Design of Monovalent Ion Selective Membranes for Reducing the Impacts of Multivalent Ions in Reverse Electrodialysis
Abstract
:1. Introduction
2. Transport Phenomenon in RED
2.1. Key Performance Parameters of RED
2.2. Co-Ion Transport
2.3. Osmotic Transport
2.4. Electro-Osmosis
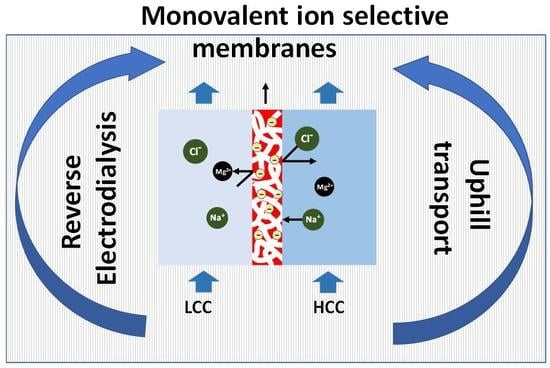
2.5. Uphill Transport
3. Impact of Multivalent Ions at Different Salinity Levels
3.1. Impact under Low Feed Salinity Conditions
3.2. Impact under High Feed Salinity Conditions
4. Strategies to Alleviate the Impact of Multivalent Ions on RED
4.1. Feed Pre-Treatment
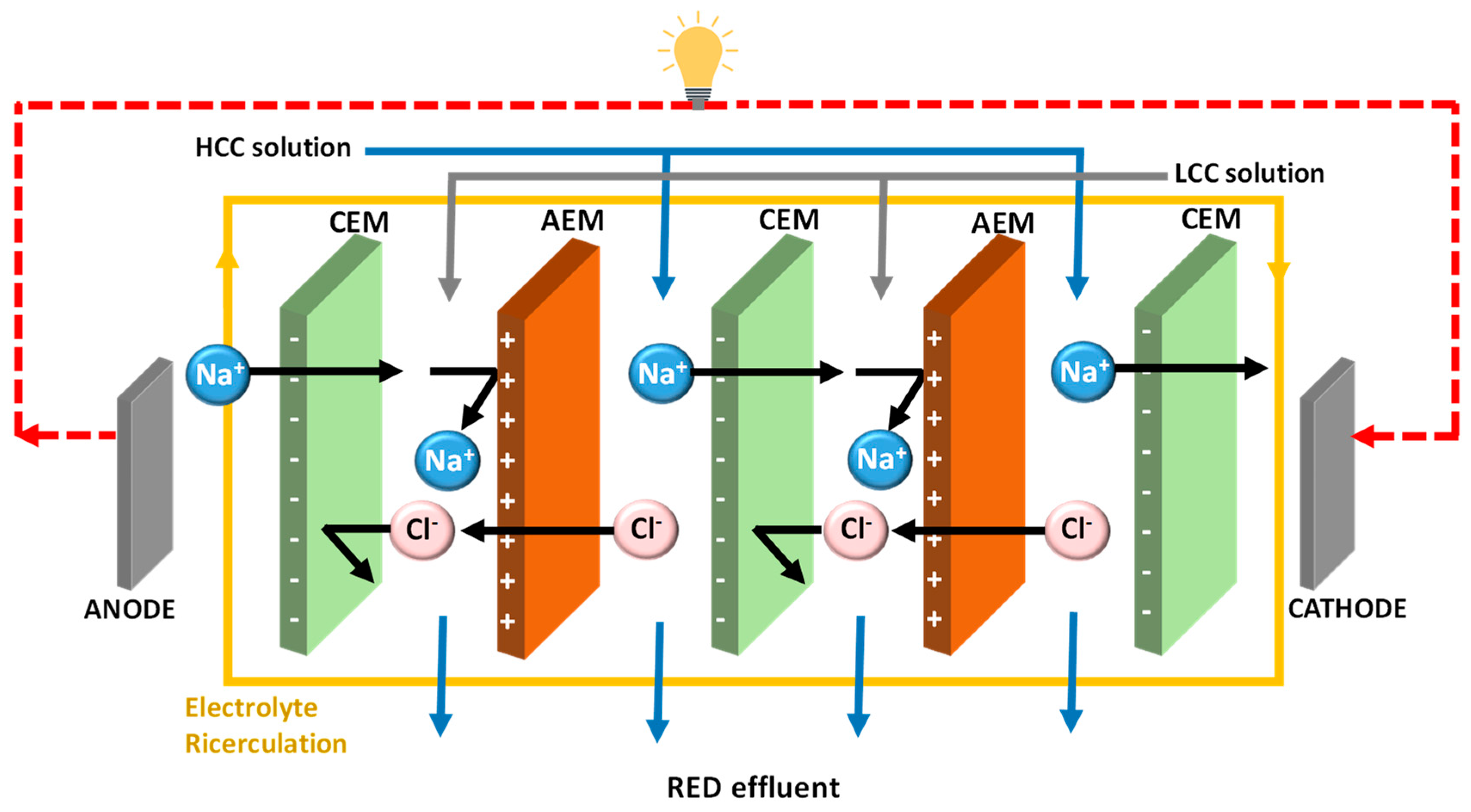
4.2. Monovalent Selective Ion Exchange Membranes
5. Strategies for Developing Monovalent Ion Selective Membranes
5.1. Surface Modification
5.2. Bulk Modification
5.3. Layer-By-Layer Deposition
6. Prospects in the Use of Conducting Polymers for RED
7. Other Prospects of Selective Ion Exchange Membranes
8. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations and Symbols
| AEM | Anion exchange membrane |
| CEM | Cation exchange membrane |
| ED | Electrodialysis |
| HCC | High concentration compartment |
| IEM | Ion exchange membrane |
| LbL | Layer by layer |
| LCC | Low concentration compartment |
| NOM | Natural organic matter |
| Pd,max | Maximum power density |
| Rnon-omic | Non-ohmic resistance |
| Rohmic | Ohmic resistance |
| OCV | Open-circuit voltage |
| α | Permselectivity |
| Pd | Power density |
| RED | Reverse electrodialysis |
| RO | Reverse osmosis |
| SGP | Salinity gradient power |
| SWRO | Seawater reverse osmosis |
| Rstack | Stack resistance |
References
- BP. BP Statstical Review of World Energy. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2019-full-report.pdf (accessed on 4 October 2019).
- Tufa, R.A.; Curcio, E.; Fontananova, E.; Di Profio, G. 3.8 Membrane-based processes for sustainable power generation using water: Pressure-retarded osmosis (PRO), reverse electrodialysis (RED), and capacitive mixing (CAPMIX). Compr. Membr. Sci. Eng. 2017, 2, 206–248. [Google Scholar]
- Tufa, R.A.; Pawlowski, S.; Veerman, J.; Bouzek, K.; Fontananova, E.; di Profio, G.; Velizarov, S.; Crespo, J.G.; Nijmeijer, K.; Curcio, E. Progress and prospects in reverse electrodialysis for salinity gradient energy conversion and storage. Appl. Energy 2018, 225, 290–331. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Avci, A.H.; Sarkar, P.; Tufa, R.A.; Messana, D.; Argurio, P.; Fontananova, E.; Di Profio, G.; Curcio, E. Effect of Mg2+ ions on energy generation by Reverse Electrodialysis. J. Membr. Sci. 2016, 520, 499–506. [Google Scholar] [CrossRef]
- Avci, A.H.; Tufa, R.A.; Fontananova, E.; Di Profio, G.; Curcio, E. Reverse Electrodialysis for energy production from natural river water and seawater. Energy 2018, 165, 512–521. [Google Scholar] [CrossRef]
- Post, J.W.; Hamelers, H.V.; Buisman, C.J. Influence of multivalent ions on power production from mixing salt and fresh water with a reverse electrodialysis system. J. Membr. Sci. 2009, 330, 65–72. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Veerman, J.; Saakes, M.; Nijmeijer, K. Influence of multivalent ions on renewable energy generation in reverse electrodialysis. Energy Environ. Sci. 2014, 7, 1434–1445. [Google Scholar] [CrossRef] [Green Version]
- Tufa, R.A.; Curcio, E.; van Baak, W.; Veerman, J.; Grasman, S.; Fontananova, E.; Di Profio, G. Potential of brackish water and brine for energy generation by salinity gradient power-reverse electrodialysis (SGP-RE). RSC Adv. 2014, 4, 42617–42623. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Hong, J.G.; Zhang, B.; Glabman, S.; Uzal, N.; Dou, X.; Zhang, H.; Wei, X.; Chen, Y. Potential ion exchange membranes and system performance in reverse electrodialysis for power generation: A review. J. Membr. Sci. 2015, 486, 71–88. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, F.; Zhang, G.; Luo, H.; Liu, L.; Shen, M.; Yang, Y. A novel two-dimensional coordination polymer-polypyrrole hybrid material as a high-performance electrode for flexible supercapacitor. Chem. Eng. J. 2018, 334, 2547–2557. [Google Scholar] [CrossRef]
- Ge, L.; Wu, B.; Yu, D.; Mondal, A.N.; Hou, L.; Afsar, N.U.; Li, Q.; Xu, T.; Miao, J.; Xu, T. Monovalent cation perm-selective membranes (MCPMs): New developments and perspectives. Chin. J. Chem. Eng. 2017, 25, 1606–1615. [Google Scholar] [CrossRef]
- Veerman, J.; Vermaas, D. Reverse electrodialysis: Fundamentals. In Sustainable Energy from Salinity Gradients; Elsevier: Amsterdam, The Netherlands, 2016; pp. 77–133. [Google Scholar]
- Tedesco, M.; Hamelers, H.; Biesheuvel, P. Nernst-Planck transport theory for (reverse) electrodialysis: II. Effect of water transport through ion-exchange membranes. J. Membr. Sci. 2017, 531, 172–182. [Google Scholar] [CrossRef] [Green Version]
- Tedesco, M.; Hamelers, H.; Biesheuvel, P. Nernst-Planck transport theory for (reverse) electrodialysis: I. Effect of co-ion transport through the membranes. J. Membr. Sci. 2016, 510, 370–381. [Google Scholar] [CrossRef]
- Nikonenko, V.; Zabolotsky, V.; Larchet, C.; Auclair, B.; Pourcelly, G. Mathematical description of ion transport in membrane systems. Desalination 2002, 147, 369–374. [Google Scholar] [CrossRef]
- Kraaijeveld, G.; Sumberova, V.; Kuindersma, S.; Wesselingh, H. Modelling electrodialysis using the Maxwell-Stefan description. Chem. Eng. J. Biochem. Eng. J. 1995, 57, 163–176. [Google Scholar] [CrossRef]
- Tedesco, M.; Cipollina, A.; Tamburini, A.; Bogle, I.D.L.; Micale, G. A simulation tool for analysis and design of reverse electrodialysis using concentrated brines. Chem. Eng. Res. Des. 2015, 93, 441–456. [Google Scholar] [CrossRef] [Green Version]
- Veerman, J.; De Jong, R.; Saakes, M.; Metz, S.; Harmsen, G. Reverse electrodialysis: Comparison of six commercial membrane pairs on the thermodynamic efficiency and power density. J. Membr. Sci. 2009, 343, 7–15. [Google Scholar] [CrossRef]
- Kingsbury, R.S.; Chu, K.; Coronell, O. Energy storage by reversible electrodialysis: The concentration battery. J. Membr. Sci. 2015, 495, 502–516. [Google Scholar] [CrossRef]
- Yip, N.Y.; Vermaas, D.A.; Nijmeijer, K.; Elimelech, M. Thermodynamic, Energy Efficiency, and Power Density Analysis of Reverse Electrodialysis Power Generation with Natural Salinity Gradients. Environ. Sci. Technol. 2014, 48, 4925–4936. [Google Scholar] [CrossRef]
- Galama, A.; Vermaas, D.; Veerman, J.; Saakes, M.; Rijnaarts, H.; Post, J.; Nijmeijer, K. Membrane resistance: The effect of salinity gradients over a cation exchange membrane. J. Membr. Sci. 2014, 467, 279–291. [Google Scholar] [CrossRef]
- Moreno, J.; Díez, V.; Saakes, M.; Nijmeijer, K. Mitigation of the effects of multivalent ion transport in reverse electrodialysis. J. Membr. Sci. 2018, 550, 155–162. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Ji, Z.-Y.; Zhang, Y.-G.; Yang, F.-J.; Liu, J.; Zhao, Y.-Y.; Yuan, J.-S. Effect of ions (K+, Mg2+, Ca2+ and SO42−) and temperature on energy generation performance of reverse electrodialysis stack. Electrochim. Acta 2018, 290, 282–290. [Google Scholar] [CrossRef]
- Rijnaarts, T.; Huerta, E.; van Baak, W.; Nijmeijer, K. Effect of Divalent Cations on RED Performance and Cation Exchange Membrane Selection to Enhance Power Densities. Environ. Sci. Technol. 2017, 51, 13028–13035. [Google Scholar] [CrossRef]
- Gómez-Coma, L.; Ortiz-Martínez, V.M.; Carmona, J.; Palacio, L.; Prádanos, P.; Fallanza, M.; Ortiz, A.; Ibañez, R.; Ortiz, I. Modeling the influence of divalent ions on membrane resistance and electric power in reverse electrodialysis. J. Membr. Sci. 2019, 592, 117385. [Google Scholar] [CrossRef]
- Schaetzle, O.; Buisman, C.J. Salinity Gradient Energy: Current State and New Trends; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Daniilidis, A.; Vermaas, D.A.; Herber, R.; Nijmeijer, K. Experimentally obtainable energy from mixing river water, seawater or brines with reverse electrodialysis. Renew. Energy 2014, 64, 123–131. [Google Scholar] [CrossRef]
- Tedesco, M.; Brauns, E.; Cipollina, A.; Micale, G.; Modica, P.; Russo, G.; Helsen, J. Reverse electrodialysis with saline waters and concentrated brines: A laboratory investigation towards technology scale-up. J. Membr. Sci. 2015, 492, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Yip, N.Y.; Brogioli, D.; Hamelers, H.V.; Nijmeijer, K. Salinity gradients for sustainable energy: Primer, progress, and prospects. Environ. Sci. Technol. 2016, 50, 12072–12094. [Google Scholar] [CrossRef]
- Fontananova, E.; Messana, D.; Tufa, R.A.; Nicotera, I.; Kosma, V.; Curcio, E.; van Baak, W.; Drioli, E.; Di Profio, G. Effect of solution concentration and composition on the electrochemical properties of ion exchange membranes for energy conversion. J. Power Sources 2017, 340, 282–293. [Google Scholar] [CrossRef]
- Tedesco, M.; Scalici, C.; Vaccari, D.; Cipollina, A.; Tamburini, A.; Micale, G. Performance of the first reverse electrodialysis pilot plant for power production from saline waters and concentrated brines. J. Membr. Sci. 2016, 500, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Kingsbury, R.; Liu, F.; Zhu, S.; Boggs, C.; Armstrong, M.; Call, D.; Coronell, O. Impact of natural organic matter and inorganic solutes on energy recovery from five real salinity gradients using reverse electrodialysis. J. Membr. Sci. 2017, 541, 621–632. [Google Scholar] [CrossRef]
- Gómez-Coma, L.; Ortiz-Martínez, V.; Fallanza, M.; Ortiz, A.; Ibañez, R.; Ortiz, I. Blue energy for sustainable water reclamation in WWTPs. J. Water Process Eng. 2020, 33, 101020. [Google Scholar] [CrossRef]
- Kuno, M.; Yasukawa, M.; Kakihana, Y.; Higa, M. The Effect of Divalent Ions on Reverse Electrodialysis Power Generation System. Bull. Soc. Sea Water Sci. Jpn. 2017, 71, 350–351. [Google Scholar]
- Avci, A.; Sarkar, P.; Messana, D.; Fontananova, E.; Di, P.G.; Curcio, E. Effect of MgCl 2 on Energy Generation by Reverse Electrodialysis. Chem. Eng. Trans. 2016, 47, 361–366. [Google Scholar]
- Oh, Y.; Jeong, Y.; Han, S.-J.; Kim, C.-S.; Kim, H.; Han, J.-H.; Hwang, K.-S.; Jeong, N.; Park, J.-S.; Chae, S. Effects of Divalent Cations on Electrical Membrane Resistance in Reverse Electrodialysis for Salinity Power Generation. Ind. Eng. Chem. Res. 2018, 57, 15803–15810. [Google Scholar] [CrossRef]
- Tufa, R.A.; Piallat, T.; Hnát, J.; Fontananova, E.; Paidar, M.; Chanda, D.; Curcio, E.; di Profio, G.; Bouzek, K. Salinity gradient power reverse electrodialysis: Cation exchange membrane design based on polypyrrole-chitosan composites for enhanced monovalent selectivity. Chem. Eng. J. 2020, 380, 122461. [Google Scholar] [CrossRef]
- Tedesco, M.; Cipollina, A.; Tamburini, A.; van Baak, W.; Micale, G. Modelling the Reverse ElectroDialysis process with seawater and concentrated brines. Desalin. Water Treat. 2012, 49, 404–424. [Google Scholar] [CrossRef] [Green Version]
- Di Salvo, J.L.; Cosenza, A.; Tamburini, A.; Micale, G.; Cipollina, A. Long-run operation of a reverse electrodialysis system fed with wastewaters. J. Environ. Manag. 2018, 217, 871–887. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Kunteng, D.; Veerman, J.; Saakes, M.; Nijmeijer, K. Periodic feedwater reversal and air sparging as antifouling strategies in reverse electrodialysis. Environ. Sci. Technol. 2014, 48, 3065–3073. [Google Scholar] [CrossRef]
- Długołęcki, P.; Nymeijer, K.; Metz, S.; Wessling, M. Current status of ion exchange membranes for power generation from salinity gradients. J. Membr. Sci. 2008, 319, 214–222. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Koninckx, A.; Vandecasteele, C. Separation of monovalent and divalent ions from aqueous solution by electrodialysis and nanofiltration. Water Res. 2004, 38, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Paepen, S.; Pinoy, L.; Meesschaert, B.; Van der Bruggen, B. Selectrodialysis: Fractionation of divalent ions from monovalent ions in a novel electrodialysis stack. Sep. Purif. Technol. 2012, 88, 191–201. [Google Scholar] [CrossRef]
- Rijnaarts, T.; Shenkute, N.T.; Wood, J.A.; de Vos, W.M.; Nijmeijer, K. Divalent cation removal by Donnan dialysis for improved reverse electrodialysis. ACS Sustain. Chem. Eng. 2018, 6, 7035–7041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besha, A.T.; Gebreyohannes, A.Y.; Tufa, R.A.; Bekele, D.N.; Curcio, E.; Giorno, L. Removal of emerging micropollutants by activated sludge process and membrane bioreactors and the effects of micropollutants on membrane fouling: A review. J. Environ. Chem. Eng. 2017, 5, 2395–2414. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, B.; Tong, X.; Chen, Y. Monovalent-anion selective and antifouling polyelectrolytes multilayer anion exchange membrane for reverse electrodialysis. J. Membr. Sci. 2018, 567, 68–75. [Google Scholar] [CrossRef]
- Güler, E.; van Baak, W.; Saakes, M.; Nijmeijer, K. Monovalent-ion-selective membranes for reverse electrodialysis. J. Membr. Sci. 2014, 455, 254–270. [Google Scholar] [CrossRef]
- Mulyati, S.; Takagi, R.; Fujii, A.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Improvement of the antifouling potential of an anion exchange membrane by surface modification with a polyelectrolyte for an electrodialysis process. J. Membr. Sci. 2012, 417, 137–143. [Google Scholar] [CrossRef]
- Mulyati, S.; Takagi, R.; Fujii, A.; Ohmukai, Y.; Matsuyama, H. Simultaneous improvement of the monovalent anion selectivity and antifouling properties of an anion exchange membrane in an electrodialysis process, using polyelectrolyte multilayer deposition. J. Membr. Sci. 2013, 431, 113–120. [Google Scholar] [CrossRef]
- Hao, L.; Liao, J.; Jiang, Y.; Zhu, J.; Li, J.; Zhao, Y.; Van der Bruggen, B.; Sotto, A.; Shen, J. “Sandwich”-like structure modified anion exchange membrane with enhanced monovalent selectivity and fouling resistant. J. Membr. Sci. 2018, 556, 98–106. [Google Scholar] [CrossRef]
- White, N.; Misovich, M.; Yaroshchuk, A.; Bruening, M.L. Coating of Nafion Membranes with Polyelectrolyte Multilayers to Achieve High Monovalent/Divalent Cation Electrodialysis Selectivities. ACS Appl. Mater. Interfaces 2015, 7, 6620–6628. [Google Scholar] [CrossRef]
- Abdu, S.; Martí-Calatayud, M.-C.; Wong, J.E.; García-Gabaldón, M.; Wessling, M. Layer-by-Layer Modification of Cation Exchange Membranes Controls Ion Selectivity and Water Splitting. ACS Appl. Mater. Interfaces 2014, 6, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ding, J.; Tan, R.; Chen, G.; Zhao, Y.; Gao, C.; der Bruggen, B.V.; Shen, J. Preparation of a monovalent selective anion exchange membrane through constructing a covalently crosslinked interface by electro-deposition of polyethyleneimine. J. Membr. Sci. 2017, 539, 263–272. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, J.; Ding, J.; Van der Bruggen, B.; Shen, J.; Gao, C. Electric-pulse layer-by-layer assembled of anion exchange membrane with enhanced monovalent selectivity. J. Membr. Sci. 2018, 548, 81–90. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, Y.; Ding, J.; Shi, W.; Pan, J.; Gao, C.; Shen, J.; van der Bruggen, B. Surface layer modification of AEMs by infiltration and photo-cross-linking to induce monovalent selectivity. Aiche J. 2018, 64, 993–1000. [Google Scholar] [CrossRef]
- Liu, H.; Ruan, H.; Zhao, Y.; Pan, J.; Sotto, A.; Gao, C.; Van der Bruggen, B.; Shen, J. A facile avenue to modify polyelectrolyte multilayers on anion exchange membranes to enhance monovalent selectivity and durability simultaneously. J. Membr. Sci. 2017, 543, 310–318. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, K.; Liu, H.; Van der Bruggen, B.; Díaz, A.S.; Shen, J.; Gao, C. An anion exchange membrane modified by alternate electro-deposition layers with enhanced monovalent selectivity. J. Membr. Sci. 2016, 520, 262–271. [Google Scholar] [CrossRef]
- Pan, J.; Ding, J.; Zheng, Y.; Gao, C.; Van der Bruggen, B.; Shen, J. One-pot approach to prepare internally cross-linked monovalent selective anion exchange membranes. J. Membr. Sci. 2018, 553, 43–53. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, C.; Van der Bruggen, B. Technology-driven layer-by-layer assembly of a membrane for selective separation of monovalent anions and antifouling. Nanoscale 2019, 11, 2264–2274. [Google Scholar] [CrossRef]
- Reig, M.; Farrokhzad, H.; Van der Bruggen, B.; Gibert, O.; Cortina, J.L. Synthesis of a monovalent selective cation exchange membrane to concentrate reverse osmosis brines by electrodialysis. Desalination 2015, 375, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhu, J.; Li, J.; Zhao, Z.; Charchalac Ochoa, S.I.; Shen, J.; Gao, C.; Van der Bruggen, B. Robust Multilayer Graphene–Organic Frameworks for Selective Separation of Monovalent Anions. ACS Appl. Mater. Interfaces 2018, 10, 18426–18433. [Google Scholar] [CrossRef]
- Li, J.; Zhou, M.-L.; Lin, J.-Y.; Ye, W.-Y.; Xu, Y.-Q.; Shen, J.-N.; Gao, C.-J.; Van der Bruggen, B. Mono-valent cation selective membranes for electrodialysis by introducing polyquaternium-7 in a commercial cation exchange membrane. J. Membr. Sci. 2015, 486, 89–96. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Penkova, A.; Kuzminova, A.; Missyul, A.; Ermakov, S.; Roizard, D. Development and characterization of new pervaporation PVA membranes for the dehydration using bulk and surface modifications. Polymers 2018, 10, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tas, S.; Zoetebier, B.; Hempenius, M.A.; Vancso, G.J.; Nijmeijer, K. Monovalent cation selective crown ether containing poly (arylene ether ketone)/SPEEK blend membranes. RSC Adv. 2016, 6, 55635–55642. [Google Scholar] [CrossRef] [Green Version]
- Balster, J.; Krupenko, O.; Pünt, I.; Stamatialis, D.; Wessling, M. Preparation and characterisation of monovalent ion selective cation exchange membranes based on sulphonated poly (ether ether ketone). J. Membr. Sci. 2005, 263, 137–145. [Google Scholar] [CrossRef]
- Ge, L.; Liu, X.; Wang, G.; Wu, B.; Wu, L.; Bakangura, E.; Xu, T. Preparation of proton selective membranes through constructing H+ transfer channels by acid–base pairs. J. Membr. Sci. 2015, 475, 273–280. [Google Scholar] [CrossRef]
- Guler, E.; Nijmeijer, K. Reverse Electrodialysis for Salinity Gradient Power Generation: Challenges and Future Perspectives. J. Membr. Sci. Res. 2018, 4, 108–110. [Google Scholar]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar] [CrossRef]
- Chen, M.; Wang, M.; Yang, Z.; Ding, X.; Li, Q.; Wang, X. A novel catalyst layer structure based surface-patterned Nafion® membrane for high-performance direct methanol fuel cell. Electrochim. Acta 2018, 263, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Materialstoday 2019. [Google Scholar] [CrossRef]
- Jia, N.; Lefebvre, M.C.; Halfyard, J.; Qi, Z.; Pickup, P.G. Modification of Nafion proton exchange membranes to reduce methanol crossover in PEM fuel cells. Electrochem. Solid-State Lett. 2000, 3, 529–531. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [Green Version]
- Hnát, J.; Plevova, M.; Tufa, R.A.; Zitka, J.; Paidar, M.; Bouzek, K. Development and testing of a novel catalyst-coated membrane with platinum-free catalysts for alkaline water electrolysis. Int. J. Hydrogen Energy 2019, 44, 17493–17504. [Google Scholar] [CrossRef]
- Kraglund, M.R.; Carmo, M.; Schiller, G.; Ansar, S.A.; Aili, D.; Christensen, E.; Jensen, J.O.J.E.; Science, E. Ion-solvating membranes as a new approach towards high rate alkaline electrolyzers. Energy Environ. Sci. 2019, 12, 3313–3318. [Google Scholar] [CrossRef] [Green Version]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Tsehaye, M.T.; Alloin, F.; Iojoiu, C. Prospects for Anion-Exchange Membranes in Alkali Metal-Air Batteries. Energies 2019, 12, 4702. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-C.; Lin, S.-J.; Hsu, S.-T. Synthesis and characterization of alkaline polyvinyl alcohol and poly(epichlorohydrin) blend polymer electrolytes and performance in electrochemical cells. J. Power Sources 2003, 122, 210–218. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Luo, Q.; Li, L.; Wang, W.; Nie, Z.; Wei, X.; Li, B.; Chen, B.; Yang, Z.; Sprenkle, V. Capacity Decay and Remediation of Nafion-based All-Vanadium Redox Flow Batteries. ChemSusChem 2013, 6, 268–274. [Google Scholar] [CrossRef]
- Xi, J.; Wu, Z.; Teng, X.; Zhao, Y.; Chen, L.; Qiu, X. Self-assembled polyelectrolyte multilayer modified Nafion membrane with suppressed vanadium ion crossover for vanadium redox flow batteries. J. Mater. Chem. 2008, 18, 1232–1238. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Cao, J.; Zhang, H.Z.; Zhang, H.M. Membranes with well-defined ions transport channels fabricated via solvent-responsive layer-by-layer assembly method for vanadium flow battery. Sci. Rep. 2014, 4, 4016. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Eigenberger, G.; Strathmann, H.; Nieken, U. Flow battery based on reverse electrodialysis with bipolar membranes: Single cell experiments. J. Membr. Sci. 2018, 565, 157–168. [Google Scholar] [CrossRef]
- Luo, J.; Vermaas, D.A.; Bi, D.; Hagfeldt, A.; Smith, W.A.; Grätzel, M. Bipolar Membrane-Assisted Solar Water Splitting in Optimal pH. Adv. Energy Mater. 2016, 6, 1600100. [Google Scholar] [CrossRef]
- Vargas-Barbosa, N.M.; Geise, G.M.; Hickner, M.A.; Mallouk, T.E. Assessing the Utility of Bipolar Membranes for use in Photoelectrochemical Water-Splitting Cells. ChemSusChem 2014, 7, 3017–3020. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Wiegman, S.; Nagaki, T.; Smith, W.A. Ion transport mechanisms in bipolar membranes for (photo)electrochemical water splitting. Sustain. Energy Fuels 2018, 2, 2006–2015. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Smith, W.A. Synergistic Electrochemical CO2 Reduction and Water Oxidation with a Bipolar Membrane. ACS Energy Lett. 2016, 1, 1143–1148. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhou, D.; Yan, Z.; Gonçalves, R.H.; Salvatore, D.A.; Berlinguette, C.P.; Mallouk, T.E. Electrolysis of CO2 to Syngas in Bipolar Membrane-Based Electrochemical Cells. ACS Energy Lett. 2016, 1, 1149–1153. [Google Scholar] [CrossRef]
- Moussaoui, R.E.; Pourcelly, G.; Maeck, M.; Hurwitz, H.D.; Gavach, C. Co-ion leakage through bipolar membranes Influence on I–V responses and water-splitting efficiency. J. Membr. Sci. 1994, 90, 283–292. [Google Scholar] [CrossRef]








| LCC (M) | HCC (M) | Membranes | (%) | Rstack | Pd, max (W/m2) | OCV (V) | Ref. |
|---|---|---|---|---|---|---|---|
| NaCl: 0.025 | NaCl: 0.75 | PC-SK and PC-SA | 84 | 69.9 Ωcm2 | 0.32 | 1.393 | [34] |
| 1 BW: Na+ (0.024), Cl− (0.032), SO42− (0.002), Ca2+ (0.00) Mg2+ (0.001), K+ (0.000) | 2 SW: Na+ (0.390), Cl− (0.578), SO42− (0.024), Ca2+ (0.027) Mg2+ (0.03), K+ (0.006) | 67 | 86.5 Ωcm2 | 0.11 | 0.926 | ||
| 3 RW: Na+ (0.004), Cl− (0.008), SO42− (0.000), Ca2+ (0.000) Mg2+ (0.000), K+ (0.000) | 4 SW: Na+ (0.39), Cl− (0.578), SO42− (0.024), Ca2+ (0.027) Mg2+ (0.03), K+ (0.006) | 72 | 150 Ωcm2 | 0.17 | 1.49 | ||
| 5 GW: Na+ (0.059), Cl− (0.093), SO42− (0.003), Ca2+ (0.001), Mg2+ (0.003), K+ (0.002) | 6 RO: Na+ (0.269), Cl− (0.409), SO42− (0.009), Ca2+ (0.004) Mg2+ (0.015), K+ (0.004) | 78 | 46.7 Ωcm2 | 0.07 | 0.53 | ||
| NaCl (0.1) | NaCl (0.5) | Fuji-AEM-80045 and Fuji-CEM-80050 | 96 | 4.59 Ωcm2 | - | - | [32] |
| NaCl (0.5) | NaCl (4) | 99 | 5.68 Ωcm2 | 0.96 | 1.71 | ||
| NaCl (0.1) | NaCl (5) | 89 | 7.58 Ωcm2 | 1.95 | 3.02 | ||
| NaCl (0.340) + MgCl2 (0.054) | NaCl (2.716) + MgCl2 (0.428) | 56 | 17.3 Ωcm2 | 0.67 | 1.47 | ||
| NaCl (0.473) + MgCl2 (0.014) | NaCl (3.78) + MgCl2 (0.11) | 63 | 8.58 Ωcm2 | 0.76 | 1.64 | ||
| NaCl (0.083) + MgCl2 (0.017) | NaCl (2.708) + MgCl2 (1.458) | 33 | 54.2 Ωcm2 | 0.60 | 1.32 | ||
| NaCl (0.03) | Brine : NaCl (5) + 2–3% Non-NaCl ions | Fujifilm AEM RP1 80045-01and Fujifilm CEM RP1 80050-04 | 90 | - | 2.7 | - | [33] |
| BW: NaCl (0.03) + K+, Mg2+, Ca2+, SO42− | Brine: NaCl (4–5) + K+, Mg2+, Ca2+, SO42− | 90 | - | 1.6 | - | ||
| BW: NaCl (0.1) | Brine; NaCl (5) | Fujifilm-AEM- 80045 and Fujifilm-CEM- 80050 | - | 3.83 Ω | 3.04 | 3.4 | [9] |
| BW Na+ (0.066), Cl− (1), SO42− (0.0035), Ca2+ (0.003) Mg2+ (0.0014), K+ (0.001), HCO3− (8.3 × 10−6) | Exhaust brine: Na+ (2.9), Cl− (4.8), SO42− (0.67), Ca2+ (0.006) Mg2+ (1.6), K+ (0.2), HCO3− (0.0008) | - | 6.76 Ω | 1.13 | 2.77 | ||
| NaCl: (0.0999975) + NaHCO3 (8.5 × 10−6), [Cl−]/[HCO3−] = 11,717 | NaCl: (4.99915) + NaHCO3 (8.5 × 10−4), [Cl−]/[HCO3−] = 5841 | - | 3.79 Ω | 3.03 | 3.39 | ||
| NaCl: (0.098) + KCl (0.002), [Na+]/[K+] = 52.1 | NaCl: (4.68) + KCl (0.32), [Na+]/[K+] = 14.5 | - | 4.08 Ω | 2.84 | 3.4 | ||
| NaCl: (0.096) + CaCl2 (0.004), [Na+]/[Ca2+] = 26.4 | NaCl: (4.99) + CaCl2 (0.01), [Na+]/[Ca2+] = 474 | - | 3.84 Ω | 2.84 | 3.27 | ||
| NaCl: (0.0966) + Na2SO4 (0.0034), [Na+]/[SO42−] = 28.8 | NaCl: (4.39) + Na2SO4 (0.61), [Na+]/[SO42−] = 7.15 | - | 4.15 Ω | 2.79 | 3.40 | ||
| NaCl: (0.083) + MgCl2 (0.017), [Na+]/[Mg2+] = 4.99 | NaCl: (3.25) + MgCl2 (1.75), [Na+]/[Mg2+] = 1.86 | - | 6.69 Ω | 1.11 | 2.73 | ||
| NaCl (0.5) | NaCl: (4) | Fujifilm-AEM- 80045 and Fujifilm-CEM- 80050 | - | 2.78 Ω | 1.06 | 1.70 | [5] |
| NaCl (0.45) + MgCl2 (0.05) | NaCl (3.60) + MgCl2 (0.40) | - | 4.44 Ω | 0.43 | 1.36 | ||
| NaCl (0.40) + MgCl2 (0.10) | NaCl (3.2) + MgCl2 (0.80) | - | 4.67 Ω | 0.36 | 1.3 | ||
| NaCl (0.30) + MgCl2 (0.20) | NaCl (2.40) + MgCl2 (1.6) | - | 5.11 Ω | 0.32 | 1.29 | ||
| NaCl (0.20) + MgCl2 (0.30) | NaCl (1.60) + MgCl2 (2.40) | - | 6.38 Ω | 0.21 | 1.15 | ||
| MgCl2 (0.50) | MgCl2 (4) | - | 8.92 Ω | 0.06 | 0.72 | ||
| RW: Na+ (0.001), Cl− (0.0005) | SW: Na+ (0.78), Cl− (0.59) | Fujifilm-AEM- 80045 and Fujifilm-CEM- 80050 | 68 | 12.8 Ω | 1.41 | 4.09 | [6] |
| RW: Na+ (0.001), Cl− (0.0005), K+ (0.0001), Mg2+ (0.001), Ca2+ (0.0038), SO42− (0.0001) | SW: Na+ (0.78), Cl− (0.59), K+ (0.017), Mg2+ (0.088), Ca2+ (0.01), SO42− (0.027) | 68 | 30.5 Ω | 0.46 | 3.68 | ||
| RW: NaCl (0.017) | SW: NaCl (0.513) | Fujifilm-CEM-Type I and Fujifilm-AEM- type I | - | 1.9 Ωcm2 | - | - | [38] |
| RW: NaCl (0.017) | SW: NaCl (0.4617 + 0.02565 (MgCl2) | - | 2.77 Ωcm2 | - | - | ||
| RW: NaCl (0.017) | SW: NaCl (0.4617 + 0.02565 (CaCl2) | - | 3.29 Ωcm2 | - | - | ||
| RW: NaCl (0.017) | SW: NaCl (0.4617 + 0.02565 (BaCl2) | - | 3.8 Ωcm2 | - | - | ||
| RW: NaCl (0.017) | SW: NaCl (0.5) | Fujifilm Type I AEM and homogeneous T0 CEM | - | - | - | 1 | [26] |
| RW: NaCl (0.0153) + MgCl2 (0.0017) | SW: NaCl (0.5) | - | - | - | 0.966 | ||
| RW: NaCl (0.0153) + MgCl2 (0.0017) | RW NaCl (0.45) + MgCl2 (0.05) | - | - | - | 0.925 | ||
| Pure NaCl: (0.5) | Pure NaCl (4) | Fujifilm-CEM T1 | 87–91 | 1.69 Ω/cm2 | 1 | 0.21 | [39] |
| NaCl (0.35) + MgCl2 (0.15) | NaCl (2.8) + MgCl2 (1.2) | - | - | 0.41 | 0.15 | ||
| Pure: NaCl: (0.008) | Pure: NaCl (0.5) | Fumatech-AEM-FKS-50 and Fumatech-CEM-FAS-50 | 0.92–0.96 | - | 1.6 | - | [35] |
| RW: Na+ (0.008), Mg2+ (0.0014), Ca2+ (0.0014), SO42− (0.00026) | RW: Na+ (0.5), Mg2+ (0.056), Ca2+ (0.009), SO42− (0.03) | 0.92–0.96 | - | 1.42 | - |
| LCC | HCC | Membrane | A (%) | Δ (μm) | R (Ωcm−2) | OCV(V) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 0.0153 M NaCl, 0.0017 M Na2SO4 10 mg/L HA sodium salt | 0.459 M NaCl, 0.051 M Na2SO4 10 mg/L HA sodium salt | AEM-CJMA-2 multi | 91.71 | 89 | - | - | 1.10 | [48] |
| 0.0153 M NaCl, 0.0017 M Na2SO4 10 mg/L HA sodium salt | 0.459 M NaCl, 0.051 M Na2SO4 10 mg/L HA sodium salt | AEM-ACS mono | 93.16 | 119 | - | - | 2.70 | |
| 0.0153 M NaCl, 0.0017 M Na2SO4 10 mg/L HA sodium salt | 0.459 M NaCl, 0.051 M Na2SO4 10 mg/L HA sodium salt | a AEM-CJMA-2 momo-TM δ | 90.05 | 102.7 | - | - | 2.44 | |
| RW: 0.012 M NaCl + 0.002 M Na2SO4 | SW: 0.45 M NaCl + 0.05 M Na2SO4 | AEM-Fuji A multi | 89 | 123 | 0.93 | 1.01 | 0.841 | [49] * |
| RW: 0.012 M NaCl + 0.002 M Na2SO4 | SW: 0.45 M NaCl + 0.05 M Na2SO4 | AEM-AMX multi | 90 | 134 | 2.35 | 0.90 | 0.832 | |
| RW: 0.012 M NaCl + 0.002 M Na2SO4 | SW: 0.45 M NaCl + 0.05 M Na2SO4 | AEM-ASV mono | 96 | 110 | 3.07 | - | 0.730 | |
| RW: 0.012 M NaCl + 0.002 M Na2SO4 | SW: 0.45 M NaCl + 0.05 M Na2SO4 | AEM-ACS mono | 94 | 121 | 4.39 | 0.85 | 0.727 | |
| RW: 0.012 M NaCl + 0.002 M Na2SO4 | SW: 0.45 M NaCl + 0.05 M Na2SO4 | b AEM-Fuji A mono-TM | 91 | 124 | 1.10 | 1.01 | 0.755 | |
| NaCl 0.35 M + MgCl2 0.15 M | NaCl 2.8 M + MgCl2 1.2 M | Fuji CEM-T1 multi | 87–91 | 117 | 1.69 | 0.15 | - | [39] |
| NaCl 0.35 M + MgCl2 0.15 M | NaCl 2.8 M + MgCl2 1.2 M | c Fuji CEM-T1 mono-TM | - | 122 | 2.12 | 0.17 | - | |
| 0.05 M NaCl + 0.05 M CaCl2 | CEM-CMX Neosepta standard | - | 160 | 3.5 | - | 0.64 | [54] ** | |
| 0.05 M NaCl + 0.05 M CaCl2 | CEM-CMS Neosepta mono | - | 130 | 3.49 | - | 1.23 | ||
| 0.05 M NaCl + 0.05 M CaCl2 | CEM-CSO Selemion mono | - | 90 | 4.09 | - | 1.72 | ||
| 0.05 M NaCl + 0.05 M CaCl2 | d CEM CMX Neosepta mono-TM | - | - | - | - | 1.24 | ||
| 0.05 M NaCl + 0.05 M MgCl2 | AEM-QPPO multi | - | - | 4.63 | - | 0.79 | [55] ** | |
| 0.05 M NaCl + 0.05 M MgCl2 | e AEM- QPPO-PEI- mono-TM | - | - | 5.30 | - | 4.19 | ||
| 0.02 M NaCl + 0.02 M Na2SO4 | Fuji AEM-T1 multi | - | 125 | 1.31 | - | 0.81 | [56] ** | |
| 0.02 M NaCl + 0.02 M Na2SO4 | AEM-ACS mono | - | 120–200 | 3–6 | - | 13.6 | ||
| 0.02 M NaCl + 0.02 M Na2SO4 | AEM-ASV mono | - | 120 | 3.1 | - | 22.3 | ||
| 0.02 M NaCl + 0.02 M Na2SO4 | AEM-AMX mono | - | 120–180 | 2–3.5 | - | - | ||
| 0.02 M NaCl + 0.02 M Na2SO4 | f Fuji AEM-T1 mono-TM | - | - | 2.20 | - | 47.04 | ||
| 0.05 M NaCl + 0.05 M Na2SO4 | AEM-commercial multi | - | 166 | 3.53 | - | 0.55 | [57] ** | |
| 0.05 M NaCl + 0.05 M Na2SO4 | g AEM mono-TM | - | - | 4.50 | - | 11.21 | ||
| 0.05 M NaCl + 0.05 M Na2SO4 | Fuji AEM-T1 multi | - | 125 | 1.30 | - | 0.39 | [58] ** | |
| 0.05 M NaCl + 0.05 M Na2SO4 | h Fuji AEM-T1 mono-TM | - | - | 3.97 | - | 4.36 | ||
| 0.05 M NaCl + 0.05 M Na2SO4 | Fuji CEM-T1 multi | - | - | 1.70 | - | 0.98 | [52] ** | |
| 0.05 M NaCl + 0.05 M Na2SO4 | i Fuji CEM-T1 mono-TM | - | - | 3.93 | - | 5.1 | ||
| 0.02 M NaCl + 0.02 M Na2SO4 | Fuji AEM multi | - | 125 | 1.31 | - | 0.66 | [59] ** | |
| 0.02 M NaCl + 0.02 M Na2SO4 | j Fuji AEM mono-TM | - | - | 4.52 | - | 2.90 | ||
| 0.05 M NaCl + 0.05 M Na2SO4 (pH = 6) | QPSF multi | - | - | 2.86 | - | 1.28 | [60] ** | |
| 0.05 M NaCl + 0.05 M Na2SO4 (pH = 6) | k QPSF-SF-0.05 mono-TM | - | - | 3.19 | - | 3.98 | ||
| 0.05 M NaCl + 0.05 M Na2SO4 (pH = 6) | k QPSF-SF-0.09 mono-TM | - | - | 4.04 | - | 15.90 | ||
| 0.05 M NaCl + 0.05 M Na2SO4 (pH = 6) | k QPSF-SF-0.17 mono-TM | - | - | 7.89 | - | 3.28 | ||
| - | Fuji CEM-T1 multi | - | 125 | 2.6 | - | 0.81 | [61] ** | |
| 50 mM Cl− + 50 mM Sulphate | lAEM-AC∼LbL#1.5 mono-TM | - | ∼125 | 3.18 | - | 1.42 | ||
| - | lAEM-AC∼LbL#3.5 mono-TM | - | ∼125 | 3.88 | - | 2.11 | ||
| - | lAEM-AC∼LbL#5.5 mono-TM | - | ∼125.5 | 4.94 | - | 3.71 | ||
| - | lAEM-AC∼LbL#7.5 mono-TM | - | ∼125.5 | 6.88 | - | 4.87 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besha, A.T.; Tsehaye, M.T.; Aili, D.; Zhang, W.; Tufa, R.A. Design of Monovalent Ion Selective Membranes for Reducing the Impacts of Multivalent Ions in Reverse Electrodialysis. Membranes 2020, 10, 7. https://doi.org/10.3390/membranes10010007
Besha AT, Tsehaye MT, Aili D, Zhang W, Tufa RA. Design of Monovalent Ion Selective Membranes for Reducing the Impacts of Multivalent Ions in Reverse Electrodialysis. Membranes. 2020; 10(1):7. https://doi.org/10.3390/membranes10010007
Chicago/Turabian StyleBesha, Abreham Tesfaye, Misgina Tilahun Tsehaye, David Aili, Wenjuan Zhang, and Ramato Ashu Tufa. 2020. "Design of Monovalent Ion Selective Membranes for Reducing the Impacts of Multivalent Ions in Reverse Electrodialysis" Membranes 10, no. 1: 7. https://doi.org/10.3390/membranes10010007
APA StyleBesha, A. T., Tsehaye, M. T., Aili, D., Zhang, W., & Tufa, R. A. (2020). Design of Monovalent Ion Selective Membranes for Reducing the Impacts of Multivalent Ions in Reverse Electrodialysis. Membranes, 10(1), 7. https://doi.org/10.3390/membranes10010007