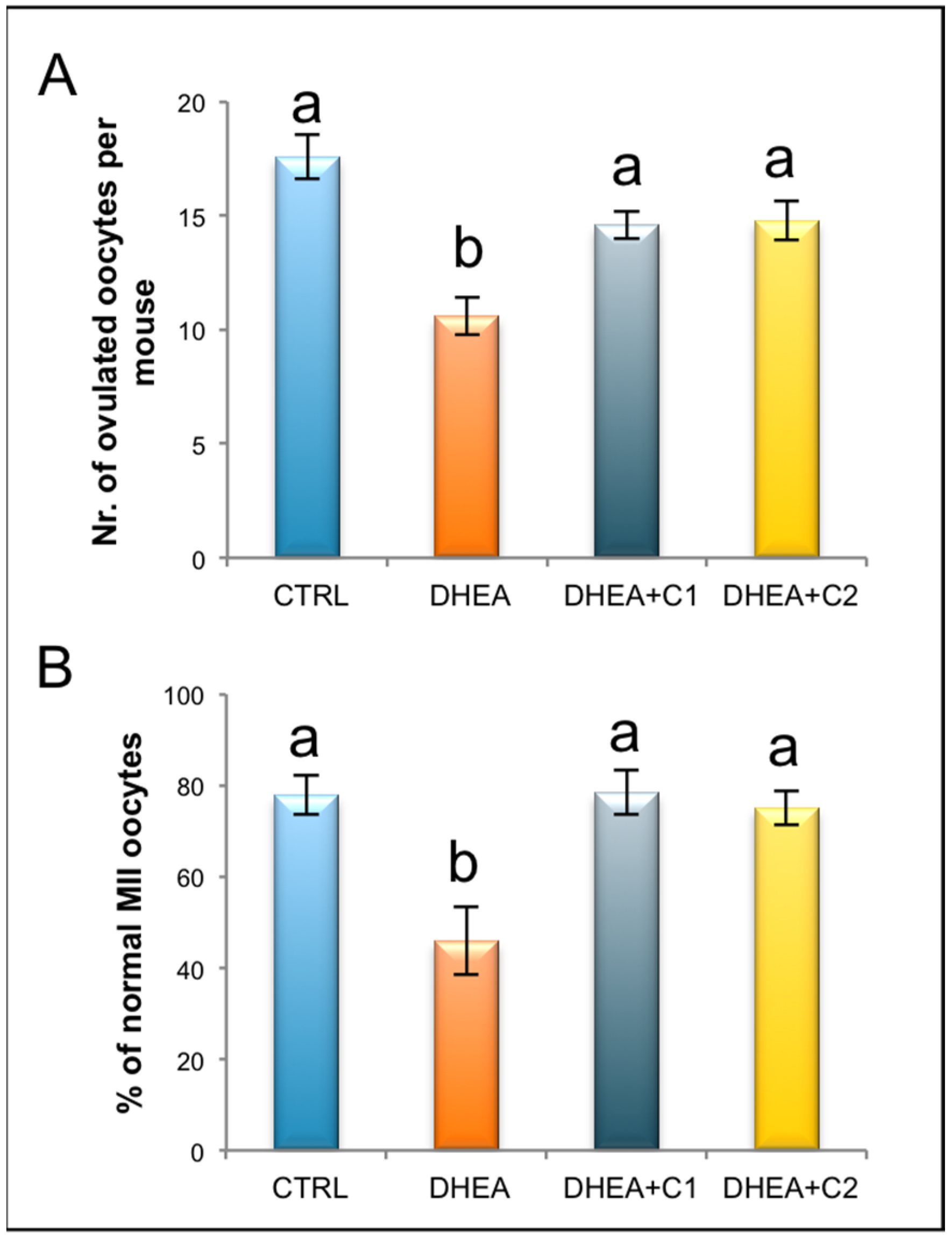
2.4. Superovulation Induction and Oocyte Collection
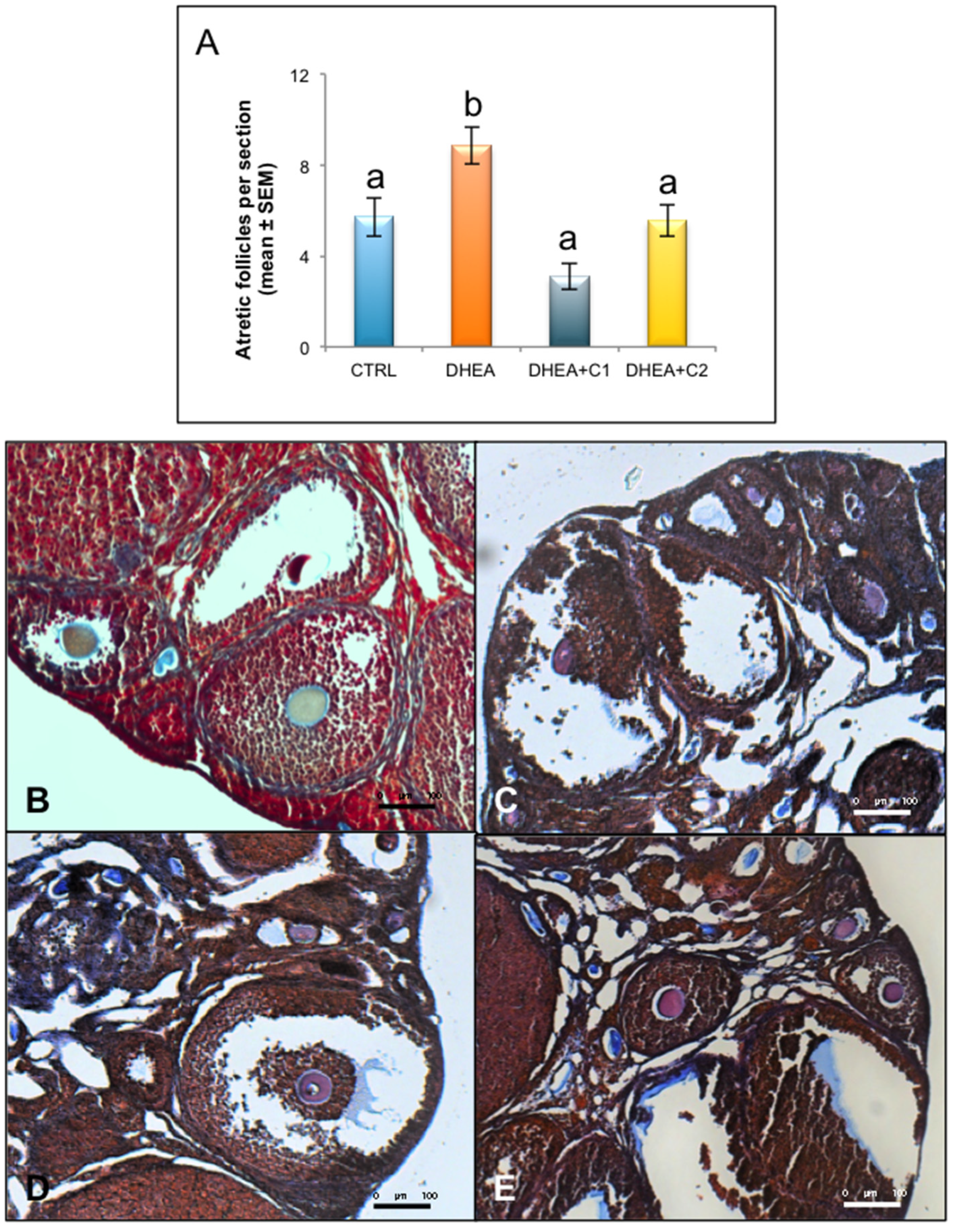
2.5. H&E Staining and Ovarian Follicle Classification and Counting
2.6. Heidenhain’s AZAN Trichrome Staining
2.7. Ovarian Analysis of Lipid Droplets
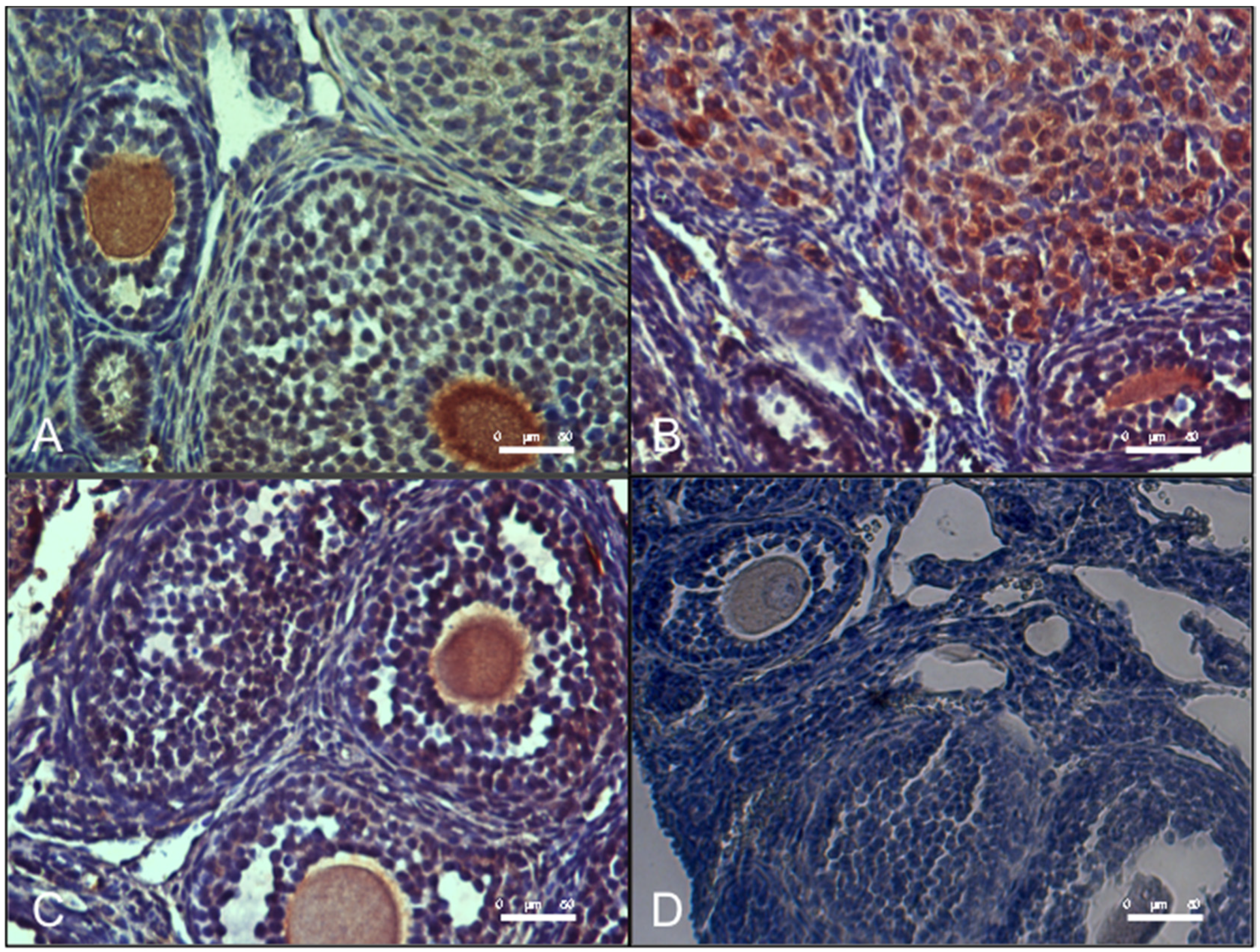
2.8. Ovarian Immunohistochemical Analysis
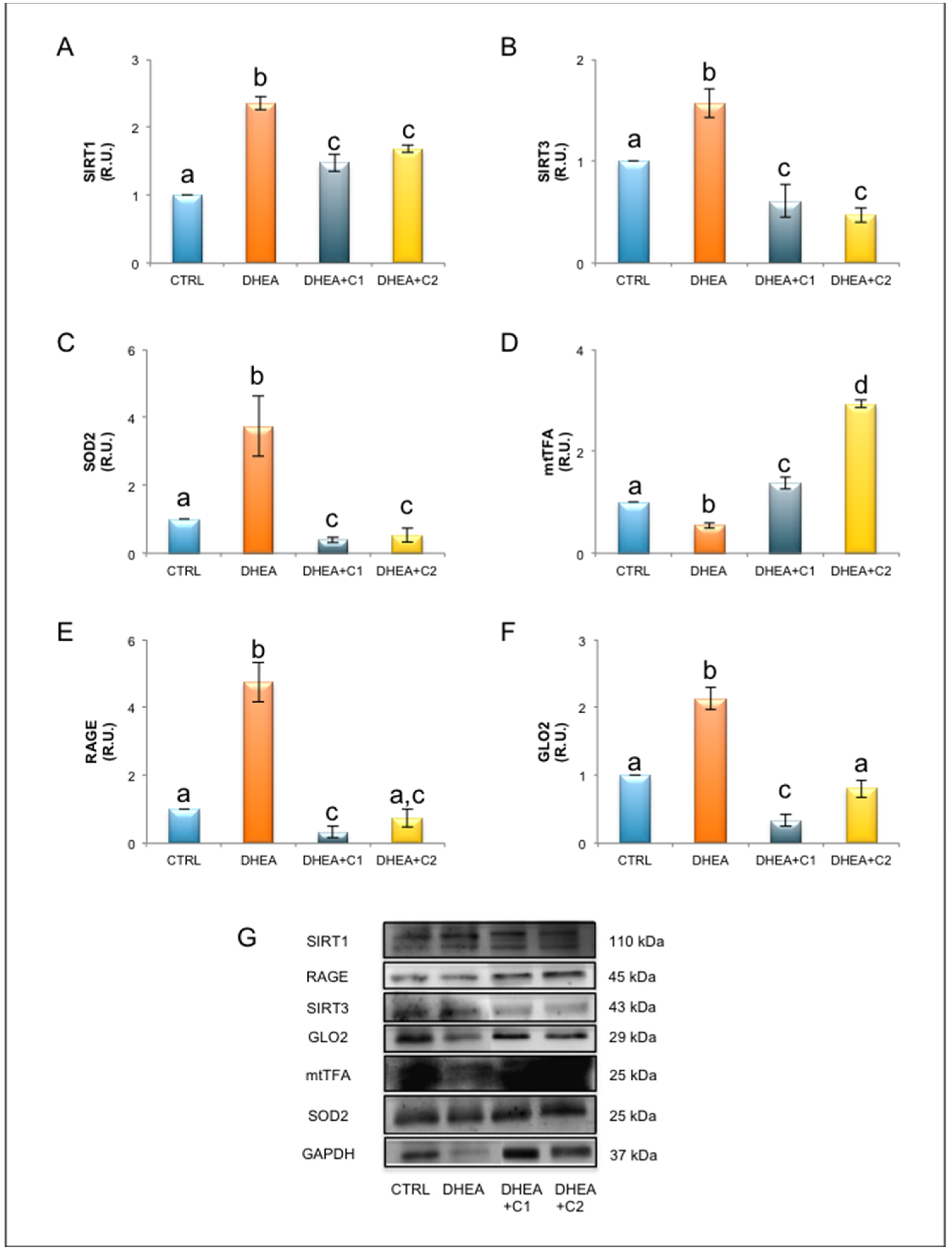
2.9. Western Blot Analysis
2.10. Analysis of DNA Distribution and Spindle Configuration of In Vivo Matured MII Oocytes
2.11. Statistical Analysis
3. Results
3.1. Administration of Carnitine Formulations Ameliorated PCOS Phenotype Induced by DHEA
3.2. Oral Administration of Carnitines Formulation 1 and 2 Modulates SIRT1 Functional Network and Mitochondrial Physiology
3.3. Oral Administration of Carnitine Formulation 2 Is Able to Prevent the Establishment of Glycative Stress of DHEA Mice, While Carnitine Formulation 1 Is Not
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trikudanathan, S. Polycystic ovarian syndrome. Med. Clin. N. Am. 2015, 99, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef]
- Sirmans, S.M.; Pate, K.A. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin. Epidemiol. 2013, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenkci, V.; Fenkci, S.; Yilmazer, M.; Serteser, M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil. Steril. 2003, 80, 123–127. [Google Scholar] [CrossRef]
- Papalou, O.; Victor, V.M.; Diamanti-Kandarakis, E. Oxidative Stress in Polycystic Ovary Syndrome. Curr. Pharm. Des. 2016, 22, 2709–2722. [Google Scholar] [CrossRef]
- Mohammadi, M. Oxidative Stress and Polycystic Ovary Syndrome: A Brief Review. Int. J. Prev. Med. 2019, 10, 86. [Google Scholar] [CrossRef]
- Di Emidio, G.; Placidi, M.; Rea, F.; Rossi, G.; Falone, S.; Cristiano, L.; Nottola, S.; D’Alessandro, A.M.; Amicarelli, F.; Palmerini, M.G.; et al. Methylglyoxal-Dependent Glycative Stress and Deregulation of SIRT1 Functional Network in the Ovary of PCOS Mice. Cells 2020, 9, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheraghi, E.; Mehranjani, M.S.; Shariatzadeh, M.A.; Esfahani, M.H.; Ebrahimi, Z. N-Acetylcysteine improves oocyte and embryo quality in polycystic ovary syndrome patients undergoing intracytoplasmic sperm injection: An alternative to metformin. Reprod. Fertil. Dev. 2016, 28, 723–731. [Google Scholar] [CrossRef]
- Ismail, A.M.; Hamed, A.H.; Saso, S.; Thabet, H.H. Adding L-carnitine to clomiphene resistant PCOS women improves the quality of ovulation and the pregnancy rate. A randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 180, 148–152. [Google Scholar] [CrossRef]
- Samimi, M.; Jamilian, M.; Ebrahimi, F.A.; Rahimi, M.; Tajbakhsh, B.; Asemi, Z. Oral carnitine supplementation reduces body weight and insulin resistance in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Endocrinol. 2016, 84, 851–857. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef]
- Kelek, S.E.; Afşar, E.; Akçay, G.; Danışman, B.; Aslan, M. Effect of chronic L-carnitine supplementation on carnitine levels, oxidative stress and apoptotic markers in peripheral organs of adult Wistar rats. Food Chem. Toxicol. 2019, 134, 110851. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sengupta, P.; Durairajanayagam, D. Role of L-carnitine in female infertility. Reprod. Biol. Endocrinol. 2018, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jia, H.; Liu, J.; Ao, N.; Yan, B.; Shen, W.; Wang, X.; Li, X.; Luo, C.; Liu, J. Combined R-alpha-lipoic acid and acetyl-L-carnitine exerts efficient preventative effects in a cellular model of Parkinson’s disease. J. Cell. Mol. Med. 2010, 14, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Shenk, J.C.; Liu, J.; Fischbach, K.; Xu, K.; Puchowicz, M.; Obrenovich, M.E.; Gasimov, E.; Alvarez, L.M.; Ames, B.N.; Lamanna, J.C.; et al. The effect of acetyl-Lcarnitine and R-alpha-lipoic acid treatment in ApoE4 mouse as a model of human Alzheimer’s disease. J. Neurol. Sci. 2009, 283, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggenenti, P.; Cattaneo, D.; Loriga, G.; Ledda, F.; Motterlini, N.; Gherardi, G.; Orisio, S.; Remuzzi, G. Ameliorating hypertension and insulin resistance in subjects at increased cardiovascular risk: Effects of acetyl-L-carnitine therapy. Hypertension 2009, 54, 567–574. [Google Scholar] [CrossRef]
- Wiseman, L.R.; Brogden, R.N. Propionyl-L-carnitine. Drugs Aging 1998, 12, 243–250. [Google Scholar] [CrossRef]
- Vanella, A.; Russo, A.; Acquaviva, R.; Campisi, A.; Di Giacomo, C.; Sorrenti, V.; Barcellona, M.L. L-propionyl-carnitine as superoxide scavenger, antioxidant, and DNA cleavage protector. Cell Biol. Toxicol. 2000, 16, 99–104. [Google Scholar] [CrossRef]
- Ceriello, A. New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care 2003, 26, 1589–1596. [Google Scholar] [CrossRef] [Green Version]
- Mingorance, C.; Rodriguez-Rodriguez, R.; Justo, M.L.; Herrera, M.D.; de Sotomayor, M.A. Pharmacological effects and clinical applications of propionyl-L-carnitine. Nutr. Rev. 2011, 69, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Ringseis, R.; Keller, J.; Eder, K. Mechanisms underlying the anti-wasting effect of L-carnitine supplementation under pathologic conditions: Evidence from experimental and clinical studies. Eur. J. Nutr. 2013, 52, 1421–1442. [Google Scholar] [CrossRef] [PubMed]
- Celik, F.; Kose, M.; Yilmazer, M.; Köken, G.N.; Arioz, D.T.; Kanat Pektas, M. Plasma L-carnitine levels of obese and non-obese polycystic ovary syndrome patients. J. Obstet. Gynaecol. 2017, 37, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, S.; Nazari, L.; Hoseini, S.; Moghaddam, P.B.; Gachkar, L. Effects of L-carnitine on Polycystic Ovary Syndrome. JBRA Assist. Reprod. 2019, 23, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Maleki, V.; Jafari-Vayghan, H.; Kashani, A.; Moradi, F.; Vajdi, M.; Kheirouri, S.; Alizadeh, M. Potential roles of carnitine in patients with polycystic ovary syndrome: A systematic review. Gynecol. Endocrinol. 2019, 35, 463–469. [Google Scholar] [CrossRef] [PubMed]
- El Sharkwy, I.; Sharaf El-Din, M. l-Carnitine plus metformin in clomiphene-resistant obese PCOS women, reproductive and metabolic effects: A randomized clinical trial. Gynecol. Endocrinol. 2019, 35, 701–705. [Google Scholar] [CrossRef]
- El Sharkwy, I.A.; Abd El Aziz, W.M. Randomized controlled trial of N-acetylcysteine versus l-carnitine among women with clomiphene-citrate-resistant polycystic ovary syndrome. Int. J. Gynaecol. Obstet. 2019, 147, 59–64. [Google Scholar] [CrossRef]
- Jamilian, M.; Foroozanfard, F.; Kavossian, E.; Kia, M.; Aghadavod, E.; Amirani, E.; Asemi, Z. Effects of Chromium and Carnitine Co-supplementation on Body Weight and Metabolic Profiles in Overweight and Obese Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2020, 193, 334–341. [Google Scholar] [CrossRef]
- Kalhori, Z.; Mehranjani, M.S.; Azadbakht, M.; Shariatzadeh, M.A. L-Carnitine improves endocrine function and folliculogenesis by reducing inflammation, oxidative stress and apoptosis in mice following induction of polycystic ovary syndrome. Reprod. Fertil. Dev. 2019, 31, 282–293. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Prati, A.; Genazzani, A.R.; Battipaglia, C.; Simoncini, T.; Szeliga, A.; Podfigurna, A.; Meczekalski, B. Synergistic effects of the integrative administration of acetyl-L-carnitine, L-carnitine, L-arginine and N-acetyl-cysteine on metabolic dynamics and on hepatic insulin extraction in overweight/obese patients with PCOS. Gynecol. Reprod. Endocrinol. Metab. 2020, 1, 56–63. [Google Scholar]
- Di Emidio, G.; Santini, S.J.; D’Alessandro, A.M.; Vetuschi, A.; Sferra, R.; Artini, P.G.; Carta, G.; Falone, S.; Amicarelli, F.; Tatone, C. SIRT1 participates in the response to methylglyoxal-dependent glycative stress in mouse oocytes and ovary. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1389–1401. [Google Scholar] [CrossRef]
- Gougeon, A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr. Rev. 1996, 17, 121–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Keefe, D.L. Ageing-associated aberration in meiosis of oocytes from senescence-accelerated mice. Hum. Reprod. 2002, 17, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Falone, S.; Santini, S., Jr.; di Loreto, S.; Cordone, V.; Grannonico, M.; Cesare, P.; Cacchio, M.; Amicarelli, F. Improved Mitochondrial and Methylglyoxal-Related Metabolisms Support Hyperproliferation Induced by 50 Hz Magnetic Field in Neuroblastoma Cells. J. Cell. Physiol. 2016, 231, 2014–2025. [Google Scholar] [CrossRef] [PubMed]
- Virmani, M.A.; Zerelli, S.; Vitullo, P.; Cossetti, C. Effect of nutrients on ovulation and oocytes quality in mice. In Proceedings of the 11th Congress of the European Society of Gynecology, Prague, Czech Republic, 21–24 October 2015. [Google Scholar]
- Busetto, G.M.; Agarwal, A.; Virmani, A.; Antonini, G.; Ragonesi, G.; Del Giudice, F.; Micic, S.; Gentile, V.; De Berardinis, E. Effect of metabolic and antioxidant supplementation on sperm parameters in oligo-astheno-teratozoospermia, with and without varicocele: A double-blind placebo-controlled study. Andrologia 2018, 50. [Google Scholar] [CrossRef] [Green Version]
- Kitano, Y.; Hashimoto, S.; Matsumoto, H.; Yamochi, T.; Yamanaka, M.; Nakaoka, Y.; Fukuda, A.; Inoue, M.; Ikeda, T.; Morimoto, Y. Oral administration of l-carnitine improves the clinical outcome of fertility in patients with IVF treatment. Gynecol. Endocr. 2018, 34, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Micic, S.; Lalic, N.; Djordjevic, D.; Bojanic, N.; Bogavac-Stanojevic, N.; Busetto, G.M.; Virmani, A.; Agarwal, A. Double-blind, randomised, placebo-controlled trial on the effect of L-carnitine and L-acetylcarnitine on sperm parameters in men with idiopathic oligoasthenozoospermia. Andrologia 2019, 51, e13267. [Google Scholar] [CrossRef] [PubMed]
- Delaney, C.L.; Spark, J.I.; Thomas, J.; Wong, Y.T.; Chan, L.T.; Miller, M.D. A systematic review to evaluate the effectiveness of carnitine supplementation in improving walking performance among individuals with intermittent claudication. Atherosclerosis 2013, 229, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, H.; Jamilian, M.; Samimi, M.; Afshar Ebrahimi, F.; Rahimi, M.; Bahmani, F.; Aghababayan, S.; Kouhi, M.; Shahabbaspour, S.; Asemi, Z. Oral carnitine supplementation influences mental health parameters and biomarkers of oxidative stress in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Gynecol. Endocrinol. 2017, 33, 442–447. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Lanzoni, C.; Ricchieri, F.; Santagni, S.; Rattighieri, E.; Chierchia, E.; Monteleone, P.; Jasonni, V.M. Acetyl-L-carnitine (ALC) administration positively affects reproductive axis in hypogonadotropic women with functional hypothalamic amenorrhea. J. Endocrinol. Investig. 2011, 34, 287–291. [Google Scholar] [CrossRef]
- Dunning, K.R.; Cashman, K.; Russell, D.L.; Thompson, J.G.; Norman, R.J.; Robker, R.L. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol. Reprod. 2010, 83, 909–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilding, M. Supply and Demand of Energy in the Oocyte and the Role of Mitochondria. Results Probl. Cell. Differ. 2017, 63, 373–387. [Google Scholar] [CrossRef]
- Dunning, K.R.; Akison, L.K.; Russell, D.L.; Norman, R.J.; Robker, R.L. Increased beta-oxidation and improved oocyte developmental competence in response to l-carnitine during ovarian in vitro follicle development in mice. Biol. Reprod. 2011, 85, 548–555. [Google Scholar] [CrossRef]
- Zare, Z.; Abouhamzeh, B.; Masteri Farahani, R.; Salehi, M.; Mohammadi, M. Supplementation of L-carnitine during in vitro maturation of mouse oocytes affects expression of genes involved in oocyte and embryo competence: An experimental study. Int. J. Reprod. Biomed. 2017, 15, 779–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentile, L.; Monti, M.; Sebastiano, V.; Merico, V.; Nicolai, R.; Calvani, M.; Garagna, S.; Redi, C.A.; Zuccotti, M. Single-cell quantitative RT-PCR analysis of Cpt1b and Cpt2 gene expression in mouse antral oocytes and in preimplantation embryos. Cytogenet. Genome Res. 2004, 105, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.T.; Kolesar, J.E.; Kaufman, B.A. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim. Biophys. Acta 2012, 1819, 921–929. [Google Scholar] [CrossRef]
- Kienhöfer, J.; Häussler, D.J.; Ruckelshausen, F.; Muessig, E.; Weber, K.; Pimentel, D.; Ullrich, V.; Bürkle, A.; Bachschmid, M.M. Association of mitochondrial antioxidant enzymes with mitochondrial DNA as integral nucleoid constituents. FASEB J. 2009, 23, 2034–2044. [Google Scholar] [CrossRef] [Green Version]
- Koh, J.H.; Johnson, M.L.; Dasari, S.; LeBrasseur, N.K.; Vuckovic, I.; Henderson, G.C.; Cooper, S.A.; Manjunatha, S.; Ruegsegger, G.N.; Shulman, G.I.; et al. TFAM Enhances Fat Oxidation and Attenuates High-Fat Diet-Induced Insulin Resistance in Skeletal Muscle [published correction appears in Diabetes. 2020 Aug;69(8):1854]. Diabetes 2019, 68, 1552–1564. [Google Scholar] [CrossRef]
- Mingorance, C.; Duluc, L.; Chalopin, M.; Simard, G.; Ducluzeau, P.H.; Herrera, M.D.; Alvarez de Sotomayor, M.; Andriantsitohaina, R. Propionyl-L-carnitine corrects metabolic and cardiovascular alterations in diet-induced obese mice and improves liver respiratory chain activity. PLoS ONE 2012, 7, e34268. [Google Scholar] [CrossRef] [Green Version]
- Tatone, C.; Eichenlaub-Ritter, U.; Amicarelli, F. Dicarbonyl stress and glyoxalases in ovarian function. Biochem. Soc. Trans. 2014, 42, 433–438. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Piperi, C.; Patsouris, E.; Korkolopoulou, P.; Panidis, D.; Pawelczyk, L.; Papavassiliou, A.G.; Duleba, A.J. Immunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovaries. Histochem. Cell Biol. 2007, 127, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Melnikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatone, C.; Di Emidio, G.; Barbonetti, A.; Carta, G.; Luciano, A.M.; Falone, S.; Amicarelli, F. Sirtuins in gamete biology and reproductive physiology: Emerging roles and therapeutic potential in female and male infertility. Hum. Reprod. Update 2018, 24, 267–289. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Emidio, G.; Rea, F.; Placidi, M.; Rossi, G.; Cocciolone, D.; Virmani, A.; Macchiarelli, G.; Palmerini, M.G.; D’Alessandro, A.M.; Artini, P.G.; et al. Regulatory Functions of L-Carnitine, Acetyl, and Propionyl L-Carnitine in a PCOS Mouse Model: Focus on Antioxidant/Antiglycative Molecular Pathways in the Ovarian Microenvironment. Antioxidants 2020, 9, 867. https://doi.org/10.3390/antiox9090867
Di Emidio G, Rea F, Placidi M, Rossi G, Cocciolone D, Virmani A, Macchiarelli G, Palmerini MG, D’Alessandro AM, Artini PG, et al. Regulatory Functions of L-Carnitine, Acetyl, and Propionyl L-Carnitine in a PCOS Mouse Model: Focus on Antioxidant/Antiglycative Molecular Pathways in the Ovarian Microenvironment. Antioxidants. 2020; 9(9):867. https://doi.org/10.3390/antiox9090867
Chicago/Turabian StyleDi Emidio, Giovanna, Francesco Rea, Martina Placidi, Giulia Rossi, Domenica Cocciolone, Ashraf Virmani, Guido Macchiarelli, Maria Grazia Palmerini, Anna Maria D’Alessandro, Paolo Giovanni Artini, and et al. 2020. "Regulatory Functions of L-Carnitine, Acetyl, and Propionyl L-Carnitine in a PCOS Mouse Model: Focus on Antioxidant/Antiglycative Molecular Pathways in the Ovarian Microenvironment" Antioxidants 9, no. 9: 867. https://doi.org/10.3390/antiox9090867
APA StyleDi Emidio, G., Rea, F., Placidi, M., Rossi, G., Cocciolone, D., Virmani, A., Macchiarelli, G., Palmerini, M. G., D’Alessandro, A. M., Artini, P. G., & Tatone, C. (2020). Regulatory Functions of L-Carnitine, Acetyl, and Propionyl L-Carnitine in a PCOS Mouse Model: Focus on Antioxidant/Antiglycative Molecular Pathways in the Ovarian Microenvironment. Antioxidants, 9(9), 867. https://doi.org/10.3390/antiox9090867







