3.1. Burst Pressure
The Müllen burst test was performed to determine the normalized burst pressure.
Figure 2 shows the normalized burst pressure as a function of treatment time at various NaOH concentrations and temperatures. The horizontal axis is plotted in logarithmic time. Regarding the electrochemical characteristics, when the AMX membrane was immersed in alkali at 40 °C for approximately 1 week, the water content increased, the electrical resistance decreased, and the ion selective permeability decreased [
1].
However, it is noteworthy that the burst pressure increased once and then decreased after 1 week of treatment. To the authors’ best knowledge, studies regarding the strength of AEMs being improved by alkali contact have not been reported.
In the alkali treatment at 40 °C, the maximum burst pressure was recorded at 24 h and 0.01 M, whereas the maximum was recorded at 0.1 M and 1 M at 3 h. These results suggest that under a higher NaOH concentration or temperature, the maximum burst pressure occurred at a shorter treatment time. At 0.1 M, the strength was almost double that of the pristine at 3 h and 150% at 168 h. The case of 0.1 M NaOH and 168 h was equivalent to 3 years of standard CIP conditions (1 h treatment with 0.1 M NaOH once a week).
In the treatment at 60 °C, the maximum value was recorded at 3 h, even at 0.01 M, and at 1 M, it was lower than the initial value after approximately 40 h.
At 80 °C, the strength increased from the initial value at treatment concentrations of 0.01 and 0.1 M for 3 h, but the strength of the samples for all the treatment concentrations decreased from the initial value after approximately 20 h.
When a membrane is subjected to the Müllen burst test, it is assumed that the tensile stress is generated in a two-dimensional direction on the membrane surface. Therefore, the increase in burst pressure can be associated with the increase in the tensile factors.
To clarify the reason for the burst pressure increase, a tensile test was performed.
3.2. Tensile Test
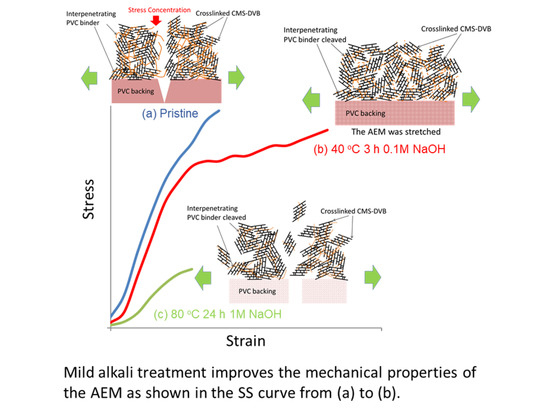
Figure 3 shows typical normalized SS curves for the AMX membrane: (a) a pristine membrane; (b) a membrane treated in 0.1 M NaOH for 3 h at 40 °C; and (c) a membrane treated in 1 M for 168 h at 80 °C. The pristine membrane showed little elongation before being fractured. In (b), a similar SS curve was observed before a yield point to the pristine membrane, but the membrane showed a higher strain. The higher strain might have contributed to the higher burst pressure. In (c), the AMX membrane indicated a much lower stress and strain.
As shown in
Figure 4, four factors were obtained from each SS curve, i.e., stress at break, strain at break, the Young’s modulus, and the area below the SS curve. Vasquez et al. investigated the area below the SS curve in the analysis of deteriorated AEMs [
4]. To determine the key factor that dominates the burst pressure, the four factors were plotted as a function of treatment time at various NaOH concentrations and temperatures; it was observed that the plots showed a similar trend to that of the burst pressure.
3.2.1. Stress at Break
Figure 5 shows the normalized stress at break as a function of treatment time at various NaOH concentrations and temperatures. As shown, the maximum point stress decreased monotonically as the alkali treatment time progressed. For 0.01, 0.1, and 1 M at 40 °C, the samples immersed in higher concentrations of alkali showed a lower stress at break. At 60 °C, the stress at break was lower than 40 °C, and it did not differ significantly between 0.1 and 1 M. However, it decreased further at 80 °C, and after 168 h, it remained almost the same for all concentrations. The plot trend of the stress at break differed from that of the burst pressure.
3.2.2. Strain at Break
Figure 6 shows the normalized strain at break as a function of treatment time at various NaOH concentrations and temperatures. Interestingly, the strain at break showed a significant increase. In the alkali treatment with 0.01 M NaOH at 40 °C, the strain at break increased with the treatment time, reaching a maximum value at 24 h, and then decreased. The maximum strain was almost twice the initial value. The maximum value was recorded at 3 h in case of 0.1 M NaOH. All points within 168 h exceeded the initial value. At 60 °C, the maximum strain at break appeared at 0.01 M for 24 h, but it decreased monotonically and decreased below the initial value for 0.1 and 1 M. At 80 °C, it decreased monotonically after 3 h. The plots of 1 M at 3 h, 0.1 M at 24 h, and 0.01 M at 168 h indicated values that were higher than the initial value. This trend differed from that of the burst pressure.
3.2.3. Young’s Modulus
Figure 7 shows the normalized Young’s modulus as a function of treatment time at various NaOH concentrations and temperatures. After the alkali treatment, the Young’s modulus decreased monotonically with time at all temperatures but decreased as the temperature increased. At 40 °C, the Young’s modulus for 0.01, 0.1, and 1 M decreased as the concentration increased; however, at 60 °C and 80 °C, no difference was observed for all the concentrations. The plot trend of the Young’s modulus differed from that of the burst pressure.
3.2.4. Area below the SS Curve
Figure 8 shows the area below the SS curve as a function of treatment time at various NaOH concentrations and temperatures. At 40 °C, the maximum value was recorded at 24 h for 0.01 M, whereas the maximum value was recorded at 3 h a for 0.1 and 1 M. At 0.1 M for 3 h, the area was almost double that of the pristine membrane. When immersed for 168 h, the value became slightly lower than the initial value. At 60 °C and 0.01 M, the maximum value was recorded at 3 h. In the case of 1 M, it decreased below the initial value after a few hours. At 80 °C, the area below the SS curve increased from the initial value for 0.01 M at 3 h, but the other samples indicated values below the initial value. At the same treatment time, the area decreased as the alkali concentration increased. The overall behavior exhibited was similar to that of the burst pressure.
The area below the SS curve in
Figure 8 was almost the same as that of the burst pressure in
Figure 2, despite some numerical differences. Therefore, as in
Figure 9, the correlation between the normalized area below the SS curve on the horizontal axis and the normalized burst pressure on the vertical axis was examined. The correlation coefficient (R
2) was 0.6709, which suggested a correlation, although weak. Meanwhile, when the normalized strain at break was represented in the vertical axis, the correlation coefficient (R
2) decreased to 0.4121. The area below the SS curve exhibited a higher correlation coefficient with the burst pressure.
Because the horizontal axis of the SS curve denoted strain and the vertical axis denoted stress, the area below the SS curve was assumed to represent the amount of work or energy until burst of a membrane.
A systematic alkali attack test on the AEM helped to confirm the following. The standard CIP condition, as recommended by the supplier, is that an AEM and 0.1 M NaOH solution should be in contact for 1 h at 40 °C. If this process is realized 52 times per year for 3 years, the cumulative immersion time will be 156 h. The equivalent load observed at the 168th hour of the immersion test conducted in this study almost corresponded to the standard CIP. At 168 h, the burst strength and area below the SS curve are higher than those observed for the pristine membrane. Hence, no problem is expected if CIP is performed solely by passing the above conditioned solution through the ED stack.
However, the AEM swells during the alkaline treatment and shrinks during the neutralization treatment. This dimensional change facilitates the deterioration of the AEM [
6]. In addition to the immersion or passing of the solution, in practical maintenance scenarios, operations such as physical rubbing to remove fouling substances may be required. If such additional operations are performed, the deterioration of the AEM will inevitably progress.
3.3. Chemical Structure Analysis
The deterioration in the mechanical properties of the AEMs is attributable to the degradation of the PVC skeleton. The PVC was extracted in THF via Soxhlet extraction, whereas changes in the molecular weight and the polydispersity of the polymeric fraction (Extract A) were determined via GPC, as shown in
Figure 10. Both the number-average molecular weight (
Mn) and weight-average molecular weight (
Mw) decreased as the alkali treatment time progressed. Meanwhile, the polydispersity (
Mw/
Mn) increased with the treatment time. The obtained results suggest the random scission of the PVC chain under alkali treatment conditions. Rapid PVC degradation appeared to have occurred in the first 5 min, from 175 to 80 kDa in
Mw; thereafter, the molecular weight decreased less with a constant polydispersity. This might be because only a hexane-insoluble polymeric fraction was collected by the extraction (Extract A), whereas the significantly lower molecular weight PVC in Extract B was outside of the analysis targets. However, the GPC results suggested that the PVC degradation in such high pH conditions resulted primarily in a change in the mechanical properties of the AEMs.
The PVC degradation owing to the alkali treatment was investigated via
1H and
13C NMR.
Figure 11a shows the
1H NMR spectra of pristine PVC in an IEM, an extract from the AMX membrane without alkali treatment, and an extract from the AMX membrane after alkali treatment for 30 min in 1 M NaOH at 80 °C. In all cases, two significant proton peaks were observed at 2.1–2.6 and 4.3–4.7 ppm, which were assigned to methylene (CH
2) and methine (CHCl) protons of the PVC backbone, respectively. Hence, it was confirmed that the extracts via Soxhlet extraction were PVC. Furthermore, small peaks at 5.5–6.5 ppm were discovered only after the alkali treatment (
Figure 11b), indicating allyl protons [
7]. In addition to the AEMs becoming darker by the treatment, C=C double bonds were generated by dehydrochlorination to form a polyene structure along the polymer backbone. Meanwhile,
Figure 11c shows the
13C NMR spectra of the corresponded specimens. The absence of peaks between 80 and 160 ppm confirmed the absence of other serious degradations that could lead to different chemical structures. In addition to the peaks of the PVC carbon skeleton (44–47 and 55–57 ppm), it was difficult to verify the polyene structure because of the low sensitivity of
13C in comparison to those of the protons from the
1H NMR spectra.
Furthermore, the effect of alkali treatment time on the mechanical properties of the AEMs was investigated via tensile testing.
Figure 12a shows the tensile properties before Soxhlet extraction. The value of the pristine sample was expressed as the dashed line. The tensile stress and tensile strain decreased monotonically as the treatment time progressed. Meanwhile, the properties after the extraction are shown in
Figure 12b. The residues extracted from the pristine membrane and following the five-minute treatments were a fibrous powder that could not tolerate the subsequent tensile testing because the PVC skeleton was removed via extraction. In other words, a membrane cannot be formed solely using the CMS-DVB copolymer; however, the shape of the membrane can be maintained using an IPN structure of PVC and CMS-DVB co-polymers. However, the residues after 10 and 30 min of alkali treatment retained their original shape such that tensile testing could be realized. The Young’s modulus and tensile stress increased with treatment time, whereas the tensile strain decreased. The brittle nature is attributable to the cross-linking of the PVC skeleton in alkali conditions, accompanied by the degradation of the polymer. Furthermore, when the alkali treatment exceeded 30 min, the shape of the membrane was maintained; however, it became extremely brittle to withstand the tensile test. This indicates that the main chain breakage has a considerable effect on the mechanical strength, although some of the chains are cross-linked. The mechanism is discussed in detail in the following section.
3.4. PVC Deterioration Mechanism
In our previous studies [
1,
8,
9], the dehydrochlorination of the PVC skeleton was confirmed to result in polyene formation under alkaline conditions; furthermore, the formation of a carbonyl group was indicated. In contrast, in this study, the main chain of PVC was partially cleaved and cross-linked. We considered the types of chemical reactions that resulted in these phenomena, as mentioned below.
As shown in
Figure 13, OH
− first subtracted β-hydrogen on the PVC, thereby inducing dechlorination to form a C=C double bond [
10].
This typical E2 reaction occurred readily along the polymer backbone, and the conjugation of the C=C double bonds or the polyene formation rendered the membranes darker. In the deprotonation by OH
−, the resulting carbanion attacked an adjacent C=C double bond to form a cross-linking point, as shown in
Figure 14.
Meanwhile, an SN2 reaction occurred in parallel with the E2 reaction, where the OH
− attacked the α-carbons of the PVC in a nucleophilic manner, inducing dechlorination. Subsequently, the OH
− further subtracted a proton from the resulting hydroxyl group with the formation of a ketone in the subsequent reaction scheme. When another OH
− reacted with a hydroxyl group immediately next to the ketone, the polymer chain was cleaved by a reverse aldol reaction [
10]. These processes are shown in
Figure 15.
As discussed in a previous study [
9], the AMX membrane contains a quaternary ammonium group and can supply OH
− continuously to PVC in the AEMs. Meanwhile, CMX contains an anionic sulfonate and prevents OH
− from approaching the PVC. This may have caused the deterioration of AMX to be more serious than that of CMX under alkaline conditions.
3.5. Deterioration Mechanism
In this study, the burst pressure of the AMX membrane with a PVC backing and binder first increased and then decreased as the alkali treatment time progressed. A similar feature was discovered in the tensile testing.
In the previous section, the effect of alkali treatment on PVC was discussed. As indicated, it caused dehydrochlorination with the formation of polyene and cross-linking structures, accompanied by PVC polymer main chain cleavage. We considered this main chain cleavage as contributing to the change in the mechanical properties of the AMX membrane, and proposed the model explaining the deterioration mechanism of the membrane as illustrated in
Figure 16.
Figure 16(a1) shows the original structure of the AMX membrane (Case I), which is a composite with a PVC backing and a chloromethylstylene and divinyl benzene (CMS-DVB) cross-linked structure containing a PVC binder (hereinafter, a cross-linked structure). The AMX membrane was prepared via the paste method [
1]. A paste containing CMS, DVB, PVC binder, and an initiator was applied to the PVC backing cloth. The PVC binder was compatible with the monomers in the paste. In the thermal polymerization of CMS and DVB in the presence of the binder, the cross-linked structure blocks were randomly formed. This is indicated as the girder bridge structure in
Figure 16(a1). The PVC binder was incorporated into the cross-linked structure, resulting in the formation of an interpenetrating network (IPN) structure to interconnect the cross-linked structure blocks, indicated as intertwined chains between the girder bridges. The PVC binder is illustrated in an orange solid line. Hence, the Case I membrane was modeled as a girder bridge cluster connected by PVC chains. In this study, the PVC backing was tough, whereas the cross-linked structure was hard and brittle. When a tensile stress was applied to the Case I membrane, not only the constrained IPN comprising the cross-linked structure blocks and PVC, but also the PVC backing exhibited a high tensile strength, owing to their hardness and brittleness before being fractured without presenting a large elongation, as shown in the SS curve in
Figure 3a. The cross-linked structure was fractured first by the stress concentration, followed by the degradation of the PVC backing as shown in
Figure 16(a2).
In the Müllen burst test, the fracture of the pristine membrane was accompanied by a loud rupture sound.
The interpenetrating PVC was degraded by the alkali treatment. In the initial stage of degradation, the PVC chain between the cross-linked structure blocks was partially cleaved. It is illustrated in an orange broken line, as shown in
Figure 16(b1) (Case II); hence, the girder bridges of the Case II membrane can be transformed more easily than those of the Case I membrane. When stress was applied to the Case II membrane, the girder bridges in the cluster shifted to decrease the stress concentration (
Figure 16(b2)), resulting in a yield point and elongation, as shown in the curve in
Figure 3b. With further stretching, the resulting partially degraded IPN structure showed multiple yield points until the PVC backing was degraded seriously, as shown in
Figure 16(b3). Although the maximum stress at break decreased, the strain to break increased to 189% compared with that of the pristine membrane, and the area below the SS curve increased, resulting in an improvement in the burst pressure. In addition, when the Case II membrane was subjected to a burst test, a “burr” sound was indicated instead of making a cracking sound, suggesting that the backing was torn. The normalized burst pressure increased by 184% compared with that of the pristine membrane.
Not only the PVC chains between the cross-linked structure blocks, but also those in the blocks were cleaved by the further alkali treatment. It is illustrated in an orange dashed line, as shown in
Figure 16(c1) (Case III). The severely degraded PVC affected the mechanical properties, as shown in
Figure 16(c2), and the resulting membrane exhibited a lower stress at break. The cross-linking of the PVC chain in parallel with the chain degradation would not be negligible during the alkali treatment for a longer period, as it suppressed the elongation under stress. Hence, the strain at break during the tensile testing is as shown in the curve
Figure 3c. In addition, the membrane yielded a decrease in the burst pressure by 67% relative to the pristine membrane; hence, the Case III membrane broke without generating a sound.
As described above, this model could explain the improvement in burst pressure and the change in the SS curve of AMX treated with alkali solutions. However, this model should be validated. The experiment conducted in this study should be repeated for a test AEM that uses a PVC backing but does not contain a PVC binder. This will be realized in the near future.