Combined Treatment with Low Cytotoxic Ethyl Acetate Nepenthes Extract and Ultraviolet-C Improves Antiproliferation to Oral Cancer Cells via Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crude Extracts and Chemical
2.2. Cell Cultures and Proliferation Determination
2.3. UVC Irradiation and EANA Treatment
2.4. Cell Cycle Analysis
2.5. Apoptosis
2.6. Intracellular ROS
2.7. Mitochondrial Superoxide (MitoSOX)
2.8. Mitochondrial Membrane Potential (MitoMP)
2.9. Antioxidant Gene Expressions
2.10. γH2AX
2.11. 8-Hydroxy-2′-Deoxyguanosine (8-OxodG)
2.12. Statistics
3. Results
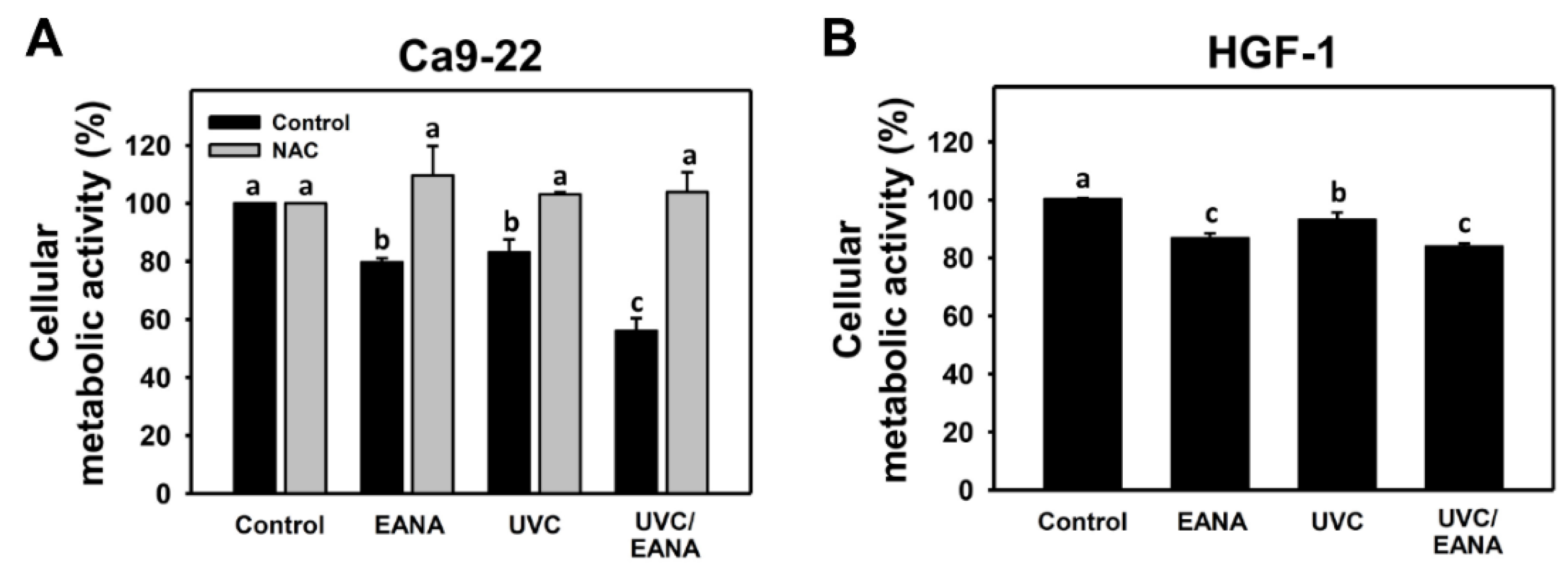
3.1. UVC Sensitizing Responses of EANA in Oral Cancer Cells
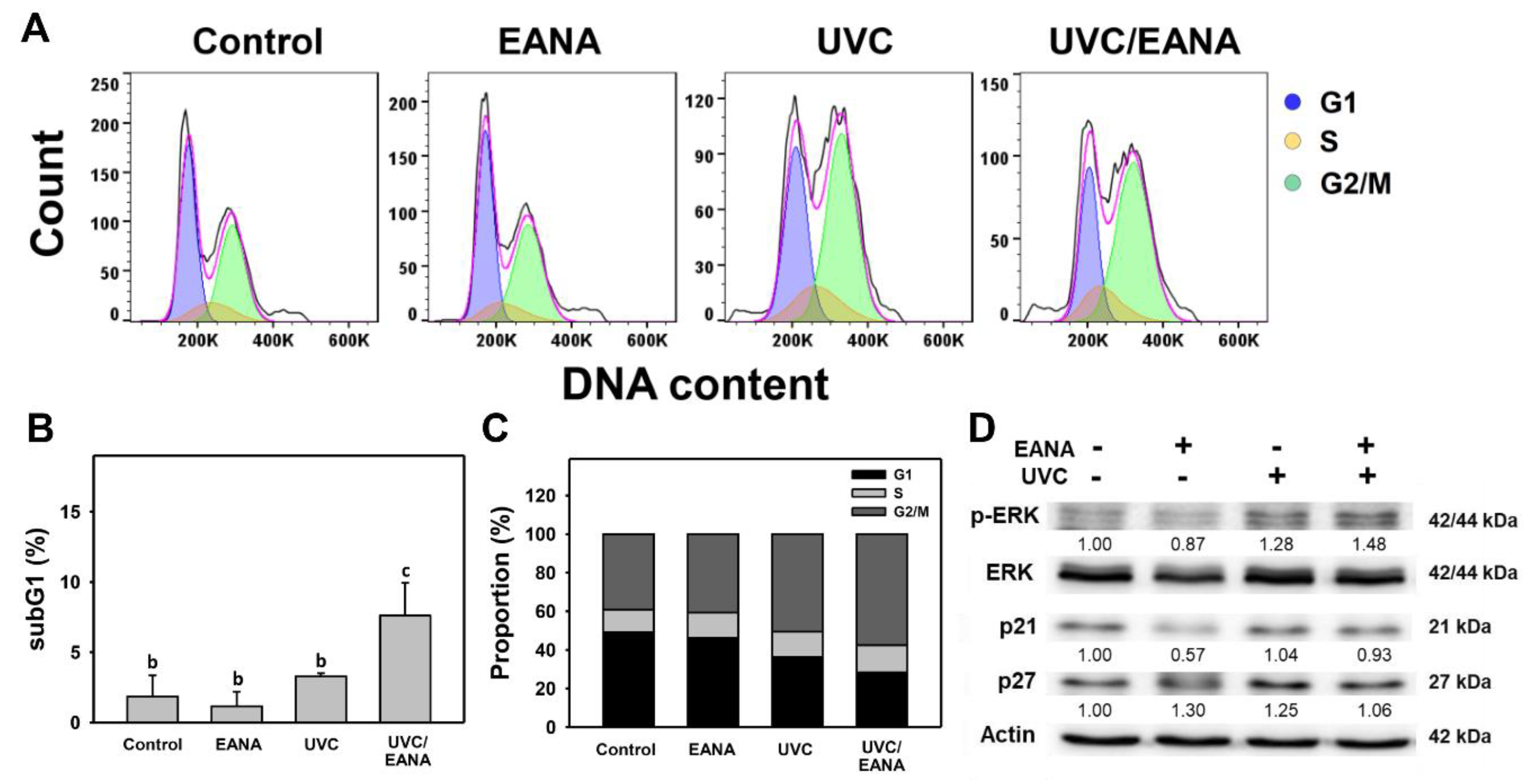
3.2. Cell Cycle Analysis of UVC and/or EANA Treatments in Oral Cancer Cells
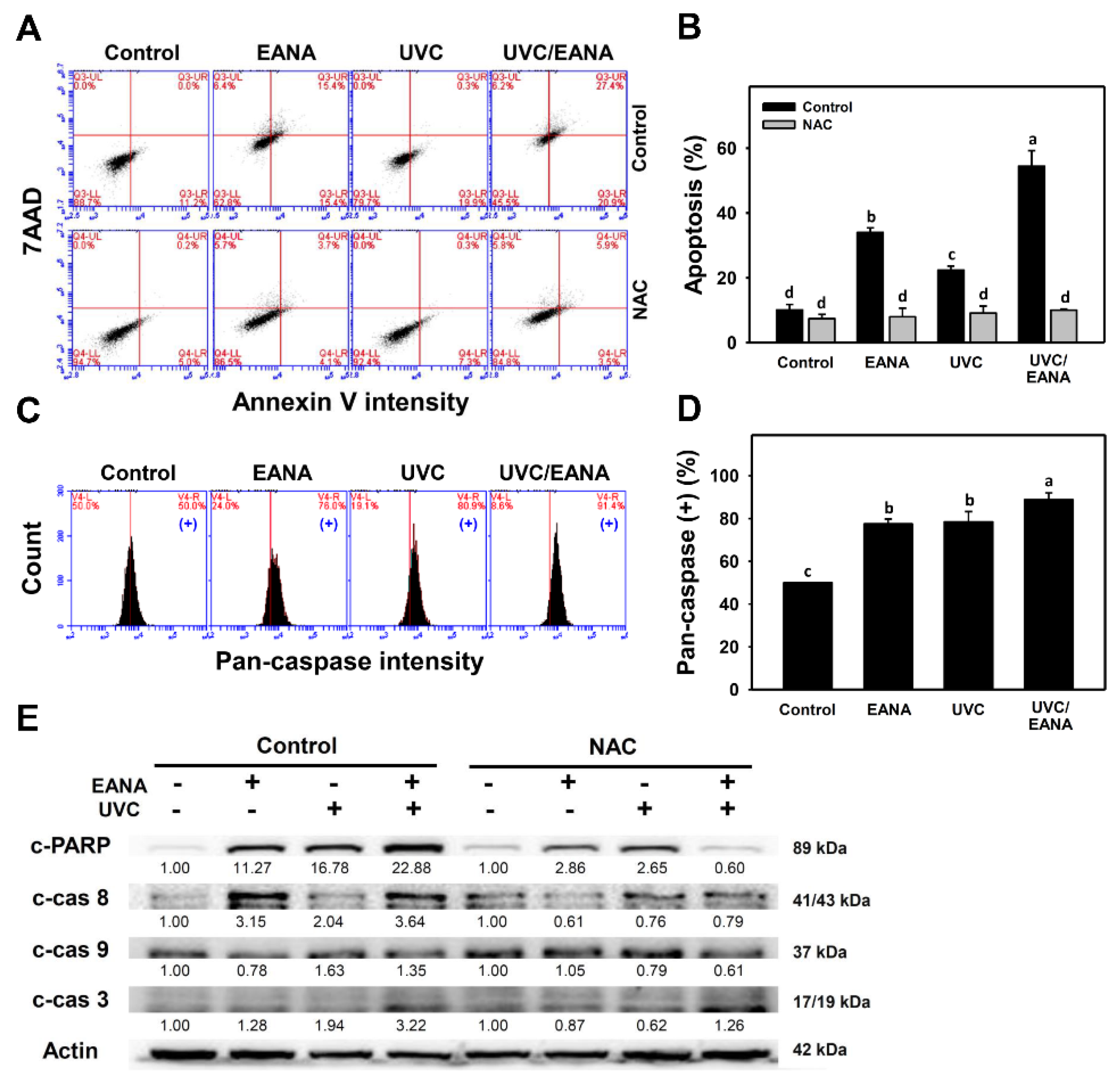
3.3. Annexin V/7AAD Changes of UVC and/or EANA Treatments in Oral Cancer Cells
3.4. Caspase Activation of UVC and/or EANA Treatments in Oral Cancer Cells
3.5. ROS Changes of UVC and/or EANA Treatments in Oral Cancer Cells
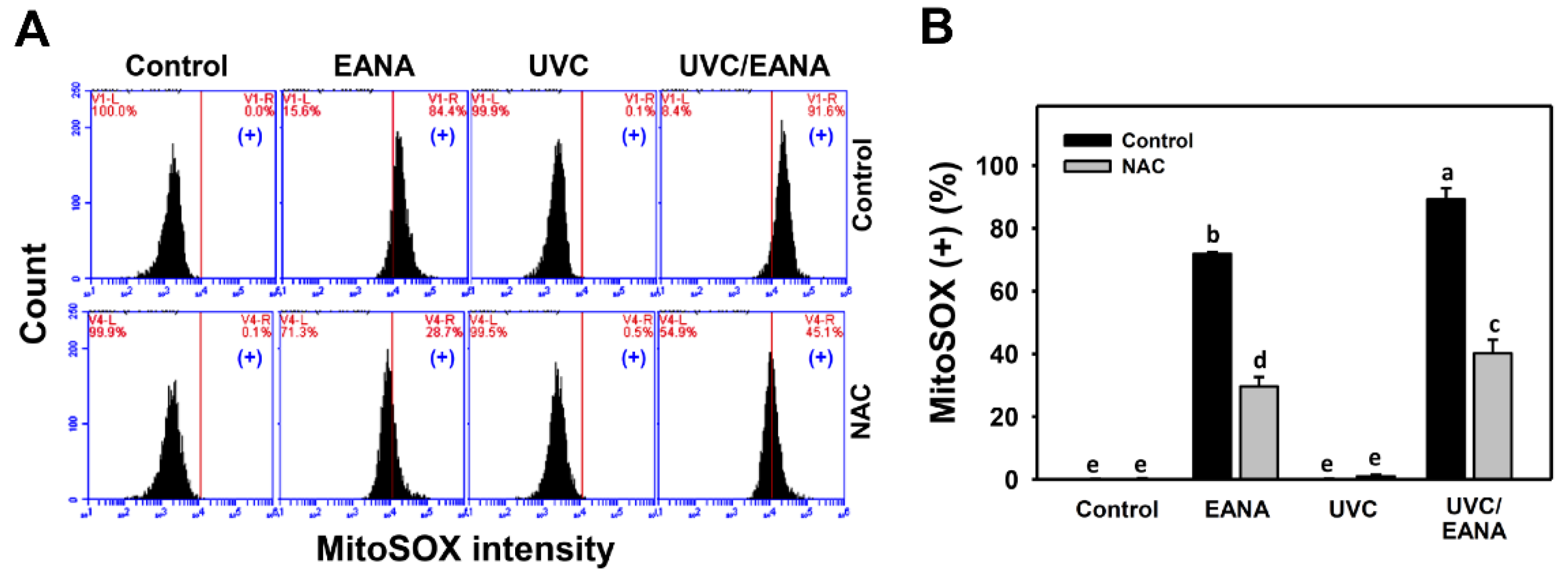
3.6. MitoSOX Changes of UVC and/or EANA Treatments in Oral Cancer Cells
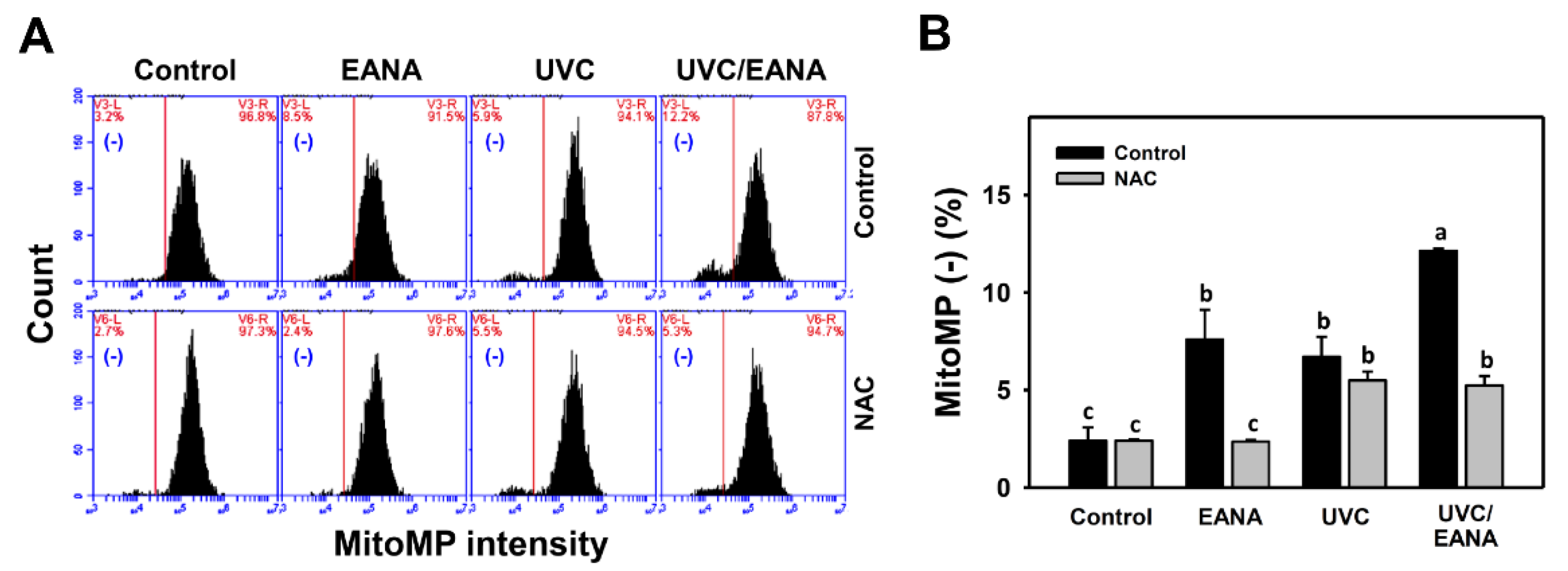
3.7. MitoMP Changes of UVC and/or EANA Treatments in Oral Cancer Cells
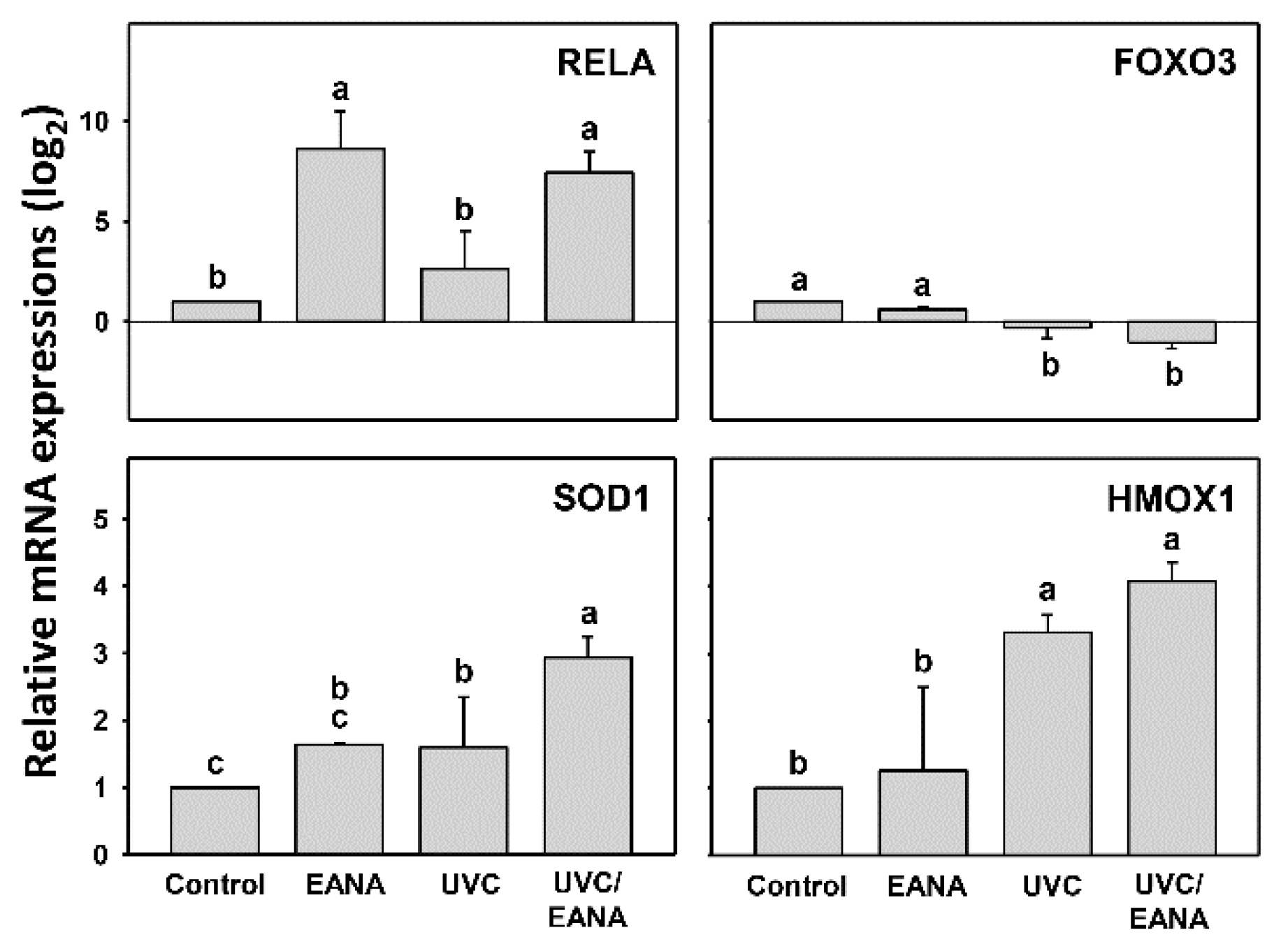
3.8. Antioxidant Gene Expressions of UVC and/or EANA Treatments in Oral Cancer Cells
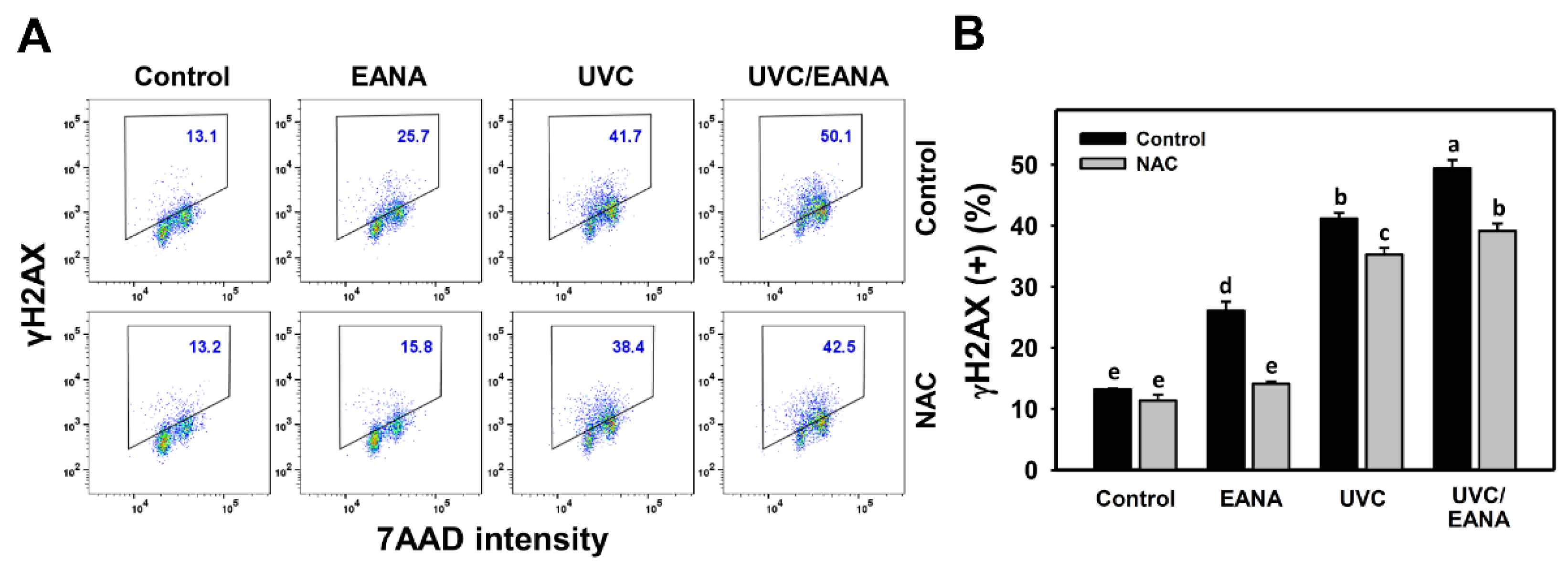
3.9. γH2AX Changes of UVC and/or EANA Treatments in Oral Cancer Cells
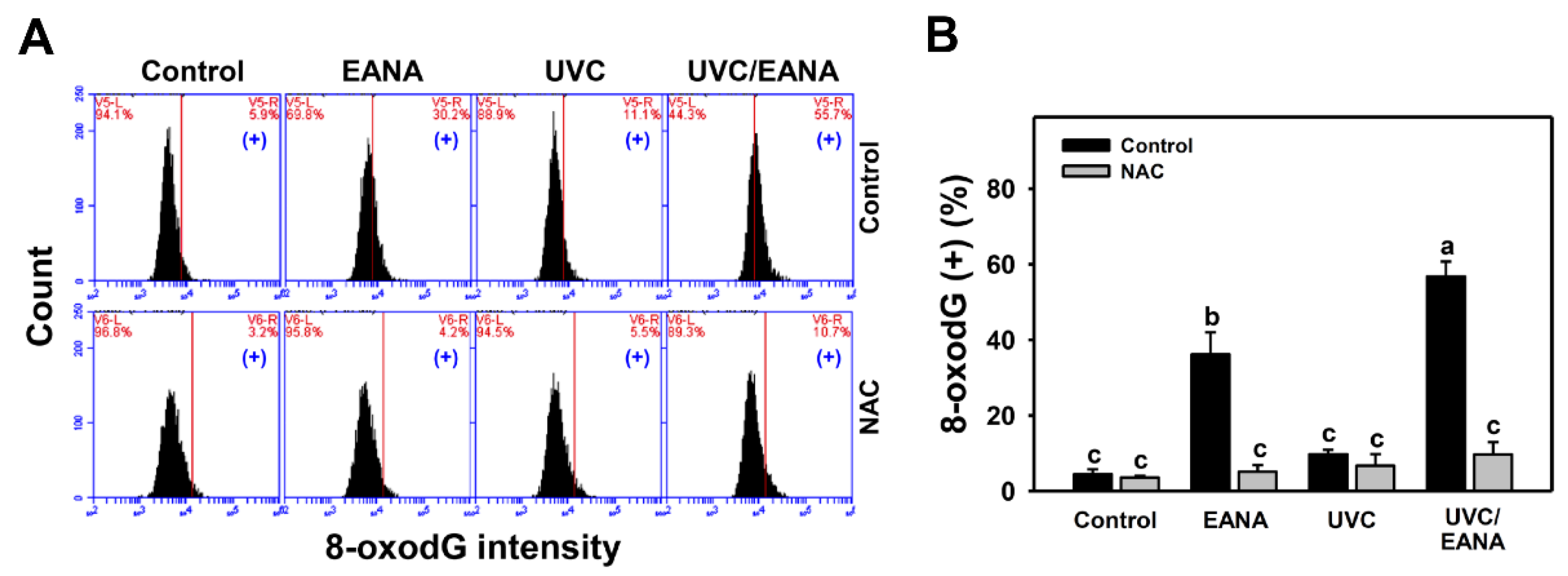
3.10. 8-oxodG Changes of UVC and/or EANA Treatments in Oral Cancer Cells
4. Discussion
4.1. Radiosensitizers with Low Side Effects Are Helpful in Cancer Therapy
4.2. Oxidative Stresses Are Synergistically Increased in the Combined Treatment (UVC/EANA) of Oral Cancer Cells
4.3. DNA Damages Are Synergistically Increased in the Combined Treatment (UVC/EANA) of Oral Cancer Cells
4.4. Apoptosis Is Synergistically Increased in the Combined Treatment (UVC/EANA) of Oral Cancer Cells
4.5. Cell Cycle Arresting at G2/M Is Synergistically Increased in the Combined Treatment (UVC/EANA) of Oral Cancer Cells
4.6. All Separate and Synergistic Changes in UVC and/or EANA of Oral Cancer Cells Are Oxidative Stress Dependent
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.J.; Richardson, R.A.; Dewhirst, M.W. Hypoxia and radiotherapy: Opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev. 2007, 26, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ruan, S.; Jia, F.; Chu, J.; Zhu, Y.; Huang, Y.; Liu, G. In vitro and in vivo superior radiosensitizing effect of berbamine for head and neck squamous cell carcinoma. Onco Targets Ther. 2018, 11, 8117–8125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotowski, U.; Heiduschka, G.; Brunner, M.; Czembirek, C.; Eder-Czembirek, C.; Schmidt, R.; Fahim, T.; Thurnher, D. Radiosensitization of head and neck cancer cells by the phytochemical agent sulforaphane. Strahlenther Onkol. 2011, 187, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Ferdous, T.; Ueyama, Y. Gimeracil exerts radiosensitizing effects on oral squamous cell carcinoma cells in vitro and in vivo. Anticancer Res. 2016, 36, 5923–5930. [Google Scholar] [CrossRef]
- Yamauchi, T.; Adachi, S.; Yasuda, I.; Nakashima, M.; Kawaguchi, J.; Yoshioka, T.; Hirose, Y.; Kozawa, O.; Moriwaki, H. Ultra-violet irradiation induces apoptosis via mitochondrial pathway in pancreatic cancer cells. Int. J. Oncol. 2011, 39, 1375–1380. [Google Scholar]
- Yamauchi, T.; Adachi, S.; Yasuda, I.; Nakashima, M.; Kawaguchi, J.; Nishii, Y.; Yoshioka, T.; Okano, Y.; Hirose, Y.; Kozawa, O.; et al. UVC radiation induces downregulation of EGF receptor via phosphorylation at serine 1046/1047 in human pancreatic cancer cells. Radiat. Res. 2011, 176, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Adachi, S.; Yasuda, I.; Nakashima, M.; Yamauchi, T.; Kawaguchi, J.; Shimizu, M.; Itani, M.; Nakamura, M.; Nishii, Y.; Yoshioka, T.; et al. Ultraviolet irradiation can induce evasion of colon cancer cells from stimulation of epidermal growth factor. J. Biol. Chem. 2011, 286, 26178–26187. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, J.; Adachi, S.; Yasuda, I.; Yamauchi, T.; Nakashima, M.; Ohno, T.; Shimizu, M.; Yoshioka, T.; Itani, M.; Kozawa, O.; et al. Cisplatin and ultra-violet-C synergistically down-regulate receptor tyrosine kinases in human colorectal cancer cells. Mol. Cancer 2012, 11, 45. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.W.; Tang, J.Y.; Yen, C.Y.; Chang, H.S.; Huang, H.W.; Chung, Y.A.; Chen, I.S.; Huang, M.Y. Synergistic anti-oral cancer effects of UVC and methanolic extracts of Cryptocarya concinna roots via apoptosis, oxidative stress and DNA damage. Int. J. Radiat. Biol. 2016, 92, 263–272. [Google Scholar] [CrossRef]
- Chi, V.V. Dictionary of Vietnamese Medicinal Plants; Publishing House Medicine: Ha Noi, Vietnam, 2012; Volume 2. [Google Scholar]
- Buch, F.; Pauchet, Y.; Rott, M.; Mithofer, A. Characterization and heterologous expression of a PR-1 protein from traps of the carnivorous plant Nepenthes mirabilis. Phytochemistry 2014, 100, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Gwee, P.S.; Khoo, K.S.; Ong, H.C.; Sit, N.W. Bioactivity-guided isolation and structural characterization of the antifungal compound, plumbagin, from Nepenthes gracilis. Pharm. Biol. 2014, 52, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Likhitwitayawuid, K.; Kaewamatawong, R.; Ruangrungsi, N.; Krungkrai, J. Antimalarial naphthoquinones from Nepenthes thorelii. Planta Med. 1998, 64, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Luyen, B.T.; Koo, J.E.; Kim, S.; Koh, Y.S.; Thanh, N.V.; Cuong, N.X.; Kiem, P.V.; Minh, C.V.; Kim, Y.H. In vitro anti-inflammatory components isolated from the carnivorous plant Nepenthes mirabilis (Lour.) Rafarin. Pharm. Biol. 2016, 54, 588–594. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.Y.; Peng, S.Y.; Cheng, Y.B.; Wang, C.L.; Farooqi, A.A.; Yu, T.J.; Hou, M.F.; Wang, S.C.; Yen, C.H.; Chan, L.P.; et al. Ethyl acetate extract of Nepenthes adrianii x clipeata induces antiproliferation, apoptosis, and DNA damage against oral cancer cells through oxidative stress. Environ. Toxicol. 2019, 34, 891–901. [Google Scholar] [CrossRef]
- Huang, C.H.; Yeh, J.M.; Chan, W.H. Hazardous impacts of silver nanoparticles on mouse oocyte maturation and fertilization and fetal development through induction of apoptotic processes. Environ. Toxicol. 2018, 33, 1039–1049. [Google Scholar] [CrossRef]
- Hung, J.H.; Chen, C.Y.; Omar, H.A.; Huang, K.Y.; Tsao, C.C.; Chiu, C.C.; Chen, Y.L.; Chen, P.H.; Teng, Y.N. Reactive oxygen species mediate Terbufos-induced apoptosis in mouse testicular cell lines via the modulation of cell cycle and pro-apoptotic proteins. Environ. Toxicol. 2016, 31, 1888–1898. [Google Scholar] [CrossRef]
- Wang, T.S.; Lin, C.P.; Chen, Y.P.; Chao, M.R.; Li, C.C.; Liu, K.L. CYP450-mediated mitochondrial ROS production involved in arecoline N-oxide-induced oxidative damage in liver cell lines. Environ. Toxicol. 2018, 33, 1029–1038. [Google Scholar] [CrossRef]
- Rodriguez, L.R.; Bui, S.N.; Beuschel, R.T.; Ellis, E.; Liberti, E.M.; Chhina, M.K.; Cannon, B.; Lemma, M.; Nathan, S.D.; Grant, G.M. Curcumin induced oxidative stress attenuation by N-acetylcysteine co-treatment: A fibroblast and epithelial cell in-vitro study in idiopathic pulmonary fibrosis. Mol. Med. 2019, 25, 27. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.C.; Haung, J.W.; Chang, F.R.; Huang, K.J.; Huang, H.M.; Huang, H.W.; Chou, C.K.; Wu, Y.C.; Chang, H.W. Golden berry-derived 4beta-hydroxywithanolide E for selectively killing oral cancer cells by generating ROS, DNA damage, and apoptotic pathways. PLoS ONE 2013, 8, e64739. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.C.; Tseng, C.N.; Yang, J.I.; Huang, H.W.; Fang, Y.; Tang, J.Y.; Chang, F.R.; Chang, H.W. Antiproliferation and induction of apoptosis in Ca9-22 oral cancer cells by ethanolic extract of Gracilaria tenuistipitata. Molecules 2012, 17, 10916–10927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignon, C.; Debeissat, C.; Georget, M.T.; Bouscary, D.; Gyan, E.; Rosset, P.; Herault, O. Flow cytometric quantification of all phases of the cell cycle and apoptosis in a two-color fluorescence plot. PLoS ONE 2013, 8, e68425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.Y.; Xu, Y.H.; Lin, L.C.; Ou-Yang, F.; Wu, K.H.; Tsao, L.Y.; Yu, T.J.; Huang, H.W.; Wang, H.R.; Liu, W.; et al. LY303511 displays antiproliferation potential against oral cancer cells in vitro and in vivo. Environ. Toxicol. 2019, 34, 958–967. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yen, C.Y.; Wang, H.R.; Yang, H.P.; Tang, J.Y.; Huang, H.W.; Hsu, S.H.; Chang, H.W. Tenuifolide B from Cinnamomum tenuifolium stem selectively inhibits proliferation of oral cancer cells via apoptosis, ROS generation, mitochondrial depolarization, and DNA damage. Toxins 2016, 8, 319. [Google Scholar] [CrossRef]
- Chang, H.W.; Li, R.N.; Wang, H.R.; Liu, J.R.; Tang, J.Y.; Huang, H.W.; Chan, Y.H.; Yen, C.Y. Withaferin A induces oxidative stress-mediated apoptosis and DNA damage in oral cancer cells. Front. Physiol. 2017, 8, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.T.; Huang, C.Y.; Tang, J.Y.; Liaw, C.C.; Li, R.N.; Liu, J.R.; Sheu, J.H.; Chang, H.W. Reactive oxygen species mediate soft corals-derived sinuleptolide-induced antiproliferation and DNA damage in oral cancer cells. Onco Targets Ther. 2017, 10, 3289–3297. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.W.; Tang, J.Y.; Ou-Yang, F.; Wang, H.R.; Guan, P.Y.; Huang, C.Y.; Chen, C.Y.; Hou, M.F.; Sheu, J.H.; Chang, H.W. Sinularin selectively kills breast cancer cells showing G2/M arrest, apoptosis, and oxidative DNA damage. Molecules 2018, 23, 849. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.Y.; Wu, C.Y.; Shu, C.W.; Wang, S.C.; Chang, M.Y.; Chang, H.W. A novel sulfonyl chromen-4-ones (CHW09) preferentially kills oral cancer cells showing apoptosis, oxidative stress, and DNA damage. Environ. Toxicol. 2018, 33, 1195–1203. [Google Scholar] [CrossRef]
- Chang, H.W.; Yen, C.Y.; Chen, C.H.; Tsai, J.H.; Tang, J.Y.; Chang, Y.T.; Kao, Y.H.; Wang, Y.Y.; Yuan, S.F.; Lee, S.Y. Evaluation of the mRNA expression levels of integrins alpha3, alpha5, beta1 and beta6 as tumor biomarkers of oral squamous cell carcinoma. Oncol. Lett. 2018, 16, 4773–4781. [Google Scholar]
- Stagos, D.; Balabanos, D.; Savva, S.; Skaperda, Z.; Priftis, A.; Kerasioti, E.; Mikropoulou, E.V.; Vougogiannopoulou, K.; Mitakou, S.; Halabalaki, M.; et al. Extracts from the mediterranean food plants Carthamus lanatus, Cichorium intybus, and Cichorium spinosum enhanced GSH levels and increased Nrf2 expression in human endothelial cells. Oxid. Med. Cell Longev. 2018, 2018, 6594101. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, Z.; Gomes, A.R.; Lee, H.J.; Aimjongjun, S.; Jiramongkol, Y.; Yao, S.; Zona, S.; Alasiri, G.; Gong, G.; Yague, E.; et al. EP300 and SIRT1/6 Co-Regulate Lapatinib Sensitivity Via Modulating FOXO3-Acetylation and Activity in Breast Cancer. Cancers 2019, 11, 1067. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wu, Y.H.; Zhang, L.; Xue, B.; Wang, Y.; Liu, B.; Liu, X.Y.; Zuo, F.; Yang, X.Y.; Chen, F.Y.; et al. MicroRNA-146a suppresses rheumatoid arthritis fibroblast-like synoviocytes proliferation and inflammatory responses by inhibiting the TLR4/NF-kB signaling. Oncotarget 2018, 9, 23944–23959. [Google Scholar] [CrossRef] [Green Version]
- Yen, C.Y.; Huang, C.Y.; Hou, M.F.; Yang, Y.H.; Chang, C.H.; Huang, H.W.; Chen, C.H.; Chang, H.W. Evaluating the performance of fibronectin 1 (FN1), integrin alpha4beta1 (ITGA4), syndecan-2 (SDC2), and glycoprotein CD44 as the potential biomarkers of oral squamous cell carcinoma (OSCC). Biomarkers 2013, 18, 63–72. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Nishio, S.; Teshima, Y.; Takahashi, N.; Thuc, L.C.; Saito, S.; Fukui, A.; Kume, O.; Fukunaga, N.; Hara, M.; Nakagawa, M.; et al. Activation of CaMKII as a key regulator of reactive oxygen species production in diabetic rat heart. J. Mol. Cell Cardiol. 2012, 52, 1103–1111. [Google Scholar] [CrossRef]
- Yu, T.J.; Tang, J.Y.; Ou-Yang, F.; Wang, Y.Y.; Yuan, S.F.; Tseng, K.; Lin, L.C.; Chang, H.W. Low concentration of withaferin A inhibits oxidative stress-mediated migration and invasion in oral cancer cells. Biomolecules 2020, 10, 777. [Google Scholar] [CrossRef]
- Fujii, Y.; Yoshihashi, K.; Suzuki, H.; Tsutsumi, S.; Mutoh, H.; Maeda, S.; Yamagata, Y.; Seto, Y.; Aburatani, H.; Hatakeyama, M. CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc. Natl. Acad. Sci. USA 2012, 109, 20584–20589. [Google Scholar] [CrossRef] [Green Version]
- Laddha, N.C.; Dwivedi, M.; Mansuri, M.S.; Singh, M.; Patel, H.H.; Agarwal, N.; Shah, A.M.; Begum, R. Association of neuropeptide Y (NPY), interleukin-1B (IL1B) genetic variants and correlation of IL1B transcript levels with vitiligo susceptibility. PLoS ONE 2014, 9, e107020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, C.Y.; Hou, M.F.; Yang, Z.W.; Tang, J.Y.; Li, K.T.; Huang, H.W.; Huang, Y.H.; Lee, S.Y.; Fu, T.F.; Hsieh, C.Y.; et al. Concentration effects of grape seed extracts in anti-oral cancer cells involving differential apoptosis, oxidative stress, and DNA damage. BMC Complement. Altern. Med. 2015, 15, 94. [Google Scholar] [CrossRef] [Green Version]
- Chambard, J.-C.; Lefloch, R.; Pouysségur, J.; Lenormand, P. ERK implication in cell cycle regulation. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2007, 1773, 1299–1310. [Google Scholar] [CrossRef]
- Florea, A.M.; Busselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Farooqi, A.A.; Ou-Yang, F.; Hou, M.F.; Huang, H.W.; Wang, H.R.; Li, K.T.; Fayyaz, S.; Shu, C.W.; Chang, H.W. Oxidative stress-modulating drugs have preferential anticancer effects—Involving the regulation of apoptosis, DNA damage, endoplasmic reticulum stress, autophagy, metabolism, and migration. Semin. Cancer Biol. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.H.; Yu, J.S. Inhibition of UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermal carcinoma A431 cells by genistein. J. Cell Biochem. 2000, 78, 73–84. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M. Heme oxygenase-1: Redox regulation of a stress protein in lung and cell culture models. Antioxid. Redox Signal. 2005, 7, 80–91. [Google Scholar] [CrossRef]
- Ferber, E.C.; Peck, B.; Delpuech, O.; Bell, G.P.; East, P.; Schulze, A. FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ. 2012, 19, 968–979. [Google Scholar] [CrossRef]
- Szoltysek, K.; Walaszczyk, A.; Janus, P.; Kimmel, M.; Widlak, P. Irradiation with UV-C inhibits TNF-α-dependent activation of the NF-κB pathway in a mechanism potentially mediated by reactive oxygen species. Genes Cells 2017, 22, 45–58. [Google Scholar] [CrossRef] [Green Version]
- Nishijima, Y.; Ibuki, A.; Minematsu, T.; Sanada, H. Expression profiles of the antioxidant enzymes gene (SOD1, CAT, GPX, and HMOX1) in the skin of UV-irradiated and obese mice. J. Nurs. Sci. Eng. 2016, 3, 13–20. [Google Scholar]
- Cadet, J.; Sage, E.; Douki, T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat. Res. 2005, 571, 3–17. [Google Scholar] [CrossRef]
- Evans, M.D.; Cooke, M.S.; Podmore, I.D.; Zheng, Q.; Herbert, K.E.; Lunec, J. Discrepancies in the measurement of UVC-induced 8-oxo-2′-deoxyguanosine: Implications for the analysis of oxidative DNA damage. Biochem. Biophys. Res. Commun. 1999, 259, 374–378. [Google Scholar] [CrossRef]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef] [Green Version]

| Genes | Forward Primers (5′→3′) | Reverse Primers (5′→3′) | Length |
|---|---|---|---|
| RELA | TCAAGATCTGCCGAGTGAAC [33] | GACGATCGTCTGTATCTGGC | 306 bp |
| FOXO3 | TACGAGTGGATGGTGCGTT | GGCTTTTCCGCTCTTCC | 189 bp |
| SOD1 | AGGGCATCATCAATTTCGAGC [36] | CCCAAGTCTCCAACATGCCTC [37] | 211 bp |
| HMOX1 | CCTTCTTCACCTTCCCCAACAT [37] | GGCAGAATCTTGCACTTTGTTGC [37] | 251 bp |
| GAPDH | CCTCAACTACATGGTTTACATGTTCC [38] | CAAATGAGCCCCAGCCTTCT [39] | 220 bp |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.-Y.; Lin, L.-C.; Yang, Z.-W.; Chang, F.-R.; Cheng, Y.-B.; Tang, J.-Y.; Chang, H.-W. Combined Treatment with Low Cytotoxic Ethyl Acetate Nepenthes Extract and Ultraviolet-C Improves Antiproliferation to Oral Cancer Cells via Oxidative Stress. Antioxidants 2020, 9, 876. https://doi.org/10.3390/antiox9090876
Peng S-Y, Lin L-C, Yang Z-W, Chang F-R, Cheng Y-B, Tang J-Y, Chang H-W. Combined Treatment with Low Cytotoxic Ethyl Acetate Nepenthes Extract and Ultraviolet-C Improves Antiproliferation to Oral Cancer Cells via Oxidative Stress. Antioxidants. 2020; 9(9):876. https://doi.org/10.3390/antiox9090876
Chicago/Turabian StylePeng, Sheng-Yao, Li-Ching Lin, Zhe-Wei Yang, Fang-Rong Chang, Yuan-Bin Cheng, Jen-Yang Tang, and Hsueh-Wei Chang. 2020. "Combined Treatment with Low Cytotoxic Ethyl Acetate Nepenthes Extract and Ultraviolet-C Improves Antiproliferation to Oral Cancer Cells via Oxidative Stress" Antioxidants 9, no. 9: 876. https://doi.org/10.3390/antiox9090876
APA StylePeng, S.-Y., Lin, L.-C., Yang, Z.-W., Chang, F.-R., Cheng, Y.-B., Tang, J.-Y., & Chang, H.-W. (2020). Combined Treatment with Low Cytotoxic Ethyl Acetate Nepenthes Extract and Ultraviolet-C Improves Antiproliferation to Oral Cancer Cells via Oxidative Stress. Antioxidants, 9(9), 876. https://doi.org/10.3390/antiox9090876







