Semen Thresholds of Normality Established by the WHO Do Not Reveal Genome Instability—A Potential Occult Male Factor
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Participants
2.3. Semen Analysis
2.4. Sperm DNA Fragmentation (SDF)
2.5. Sperm Nuclear Chromatin Condensation and Decondensation Assessment
- Condensed chromatin—histones replaced by protamines transforming the nucleus into a highly compact structure.
- Hypocondensed chromatin—insufficient chromatin condensation or a potential condition of underprotamination rendering the paternal genome susceptible to damage.
- Decondensed chromatin—ability of compacted chromatin to decondense in vitro after SDS and EDTA treatment.
- Hypercondensed chromatin—resistance to decondensation achieving a state of hyperstability making the paternal genome unavailable for further fertilization.
2.6. Fluorescence In Situ Hybridization (FISH) Analysis
2.7. Statistical Analysis
3. Results
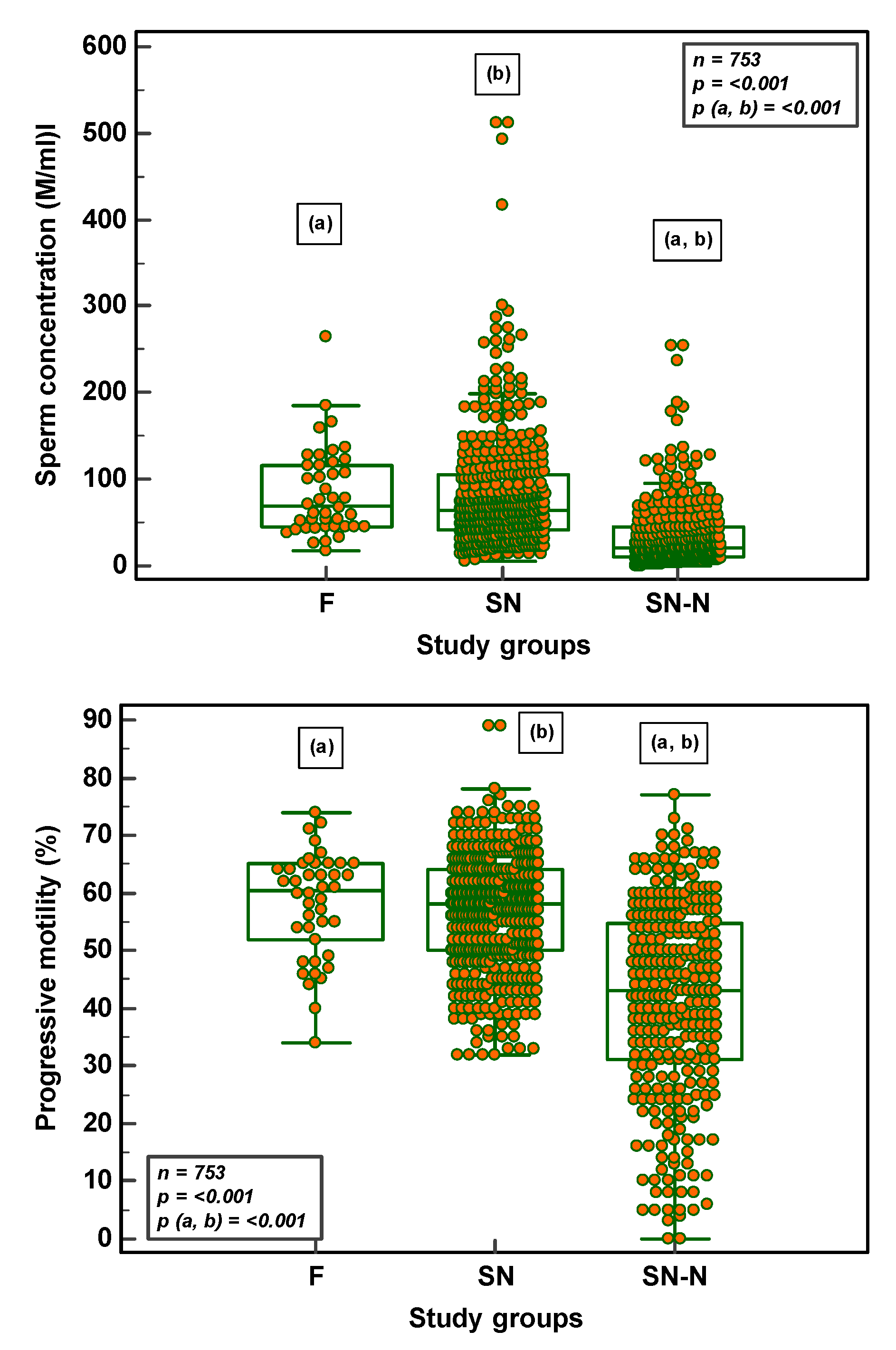
3.1. Semen Parameters in Fertile, Subfertile Normozoospermic and Subfertile Non-Normozoospermic Males
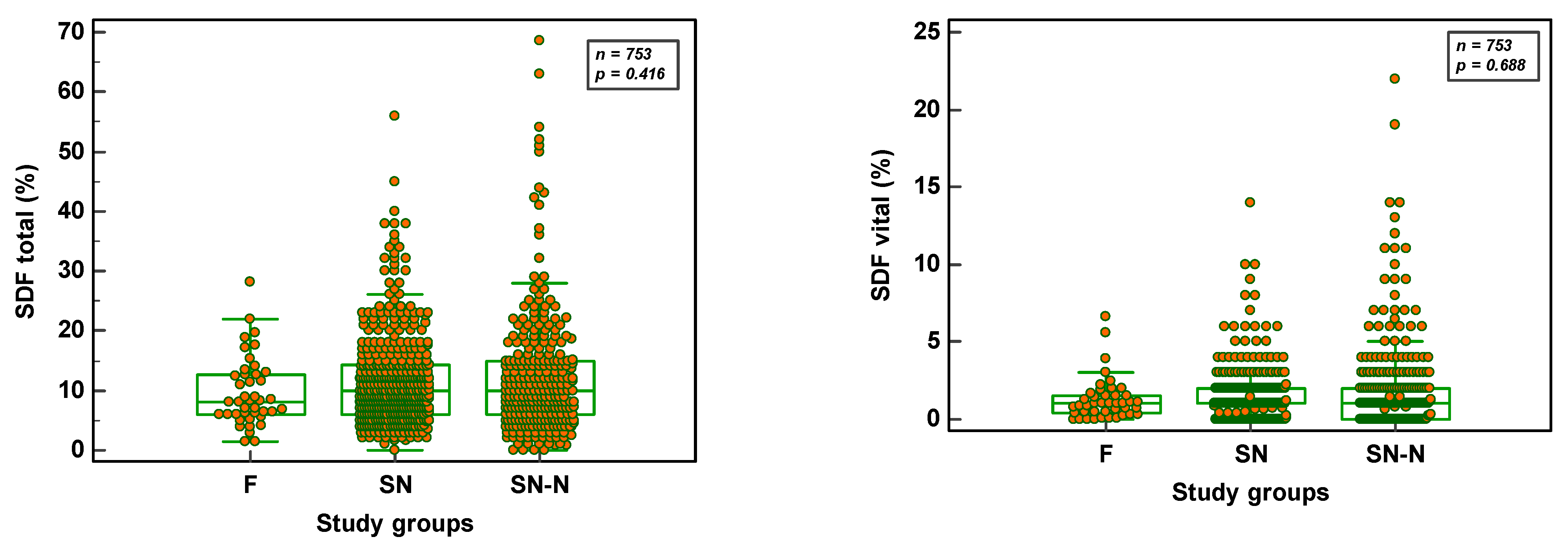
3.2. SDF Parameters in Fertile, Subfertile Normozoospermic and Subfertile Non-normozoospermic Males
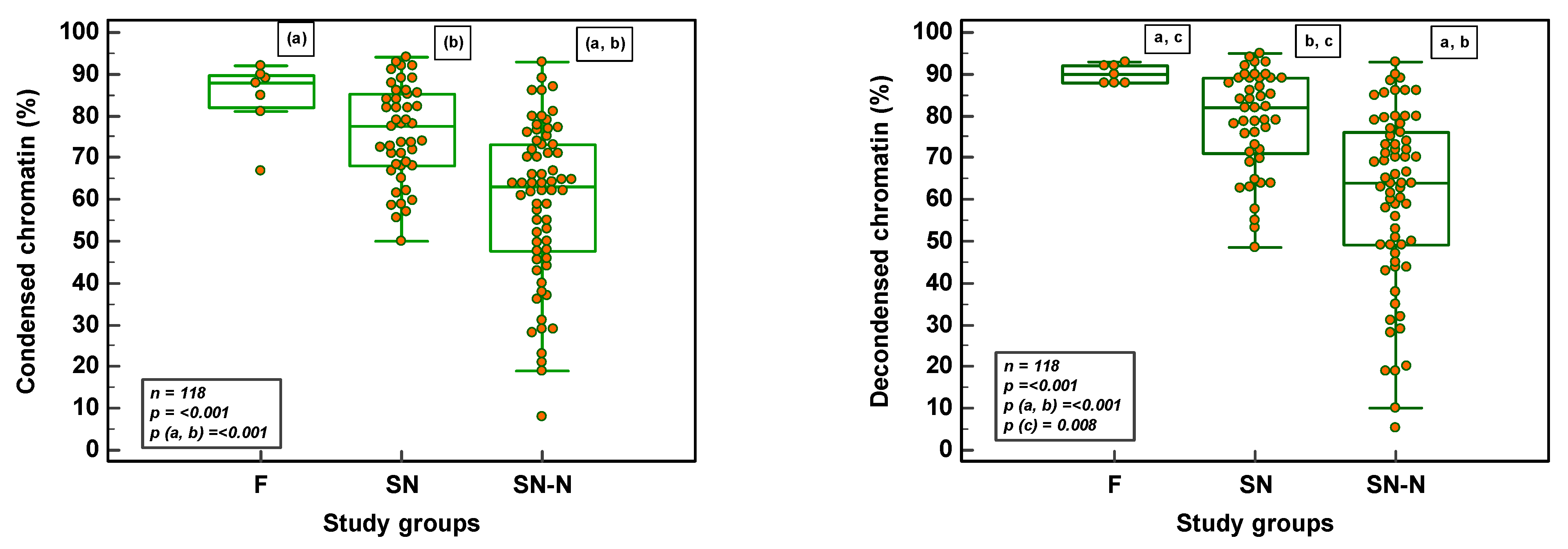
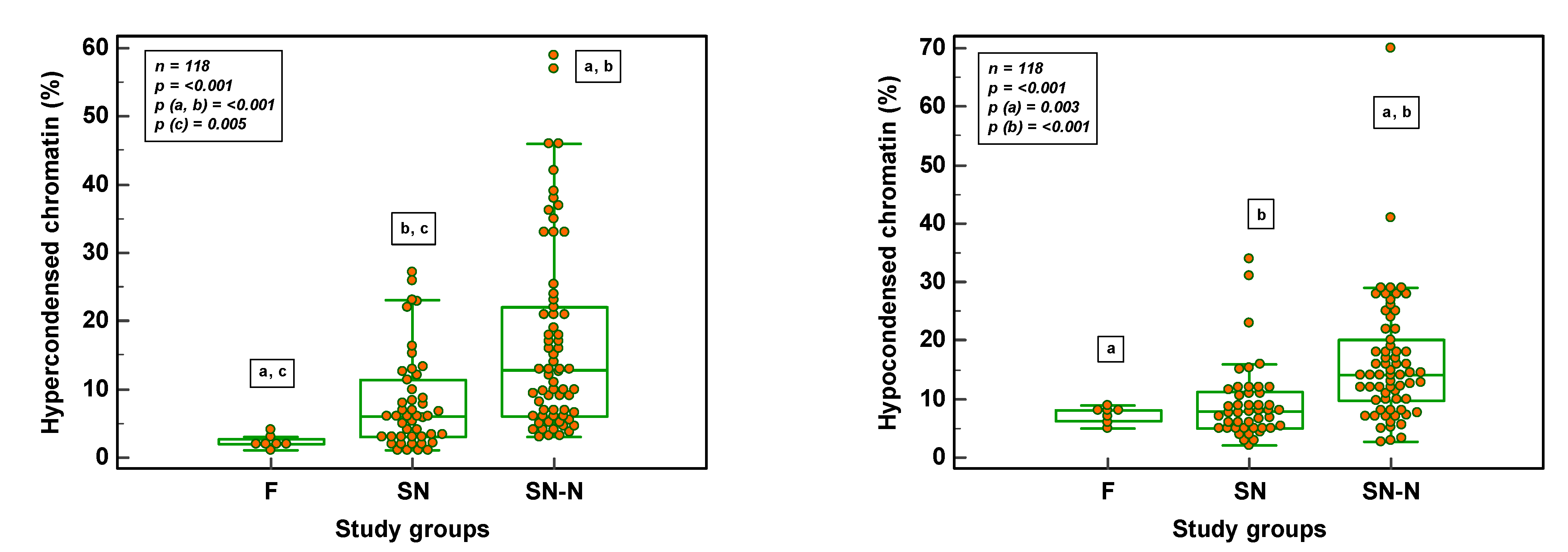
3.3. Chromatin Maturity and Stability in Fertile, Subfertile Normozoospermic and Subfertile Non-Normozoospermic Males
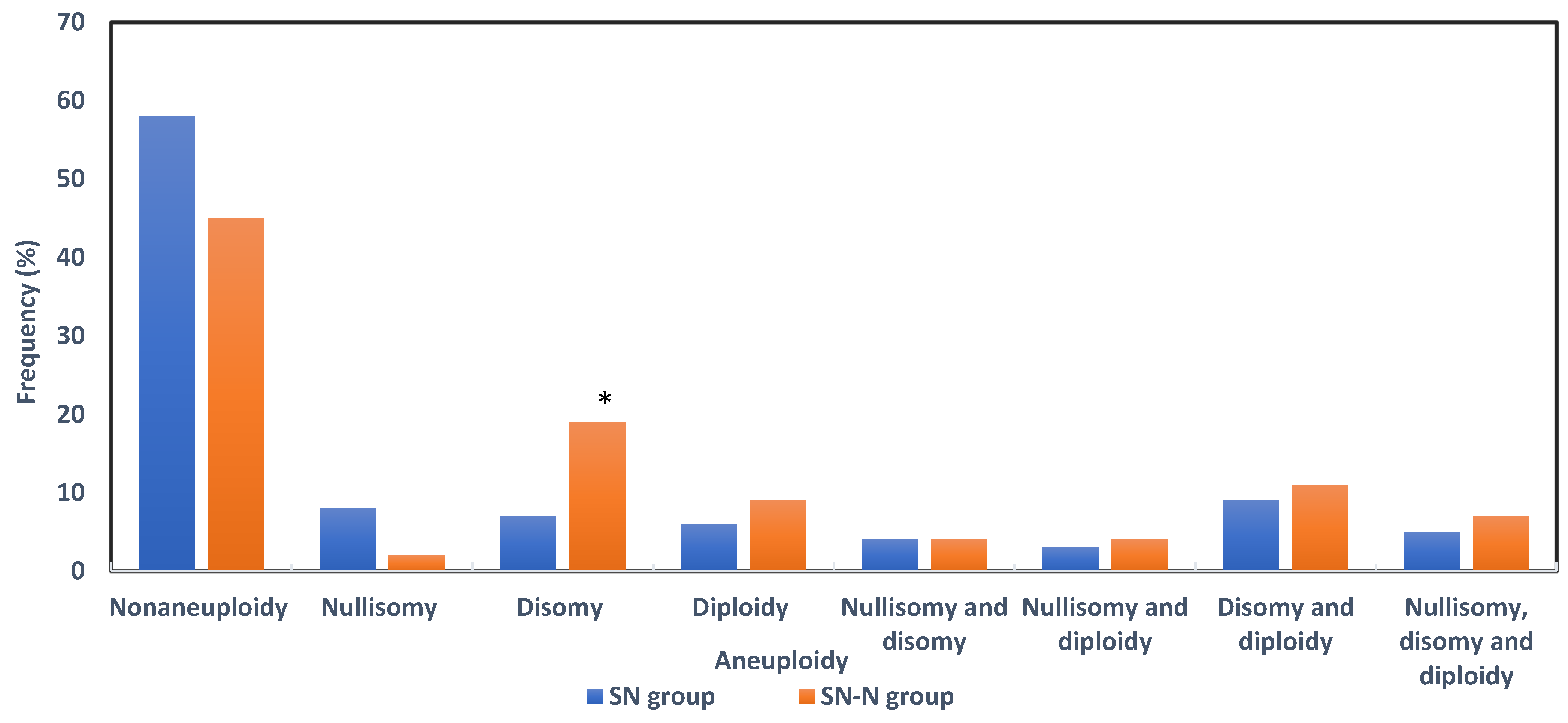
3.4. Sperm Aneuploidy in Fertile, Subfertile Normozoospermic and Subfertile Non-Normozoospermic Males
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aitken, R.J. The Male Is Significantly Implicated as the Cause of Unexplained Infertility. Semin. Reprod. Med. 2020, 38, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Irvine, D.S.; Wu, F.C. Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. Am. J. Obstet. Gynecol. 1991, 164, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Kini, S.; Morrell, D.; Thong, K.J.; Kopakaki, A.; Hillier, S.; Irvine, D.S. Lack of impact of semen quality on fertilization in assisted conception. Scott. Med. J. 2010, 55, 20–23. [Google Scholar] [CrossRef]
- Van Den Hoven, L.; Hendriks, J.C.; Verbeet, J.G.; Westphal, J.R.; Wetzels, A.M. Status of sperm 500 morphology assessment: An evaluation of methodology and clinical value. Fertil. Steril. 2015, 103, 53–58. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed; WHO Press: Geneva, Switzerland, 2021. Available online: https://www.who.int/publications/i/item/9789240030787 (accessed on 29 January 2021).
- World Health Organization. Laboratory Manual for the Examination of Human Semen and Semen-cervical Mucus Interaction, 5th ed.; Cambridge University Press: Cambridge, UK, 2010.
- Barratt, C.L.R.; Mortimer, D.; Amer, M.; Cairo Consensus Workshop Group. The current status and future of andrology: A consensus report from the Cairo workshop group. Andrology 2020, 8, 27–52. [Google Scholar] [CrossRef]
- Vasconcelos, A.L.; Campbell, M.J.; Barratt, C.L.R.; Gellatly, S.A. Do studies published in two leading reproduction journals between 2011 and 2020 demonstrate that they followed WHO5 recommendations for basic semen analysis? Hum. Reprod. 2022, 37, 2255–2263. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef]
- Jensen, T.K.; Lindahl-Jacobsen, R.; Christensen, K.; Nielsen, N.C.; Bostofte, E. Good Semen Quality and Life Expectancy: A Cohort Study of 43,277 Men. Am. J. Epidemiol. 2009, 170, 559–565. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Li, S.; Behr, B.; Cullen, M.R.; Galusha, D.; Lamb, D.J.; Lipshultz, L.I. Semen quality, infertility and mortality in the USA. Hum. Reprod. 2014, 29, 1567–1574. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Li, S.; Behr, B.; Pera, R.R.; Cullen, M.R. Relationship between semen production and medical comorbidity. Fertil. Steril. 2015, 103, 66–71. [Google Scholar] [CrossRef]
- Latif, T.; Jensen, T.K.; Mehlsen, J.; Holmboe, S.A.; Brinth, L.; Pors, K.; Skouby, S.O.; Jørgensen, N.; Lindahl-Jacobsen, R. Semen Quality as a Predictor of Subsequent Morbidity: A Danish Cohort Study of 4712 Men With Long-Term Follow-up. Am. J. Epidemiol. 2017, 186, 910–917. [Google Scholar] [CrossRef]
- Salonia, A.; Matloob, R.; Gallina, A.; Abdollah, F.; Saccà, A.; Briganti, A.; Suardi, N.; Colombo, R.; Rocchini, L.; Guazzoni, G.; et al. Are infertile men less healthy than fertile men? Results of a prospective case-control survey. Eur. Urol. 2009, 56, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Aston, K.I.; Carrell, D.T. Emerging evidence for the role of genomic instability in male factor infertility. Syst. Biol. Reprod. Med. 2012, 58, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Ambar, R.F.; Agarwal, A.; Henkel, R. Etiologies of sperm DNA damage and its impact on male infertility. Andrologia 2021, 53, e13706. [Google Scholar] [CrossRef] [PubMed]
- González-Marín, C.; Goasálvez, J.; Roy, R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int. J. Mol. Sci. 2012, 13, 14026–14052. [Google Scholar] [CrossRef] [PubMed]
- Mengual, L.; Ballescá, J.L.; Ascaso, C.; Oliva, R. Marked differences in protamine content and P1/P2 ratios in sperm cells from percoll fractions between patients and controls. J. Androl. 2003, 24, 438–447. [Google Scholar] [CrossRef]
- Carrell, D.T.; Hammoud, S.S. The human sperm epigenome and its potential role in embryonic development. Mol. Hum. Reprod. 2010, 16, 37–47. [Google Scholar] [CrossRef]
- Zhang, X.; San Gabriel, M.; Zini, A. Sperm nuclear histone to protamine ratio in fertile and infertile men: Evidence of heterogeneous subpopulations of spermatozoa in the ejaculate. J. Androl. 2006, 27, 414–420. [Google Scholar] [CrossRef]
- Zini, A.; Gabriel, M.S.; Zhang, X. The histone to protamine ratio in human spermatozoa: Comparative study of whole and processed semen. Fertil. Steril. 2007, 87, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, S.; Reynoir, N.; Escoffier, E.; Thevenon, J.; Caron, C.; Khochbin, S. Epigenetic reprogramming of the male genome during gametogenesis and in the zygote. Reprod. Biomed. Online 2008, 16, 492–503. [Google Scholar] [CrossRef]
- Ward, W.S. Function of sperm chromatin structural elements in fertilization and development. Mol. Hum. Reprod. 2010, 16, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Dale, B.; Cohen, M. DNA damage and repair in human oocytes and embryos: A review. Zygote 2010, 18, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Evenson, D.; Cohen, M.; Dale, B. Effect of antioxidants on sperm genetic damage. Adv. Exp. Med. Biol. 2014, 791, 173–189. [Google Scholar]
- Burrello, N.; Arcidiacono, G.; Vicari, E.; Asero, P.; Di Benedetto, D.; De Palma, A.; Romeo, R.; D’Agata, R.; Calogero, A.E. Morphologically normal spermatozoa of patients with secretory oligo-astheno-teratozoospermia have an increased aneuploidy rate. Hum. Reprod. 2004, 19, 2298–2302. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, X.; Ferrer, M.; García-Mengual, E.; Muñoz, P.; Triviño, J.C.; Calatayud, C.; Rawe, V.Y.; Ruiz-Jorro, M. Correlation between aneuploidy, apoptotic markers and DNA fragmentation in spermatozoa from normozoospermic patients. Reprod. Biomed. Online 2014, 28, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Egozcue, J.; Blanco, J.; Anton, E.; Egozcue, S.; Sarrate, Z.; Vidal, F. Genetic analysis of sperm and implications of severe male infertility—A review. Placenta 2003, 24 (Suppl. B), S62–S65. [Google Scholar] [CrossRef]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Björndahl, L. A paradigmatic shift in the care of male factor infertility: How can the recommendations for basic semen examination in the sixth edition of the WHO manual and the ISO 23162:2021 standard help? Reprod. Biomed. Online 2022, 45, 731–736. [Google Scholar] [CrossRef]
- Björndahl, L.; Barratt, C.L.R.; Mortimer, D.; Agarwal, A.; Aitken, R.J.; Alvarez, J.G.; Aneck-Hahn, N.; Arver, S.; Baldi, E.; Bassas, L.; et al. Standards in semen examination: Publishing reproducible and reliable data based on high-quality methodology. Hum. Reprod. 2022, 37, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, U.; Spiessens, C. Basic semen analysis courses: Experience in Belgium. In Modern ART in the 2000’s—Andrology in the Nineties; Ombelet, W., Bosmans, E., Vandeput, H., Vereecken, A., Renier, M., Hoomans, E., Eds.; The Parthenon Publishing Group: London, UK, 1998; pp. 107–113. [Google Scholar]
- Björndahl, L.; Barratt, C.L.; Fraser, L.R.; Kvist, U.; Mortimer, D. ESHRE basic semen analysis courses 1995–1999: Immediate beneficial effects of standardized training. Hum. Reprod. 2002, 17, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, U.; Wyns, C.; Mahmoud, A.; Vernelen, K.; China, B.; Verheyen, G. Fifteen years of Belgian experience with external quality assessment of semen analysis. Andrology 2016, 4, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.A.; De Iuliis, G.N.; Aitken, R.J. The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: Development of an improved methodology. Int. J. Androl. 2010, 34, 2–13. [Google Scholar] [CrossRef]
- Bonde, J.P.E.; Ernest, E.; Jensen, T.K.; Hjolland, N.; Kolstad, H.; Henriksen, T.; Scheike, T.; Giwercman, A.; Olsen, J.; Skakkebæk, N. Relation between semen quality and fertility: A population-based study of 430 first-pregnancy planners. Lancet 1998, 352, 1172–1177. [Google Scholar] [CrossRef]
- Punjabi, U.; Van Mulders, H.; Goovaerts, I.; Peeters, K.; Clasen, K.; Janssens, P.; Zemtsova, O.; De Neubourg, D. Sperm DNA fragmentation in the total and vital fractions before and after density gradient centrifugation: Significance in male fertility diagnosis. Clin. Biochem. 2018, 62, 47–54. [Google Scholar] [CrossRef]
- Punjabi, U.; Van Mulders, H.; Goovaerts, I.; Peeters, K.; Roelant, E.; De Neubourg, D. DNA fragmentation in concert with the simultaneous assessment of cell viability in a subfertile population: Establishing thresholds of normality both before and after density gradient centrifugation. J. Assist. Reprod. Genet. 2019, 36, 1413–1421. [Google Scholar] [CrossRef]
- Molina, J.; Castilla, J.A.; Gil, T.; Hortas, M.L.; Vergara, F.; Herruzo, A. Influence of incubation on the chromatin condensation and nuclear stability of human spermatozoa by flow cytometry. Hum. Reprod. 1995, 10, 1280–1286. [Google Scholar] [CrossRef]
- Punjabi, U.; Peeters, K.; De Neubourg, D. Sperm nuclear maturity and chromatin stability in subfertile patients: Density gradient centrifugation is fair but non-discriminative in selecting the right population. Reprod. Biol. 2019, 19, 316–321. [Google Scholar] [CrossRef]
- Vegetti, W.; Van Assche, E.; Frias, A.; Verheyen, G.; Bianchi, M.M.; Bonduelle, M.; Liebaers, I.; Van Steirteghem, A. Correlation between semen parameters and sperm aneuploidy rates investigated by fluorescence in-situ hybridization in infertile men. Hum. Reprod. 2000, 15, 351–365. [Google Scholar] [CrossRef]
- Romero Herrera, J.A.; Bang, A.K.; Priskorn, L.; Izarzugaza, J.M.G.; Brunak, S.; Jørgensen, N. Semen quality and waiting time to pregnancy explored using association mining. Andrology 2021, 9, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Coban, O.; Serdarogullari, M.; Sekerci, Z.O.; Bilgina, E.M.; Serakinci, N. Evaluation of the impact of sperm morphology on embryo aneuploidy rates in a donor oocyte program. Syst. Biol. Reprod. Med. 2018, 64, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, M.; Moffatt, O.; Manicardi, G.C.; Bizzaro, D.; Afnan, M.; Sakkas, D. Interrelationships between seminal parameters and sperm nuclear DNA damage before and after density gradient centrifugation: Implications for assisted conception. Hum. Reprod. 2001, 16, 2160–2165. [Google Scholar] [CrossRef] [PubMed]
- Zini, A.; Bielecki, R.; Phang, D.; Zenzes, M.T. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil. Steril. 2001, 75, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Oleszczuk, K.; Augustinsson, L.; Bayat, N.; Giwercman, A.; Bungum, M. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology 2013, 1, 357–360. [Google Scholar] [CrossRef]
- Feijó, C.M.; Esteves, S.C. Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertil. Steril. 2014, 101, 58–63.e3. [Google Scholar] [CrossRef]
- Barratt, C.L.; Aitken, R.J.; Björndahl, L.; Carrell, D.T.; de Boer, P.; Kvist, U.; Lewis, S.E.; Perreault, S.D.; Perry, M.J.; Ramos, L.; et al. Sperm DNA: Organization, protection and vulnerability: From basic science to clinical applications—A position report. Hum. Reprod. 2010, 25, 824–838. [Google Scholar] [CrossRef]
- Johnson, S.L.; Dunleavy, J.; Gemmell, N.J.; Nakagawas, S. Consistent age-dependent declines in human semen quality: A systematic review and meta-analysis. Ageing Res. Rev. 2015, 19, 22–33. [Google Scholar] [CrossRef]
- Print, C.G.; Loveland, K.L. Germ cell suicide: New insights into apoptosis during spermatogenesis. Bioassays 2000, 22, 423–430. [Google Scholar] [CrossRef]
- Caroppo, E.; Dattilo, M. Sperm redox biology challenges the role of antioxidants as a treatment for male factor infertility. F&S Rev. 2022, 3, 90–104. [Google Scholar]
- Muratori, M.; Tamburrino, L.; Marchiani, S.; Cambi, M.; Olivito, B.; Azzari, C.; Forti, G.; Baldi, E. Investigation on the Origin of Sperm DNA Fragmentation: Role of Apoptosis, Immaturity and Oxidative Stress. Mol. Med. 2015, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Björndahl, L.; Kvist, U. Human sperm chromatin stabilization: A proposed model including zinc bridges. Mol. Hum. Reprod. 2010, 16, 23–29. [Google Scholar] [CrossRef]
- Borini, A.; Tarozzi, N.; Bizzaro, D.; Bonu, M.; Fava, L.; Flamigni, C.; Coticchio, G. Sperm DNA fragmentation: Paternal effect on early post-implantation embryo development in ART. Hum. Reprod. 2006, 21, 2876–2881. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Trpkov, K.; Rademaker, A.; Ko, E.; Martin, R.H. Variation in meiotic recombination frequencies among human males. Hum. Genet. 2005, 116, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.A.; Wong, E.C.; Chow, V.; Nigro, M.; Ma, S. Abnormal meiotic recombination in infertile men and its association with sperm aneuploidy. Hum. Mol. Genet. 2007, 16, 2870–2879. [Google Scholar] [CrossRef]
- Gonsalves, J.; Sun, F.; Schlegel, P.N.; Turek, P.J.; Hopps, C.V.; Greene, C.; Martin, R.; Reijo Perra, R.A. Defective recombination in infertile men. Hum. Mol. Genet. 2004, 13, 2875–2883. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Turek, P.; Greene, C.; Ko, E.; Rademaker, A.; Martin, R.H. Abnormal progression through meiosis in men with nonobstructive azoospermia. Fertil. Steril. 2007, 87, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Ferguson, K.; Arsovska, S.; Moens, P.; Chow, V. Reduced recombination associated with aneuploid sperm in infertile man: A case report. Hum. Reprod. 2006, 21, 980–985. [Google Scholar] [CrossRef]
- Kirkpatrick, G.; Ferguson, K.A.; Gao, H.; Tang, S.; Chow, V.; Yuen, B.H.; Ma, S. A comparison of sperm aneuploidy rates between infertile men with normal and abnormal karyotypes. Hum. Reprod. 2008, 23, 1679–1683. [Google Scholar] [CrossRef]
- Ramasamy, R.; Scovell, J.M.; Kovac, J.R.; Cook, P.; Lamb, D.J.; Lipshultz, L.I. Fluorescence in situ hybridization detects increased sperm aneuploidy in men with recurrent pregnancy loss. Fertil. Steril. 2015, 103, 906–909.e1. [Google Scholar] [CrossRef]
- Tempest, H.G.; Ko, E.; Rademaker, A.; Chan, P.; Robaire, B.; Martin, R.H. Intra-individual and inter-individual variations in sperm aneuploidy frequencies in normal men. Fertil. Steril. 2009, 91, 185–192. [Google Scholar] [CrossRef]
- Sharma, R.; Agarwal, A.; Rohra, V.K.; Assidi, M.; Abu-Elmagd, M.; Turki, R.F. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod. Biol. Endocrinol. 2015, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- García-Ferreyra, J.; Luna, D.; Villegas, L.; Romero, R.; Zavala, P.; Hilario, R.; Dueñas-Chacón, J. High Aneuploidy Rates Observed in Embryos Derived from Donated Oocytes are Related to Male Aging and High Percentages of Sperm DNA Fragmentation. Clin. Med. Insights Reprod. Health 2015, 9, 21–27. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.M.; Al-Din, A.B.; Barakat, M.T.; El-Fakahany, H.M.; Xu, J.; Sakkas, D. Deoxyribonucleic acid repair and apoptosis in testicular germ cells of aging fertile men: The role of the poly(adenosine diphosphate-ribosyl)ation pathway. Fertil. Steril. 2009, 91 (Suppl. 5), 2221–2229. [Google Scholar] [CrossRef]
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef] [PubMed]



| Sperm Aneuploidy | Study Groups | p Value * | ||
|---|---|---|---|---|
| F (n = 17) | SN (n = 100) | SN-N (n = 78) | ||
| Chromosome 13 | ||||
| nullisomy (%) | 0.12 ± 0.16 | 0.17 ± 0.27 | 0.13 ± 0.16 | 0.632 |
| disomy (%) | 0.15± 0.11 | 0.15 ± 0.28 | 0.18 ± 0.29 | 0.188 |
| Chromosome 18 | ||||
| nullisomy (%) | 0.05 ± 0.09 | 0.21 ± 0.50 | 0.13 ± 0.22 | 0.262 |
| disomy (%) | 0.11 ± 0.17 | 0.22 ± 0.58 | 0.19 ± 0.25 | 0.237 |
| Chromosome 21 | ||||
| nullisomy (%) | 0.08 ± 0.12 | 0.14 ± 0.15 | 0.12 ± 0.17 | 0.173 |
| disomy (%) | 0.09 ± 0.09 | 0.15 ± 0.40 | 0.19 ± 0.30 | 0.141 |
| Chromosome X/Y | ||||
| nullisomy (%) | 0.28 ± 0.31 | 0.26 ± 0.39 | 0.31 ± 0.54 | 0.329 |
| disomy XX (%) | 0.15 ± 0.22 | 0.11 ± 0.15 | 0.10 ± 0.17 | 0.605 |
| disomy XY (%) | 0.11 ± 0.13 | 0.24 ± 0.89 | 0.24 ± 0.34 | 0.376 |
| disomy YY (%) | 0.06 ± 0.10 | 0.07 ± 0.16 | 0.07 ± 0.13 | 0.648 |
| Autosomal aneuploidy (%) | 0.60 ± 0.35 | 1.04 ± 1.24 | 0.95 ± 0.84 | 0.347 |
| Sex aneuploidy (%) | 0.61 ± 0.42 | 0.68 ± 1.14 | 0.72 ± 0.86 | 0.605 |
| Diploidy (%) | 0.49 ± 0.35 | 0.93 ±01.95 | 1.59 ± 2.73 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punjabi, U.; Goovaerts, I.; Peeters, K.; De Neubourg, D. Semen Thresholds of Normality Established by the WHO Do Not Reveal Genome Instability—A Potential Occult Male Factor. Genes 2023, 14, 239. https://doi.org/10.3390/genes14020239
Punjabi U, Goovaerts I, Peeters K, De Neubourg D. Semen Thresholds of Normality Established by the WHO Do Not Reveal Genome Instability—A Potential Occult Male Factor. Genes. 2023; 14(2):239. https://doi.org/10.3390/genes14020239
Chicago/Turabian StylePunjabi, Usha, Ilse Goovaerts, Kris Peeters, and Diane De Neubourg. 2023. "Semen Thresholds of Normality Established by the WHO Do Not Reveal Genome Instability—A Potential Occult Male Factor" Genes 14, no. 2: 239. https://doi.org/10.3390/genes14020239
APA StylePunjabi, U., Goovaerts, I., Peeters, K., & De Neubourg, D. (2023). Semen Thresholds of Normality Established by the WHO Do Not Reveal Genome Instability—A Potential Occult Male Factor. Genes, 14(2), 239. https://doi.org/10.3390/genes14020239






