The Role of Humic Acid, PP Beads, and pH with Water Backwashing in a Hybrid Water Treatment of Multichannel Alumina Microfiltration and PP Beads
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
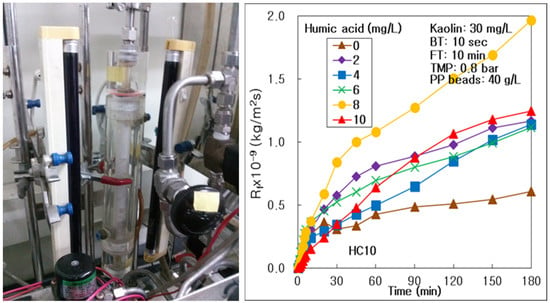
3.1. Effect of HA Concentration on Membrane Fouling and Treatment Efficiency
3.2. Effect of Pure PP Beads on Membrane Fouling and Treatment Efficiency
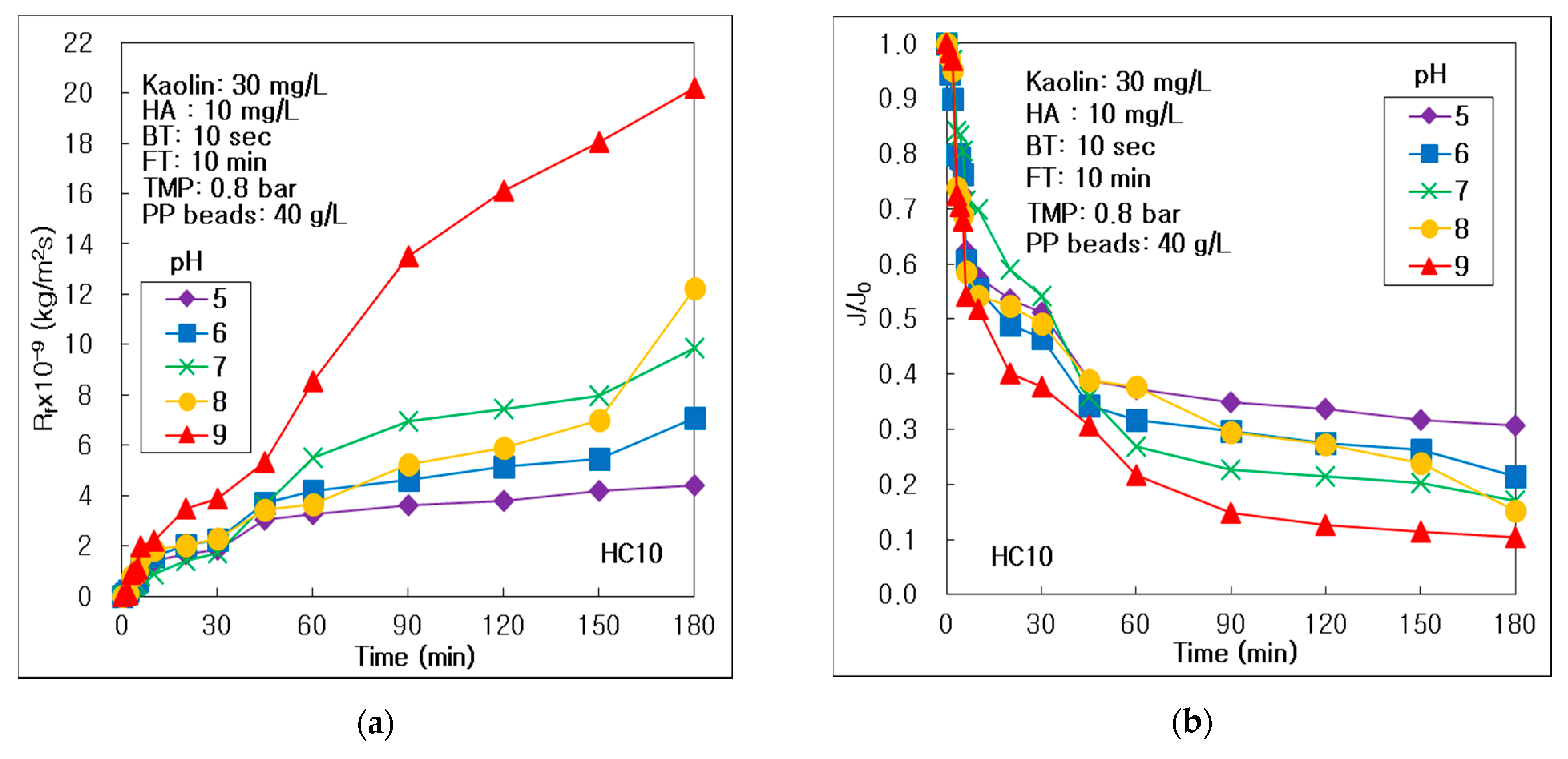
3.3. Effect of pH on Membrane Fouling and Treatment Efficiency
4. Conclusions
- (1)
- DOM, such as HA, could drive membrane fouling more severely on the surface and inside the alumina membrane, with an increasing HA concentration in water; however, the thick fouling cake on the membrane could be removed by water backwashing at 10 mg/L of HA. DOM could affect the treatment of suspended particles, such as kaolin, in the hybrid process of the multichannel alumina MF and the pure PP beads; however, it could not affect the process of tubular carbon fiber UF membrane. DOM could be treated more effectively at high DOM condition in the hybrid water treatment process of seven-channel alumina MF and pure PP beads.
- (2)
- The optimal PP beads concentration could be 5 g/L to control the membrane fouling in this hybrid process of seven-channel alumina MF HC10 and PP beads. The tubid matters could be treated effectively, independent of PP beads concentration in this hybrid process. The optimal PP beads concentration was 5 g/L to remove DOM in this hybrid process. The optimal PP beads concentration to reduce the turbid matter could be 30 g/L in the hybrid process of C005 UF membrane.
- (3)
- The reversible and irreversible membrane fouling, and concentration polarization could be inhibited at acid condition, because both the membrane and humic materials had a negative surface charge at acid conditions below pH 7. The turbid matter could be removed more effectively at an acidic pH condition in the hybrid process of the alumina HC10 MF membrane; however, pH could not affect treating the turbid matter for carbon fiber UF process. DOM could be removed effectively in alkalic condition, because of the secondary layer on the membrane surface accumulated by the most severe membrane fouling.
Author Contributions
Funding
Conflicts of Interest
References
- Meng, F.G.; Chae, S.R.; Drews, A.; Kraume, M.; Shin, H.S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Zhang, D.R.; He, Y.; Zhao, X.S.; Bai, R. Modification of membrane surface for anti-biofouling performance: Effect of anti-adhesion and anti-bacterial approaches. J. Membr. Sci. 2010, 346, 121–130. [Google Scholar] [CrossRef]
- Yoon, Y.; Lueptow, R.M. Removal of organic contaminants by RO and NF membranes. J. Membr. Sci. 2005, 261, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Choo, K.-H. Natural Organic Matter Removal and Fouling Control in Low-Pressure Membrane Filtration for Water Treatment. Environ. Eng. Res. 2014, 19, 1–8. [Google Scholar] [CrossRef]
- Tian, J.; Wu, C.; Yu, H.; Gao, S.; Li, G.; Cui, F.; Qu, F. Applying ultraviolet/persulfate (UV/PS) pre-oxidation for controlling ultrafiltration membrane fouling by natural organic matter (NOM) in surface water. Water Res. 2018, 132, 190–199. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Utomo, D.P. Performance evaluation of double stage process using nano hybrid PES/SiO2-PES membrane and PES/ZnO-PES membranes for oily waste water treatment to clean water. J. Environ. Chem. Eng. 2017, 5, 6077–6086. [Google Scholar] [CrossRef]
- Szymański, K.; Morawski, A.W.; Mozia, S. Surface water treatment in hybrid systems coupling advanced oxidation processes and ultrafiltration using ceramic membrane. Desalin. Water Treat. 2017, 64, 302–306. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Liu, P.; Liu, J.; Wang, Z.; Jiao, Y.; Bie, A.; Xia, J. Application of inorganic ceramic membrane in treatment of emulsion wastewater. Oxid. Commun. 2016, 39, 2753–2757. [Google Scholar]
- Zhang, H.; Zhong, Z.; Xing, W. Application of ceramic membranes in the treatment of oilfield-produced water: Effects of polyacrylamide and inorganic salts. Desalination 2013, 309, 84–90. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, L.; Roddick, F.A. Influence of the characteristics of soluble algal organic matter released from Microcystis aeruginosa on the fouling of a ceramic microfiltration membrane. J. Membr. Sci. 2013, 425, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Fan, L.; Roddick, F.A. Impact of the interaction between aquatic humic substances and algal organic matter on the fouling of a ceramic microfiltration membrane. Membranes 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benito, A.; Penadés, A.; Lliberia, J.L.; Gonzalez-Olmos, R. Degradation pathways of aniline in aqueous solutions during electro-oxidation with BDD electrodes and UV/H2O2 treatment. Chemosphere 2017, 166, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Khuzwayo, Z.; Chirwa, E.M.N. Analysis of catalyst photo-oxidation selectivity in the degradation of polyorganochlorinated pollutants in batch systems using UV and UV/TiO2. S. Afr. J. Chem. Eng. 2017, 23, 17–25. [Google Scholar]
- Milelli, D.; Lemont, F.; Ruffel, L.; Barral, T.; Marchand, M. Thermo- and photo-oxidation reaction scheme in a treatment system using submerged plasma. Chem. Eng. J. 2017, 317, 1083–1091. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A.; Cocca, M.; Vega, K.; Fleischer, A.; Gupta, S.K.; Mehan, M.; Takacs, G.A. Vacuum UV photo-oxidation of poly(ethylene terephthalate). J. Adhes. Sci. Technol. 2017, 31, 2542–2554. [Google Scholar] [CrossRef]
- Semitsoglou-Tsiapou, S.; Templeton, M.R.; Graham, N.J.D.; Hernández Leal, L.; Martijn, B.J.; Royce, A.; Kruithof, J.C. Low pressure UV/H2O2 treatment for the degradation of the pesticides metaldehyde, clopyralid and mecoprop—Kinetics and reaction product formation. Water Res. 2016, 91, 285–294. [Google Scholar] [CrossRef]
- Wu, X.H.; Su, P.B.; Liu, H.L.; Qi, L.L. Photocatalytic degradation of Rhodamine B under visible light with Nd-doped titanium dioxide films. J. Rare Earths 2009, 27, 739–743. [Google Scholar] [CrossRef]
- Li, A.; Zhao, X.; Liu, H.; Qu, J. Characteristic transformation of humic acid during photoelectrocatalysis process and its subsequent disinfection byproduct formation potential. Water Res. 2011, 45, 6131–6140. [Google Scholar] [CrossRef]
- Kim, N.Y.; Park, J.Y. Roles of polypropylene beads and photo-oxidation in hybrid water treatment process of alumina MF and photocatalyst-coated PP beads. Desalin. Water Treat. 2017, 58, 368–375. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, S.; Bang, T. Effect of water back-flushing time and polypropylene beads in hybrid water treatment process of photocatalyst-coated PP beads and alumina microfiltration membrane. Membr. J. 2016, 26, 301–309. [Google Scholar] [CrossRef]
- Park, Y.; Park, J.Y. Roles of adsorption and photo-oxidation in hybrid water treatment process of tubular carbon fiber ultrafiltration and PP beads with UV irradiation and water back-flushing. Desalin. Water Treat. 2017, 61, 20–28. [Google Scholar] [CrossRef]
- Song, S.; Park, Y.; Park, J.Y. Roles of polypropylene beads and pH in hybrid water treatment of carbon fiber membrane and PP beads with water back-flushing. Membr. Water Treat. 2019, 10, 155–163. [Google Scholar]
- Gao, S.C.; Park, J.Y. Advanced water treatment of high turbidity source by hybrid process of ceramic ultrafiltration and photocatalyst: 1. Effect of photocatalyst and water-back-flushing condition. Membr. J. 2011, 21, 127–140. [Google Scholar]
- Zhao, Y.; Zhou, S.; Li, M. Humic acid removal and easy-cleanability using temperature responsive ZrO2 tubular membranes grafted with poly(N-isopropylacrylamide) brush chains. Water Res. 2013, 47, 2375–2386. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, W.; Xu, N.; Wong, F.-S. Effects of inorganic salt on ceramic membrane MF of titanium dioxide suspension. J. Membr. Sci. 2005, 254, 81–88. [Google Scholar] [CrossRef]




| Membrane Model | HC10 |
|---|---|
| Pore size (μm) | 0.1 |
| No. of channels | 7 |
| Outer diameter (mm) | 20 |
| Inner diameter (mm) | 4 |
| Length (mm) | 245 |
| Surface area (cm2) | 215 |
| Material | ⍺-alumina |
| Company | Dongseo Industry (Korea) |
| Membrane | Humic Acid (mg/L) | 0 | 2 | 4 | 6 | 8 | 10 |
|---|---|---|---|---|---|---|---|
| 7 channels alumina MF (HC10) | Rm × 10−9 (kg/m2s) | 0.823 | 0.840 | 0.803 | 0.864 | 0.831 | 0.798 |
| Rb × 10−9 (kg/m2s) | 0.006 | 0.072 | 0.030 | 0.001 | 0.038 | 0.053 | |
| Rf,180 × 10−9 (kg/m2s) | 0.606 | 1.165 | 1.137 | 1.113 | 1.963 | 1.246 | |
| Rif × 10−9 (kg/m2s) | 0.432 | 0.044 | 0.228 | 0.408 | 0.453 | 1.037 | |
| Rrf × 10−9 (kg/m2s) | 0.173 | 1.121 | 0.909 | 0.705 | 1.510 | 0.209 | |
| J0 (L/m2h) | 341 | 310 | 339 | 326 | 325 | 332 | |
| J180 (L/m2h) | 197 | 136 | 143 | 143 | 100 | 135 | |
| J180/J0 | 0.578 | 0.439 | 0.423 | 0.437 | 0.307 | 0.406 | |
| VT (L) | 14.18 | 10.59 | 12.48 | 11.30 | 9.05 | 9.39 | |
| Tubular carbon fiber UF (C005) [22] | Rm × 10−9 (kg/m2s) | - | 0.429 | 0.397 | 0.418 | 0.418 | 0.415 |
| Rb × 10−9 (kg/m2s) | - | 0.133 | 0.185 | 0.174 | 0.174 | 0.237 | |
| Rf,180 × 10−9 (kg/m2s) | - | 1.775 | 2.135 | 2.676 | 5.311 | 6.998 | |
| Rif × 10−9 (kg/m2s) | - | 0.389 | 0.016 | 0.215 | 0.194 | 0.768 | |
| Rrf × 10−9 (kg/m2s) | - | 1.386 | 2.119 | 2.461 | 5.118 | 6.230 | |
| J0 (L/m2h) | - | 1129 | 1092 | 1073 | 1072 | 974 | |
| J180 (L/m2h) | - | 272 | 234 | 194 | 108 | 83 | |
| J180/J0 | - | 0.241 | 0.214 | 0.181 | 0.100 | 0.085 | |
| VT (L) | - | 4.98 | 4.89 | 3.83 | 2.67 | 1.92 |
| Kaolin (mg/L) | Humic Acid (mg/L) | Turbidity (NTU) | Average Treatment Efficiency (%) | ||||
|---|---|---|---|---|---|---|---|
| Feed Water | Treated Water | Adsorption Media | |||||
| Range | Average | Range | Average | 7 Channels Alumina MF | Tubular Carbon Fiber UF [22] | ||
| 30 | 0 | 12.1–13.5 | 12.6 | 0.342–0.396 | 0.371 | 97.0 | - |
| 2 | 12.1–16.3 | 13.6 | 0.370–0.446 | 0.396 | 97.1 | 99.1 | |
| 4 | 18.2–24.5 | 20.9 | 0.312–0.549 | 0.397 | 98.1 | 98.0 | |
| 6 | 13.2–20.2 | 15.6 | 0.187–0.469 | 0.260 | 98.3 | 98.9 | |
| 8 | 23.9–37.2 | 31.1 | 0.324–0.534 | 0.415 | 98.7 | 98.0 | |
| 10 | 24.5–32.4 | 29.4 | 0.391–0.557 | 0.457 | 98.4 | 99.0 | |
| Kaolin (mg/L) | Humic Acid (mg/L) | UV254 Absorbance (cm−1) | Average Treatment Efficiency (%) | ||||
|---|---|---|---|---|---|---|---|
| Feed Water | Treated Water | Adsorption Media | |||||
| Range | Average | Range | Average | 7 Channels Alumina MF | Tubular Carbon Fiber UF [22] | ||
| 30 | 0 | 0.003–0.005 | 0.004 | 0.002–0.004 | 0.003 | 14.3 | - |
| 2 | 0.147–0.176 | 0.161 | 0.110–0.140 | 0.131 | 18.6 | 67.2 | |
| 4 | 0.132–0.241 | 0.164 | 0.121–0.147 | 0.132 | 19.5 | 65.9 | |
| 6 | 0.164–0.211 | 0.186 | 0.131–0.151 | 0.145 | 22.0 | 69.3 | |
| 8 | 0.184–0.370 | 0.244 | 0.135–0.152 | 0.146 | 40.3 | 59.3 | |
| 10 | 0.203–0.302 | 0.247 | 0.110–0.142 | 0.124 | 49.7 | 60.9 | |
| Membrane | PP Beads (g/L) | 0 | 5 | 10 | 20 | 30 | 40 | 50 |
|---|---|---|---|---|---|---|---|---|
| 7-channel alumina MF (HC10) | Rm × 10−9 (kg/m2s) | 1.24 | 1.27 | 1.53 | 1.75 | 1.82 | 1.93 | 1.49 |
| Rb × 10−9 (kg/m2s) | 0.020 | 0.024 | 0.106 | 0.009 | 0.041 | 0.142 | 0.076 | |
| Rf,180 × 10−9 (kg/m2s) | 4.23 | 4.35 | 6.79 | 7.15 | 7.09 | 9.83 | 12.94 | |
| Rif × 10−9 (kg/m2s) | 1.75 | 1.24 | 1.20 | 3.24 | 0.66 | 0.43 | 1.44 | |
| Rrf × 10−9 (kg/m2s) | 2.486 | 3.110 | 5.587 | 3.910 | 6.423 | 9.403 | 11.500 | |
| J0 (L/m2h) | 503 | 491 | 388 | 361 | 342 | 307 | 405 | |
| J180 (L/m2h) | 116 | 113 | 75 | 71 | 71 | 53 | 44 | |
| J180/J0 | 0.230 | 0.229 | 0.194 | 0.198 | 0.208 | 0.174 | 0.108 | |
| VT (L) | 10.11 | 11.75 | 7.56 | 6.94 | 7.91 | 6.55 | 6.85 | |
| Tubular carbon fiber UF (C005) [23] | Rm × 10−9 (kg/m2s) | 0.413 | 0.409 | 0.409 | 0.411 | 0.403 | 0.405 | 0.403 |
| Rb × 10−9 (kg/m2s) | 0.062 | 0.043 | 0.119 | 0.031 | 0.042 | 0.048 | 0.009 | |
| Rf,180 × 10−9 (kg/m2s) | 3.306 | 3.596 | 3.683 | 4.767 | 4.967 | 4.918 | 4.892 | |
| Rif × 10−9 (kg/m2s) | 0.008 | 0.004 | 0.021 | 0.006 | 0.015 | 0.020 | 0.002 | |
| Rrf × 10−9 (kg/m2s) | 3.298 | 3.596 | 3.662 | 4.761 | 4.953 | 4.898 | 4.890 | |
| J0 (L/m2h) | 1336 | 1407 | 1221 | 1435 | 1426 | 1401 | 1543 | |
| J180 (L/m2h) | 168 | 157 | 151 | 122 | 117 | 118 | 120 | |
| J180/J0 | 0.126 | 0.112 | 0.124 | 0.085 | 0.082 | 0.084 | 0.078 | |
| VT (L) | 4.13 | 4.99 | 3.54 | 3.35 | 2.73 | 3.16 | 3.29 |
| PP Beads (g/L) | Turbidity (NTU) | Average Treatment Efficiency (%) | ||||
|---|---|---|---|---|---|---|
| Feed Water | Treated Water | 7 Channels Alumina MF | Tubular Carbon Fiber UF [23] | |||
| Range | Average | Range | Average | |||
| 0 | 20.6–25.9 | 23.1 | 0.207–0.326 | 0.264 | 98.9 | 98.6 |
| 5 | 22.8–27.3 | 25.5 | 0.338–0.587 | 0.438 | 98.3 | 98.5 |
| 10 | 22.8–25.7 | 24.4 | 0.297–0.434 | 0.349 | 98.6 | 99.1 |
| 20 | 20.7–28.8 | 24.0 | 0.298–0.408 | 0.341 | 98.6 | 97.9 |
| 30 | 15.3–20.4 | 17.8 | 0.353–0.542 | 0.447 | 97.5 | 99.3 |
| 40 | 22.8–25.8 | 24.1 | 0.254–0.333 | 0.297 | 98.8 | 98.7 |
| 50 | 27.3–34.3 | 29.7 | 0.308–0.354 | 0.339 | 98.9 | 98.4 |
| PP Beads (g/L) | UV254 Absorbance (cm−1) | Average Treatment Efficiency (%) | ||||
|---|---|---|---|---|---|---|
| Feed Water | Treated Water | 7 Channels Alumina MF | Tubular Carbon Fiber UF [23] | |||
| Range | Average | Range | Average | |||
| 0 | 0.339–0.411 | 0.389 | 0.145–0.265 | 0.205 | 47.4 | 75.9 |
| 5 | 0.498–0.569 | 0.526 | 0.214–0.298 | 0.256 | 51.3 | 77.0 |
| 10 | 0.399–0.487 | 0.431 | 0.185–0.251 | 0.220 | 48.9 | 77.8 |
| 20 | 0.305–0.363 | 0.342 | 0.155–0.228 | 0.182 | 46.8 | 83.2 |
| 30 | 0.403–0.598 | 0.527 | 0.192–0.423 | 0.273 | 48.1 | 82.3 |
| 40 | 0.318–0.359 | 0.335 | 0.154–0.177 | 0.168 | 49.8 | 82.4 |
| 50 | 0.310–0.410 | 0.374 | 0.159–0.215 | 0.200 | 46.5 | 84.1 |
| Membrane | pH | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|
| 7-channel alumina MF (HC10) | Rm × 10−9 (kg/m2s) | 1.87 | 1.89 | 1.97 | 2.07 | 2.27 |
| Rb × 10−9 (kg/m2s) | 0.071 | 0.060 | 0.065 | 0.136 | 0.080 | |
| Rf,180 × 10−9 (kg/m2s) | 4.41 | 7.11 | 9.86 | 12.25 | 20.20 | |
| Rif × 10−9 (kg/m2s) | 0.60 | 0.63 | 0.88 | 3.47 | 0.20 | |
| Rrf × 10−9 (kg/m2s) | 3.802 | 6.472 | 8.975 | 8.780 | 19.997 | |
| J0 (L/m2h) | 100 | 70 | 53 | 44 | 28 | |
| J180 (L/m2h) | 327 | 326 | 313 | 288 | 270 | |
| J180/J0 | 0.306 | 0.215 | 0.171 | 0.152 | 0.104 | |
| VT (L) | 8.12 | 7.00 | 6.30 | 6.26 | 3.87 | |
| Tubular carbon fiber UF (C005) [23] | Rm × 10−9 (kg/m2s) | 0.412 | 0.413 | 0.405 | 0.405 | 0.401 |
| Rb × 10−9 (kg/m2s) | 0.001 | 0.047 | 0.048 | 0.020 | 0.003 | |
| Rf,180 × 10−9 (kg/m2s) | 4.87 | 4.16 | 4.92 | 4.62 | 5.51 | |
| Rif × 10−9 (kg/m2s) | 0.003 | 0.010 | 0.020 | 0.006 | 0.020 | |
| Rrf × 10−9 (kg/m2s) | 4.865 | 4.149 | 4.898 | 4.616 | 5.487 | |
| J0 (L/m2h) | 1537 | 1382 | 1401 | 1494 | 1573 | |
| J180 (L/m2h) | 120 | 138 | 118 | 126 | 107 | |
| J180/J0 | 0.078 | 0.099 | 0.084 | 0.084 | 0.068 | |
| VT (L) | 3.30 | 3.68 | 3.17 | 3.85 | 3.21 |
| pH | Turbidity (NTU) | Average Treatment Efficiency (%) | ||||
|---|---|---|---|---|---|---|
| Feed Water | Treated Water | 7 Channels Alumina MF | Tubular Carbon Fiber UF [23] | |||
| Range | Average | Range | Average | |||
| 5 | 21.9–25.1 | 23.4 | 0.297–0.379 | 0.331 | 98.6 | 98.8 |
| 6 | 22.4–24.5 | 23.5 | 0.229–0.347 | 0.321 | 98.6 | 98.8 |
| 7 | 23.9–26.0 | 25.0 | 0.387–0.439 | 0.411 | 98.4 | 98.7 |
| 8 | 23.4–25.7 | 24.5 | 0.411–0.511 | 0.471 | 98.1 | 99.0 |
| 9 | 20.6–21.4 | 20.9 | 0.399–0.487 | 0.423 | 98.0 | 98.7 |
| pH | UV254 Absorbance (cm−1) | Average Treatment Efficiency (%) | ||||
|---|---|---|---|---|---|---|
| Feed Water | Treated Water | 7 Channels Alumina MF | Tubular Carbon Fiber UF [23] | |||
| Range | Average | Range | Average | |||
| 5 | 0.421–0.511 | 0.493 | 0.211–0.264 | 0.243 | 50.7 | 85.1 |
| 6 | 0.478–0.513 | 0.499 | 0.219–0.289 | 0.252 | 49.4 | 81.6 |
| 7 | 0.489–0.509 | 0.497 | 0.239–0.269 | 0.256 | 48.6 | 82.4 |
| 8 | 0.458–0.527 | 0.493 | 0.222–0.279 | 0.253 | 48.7 | 82.3 |
| 9 | 0.511–0.521 | 0.516 | 0.213–0.271 | 0.245 | 52.5 | 81.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Lee, Y.; Park, J.Y. The Role of Humic Acid, PP Beads, and pH with Water Backwashing in a Hybrid Water Treatment of Multichannel Alumina Microfiltration and PP Beads. Membranes 2020, 10, 3. https://doi.org/10.3390/membranes10010003
Hwang S, Lee Y, Park JY. The Role of Humic Acid, PP Beads, and pH with Water Backwashing in a Hybrid Water Treatment of Multichannel Alumina Microfiltration and PP Beads. Membranes. 2020; 10(1):3. https://doi.org/10.3390/membranes10010003
Chicago/Turabian StyleHwang, Sungju, Yooju Lee, and Jin Yong Park. 2020. "The Role of Humic Acid, PP Beads, and pH with Water Backwashing in a Hybrid Water Treatment of Multichannel Alumina Microfiltration and PP Beads" Membranes 10, no. 1: 3. https://doi.org/10.3390/membranes10010003
APA StyleHwang, S., Lee, Y., & Park, J. Y. (2020). The Role of Humic Acid, PP Beads, and pH with Water Backwashing in a Hybrid Water Treatment of Multichannel Alumina Microfiltration and PP Beads. Membranes, 10(1), 3. https://doi.org/10.3390/membranes10010003