Study of Polyaniline/Poly(Sodium 4-Styrenesulfonate) Composite Deposits Using an Electrochemical Quartz Crystal Microbalance for the Modification of a Commercial Anion Exchange Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electromicrogravimetric Study of the PAni/PSS Composite Deposits
2.1.1. Materials
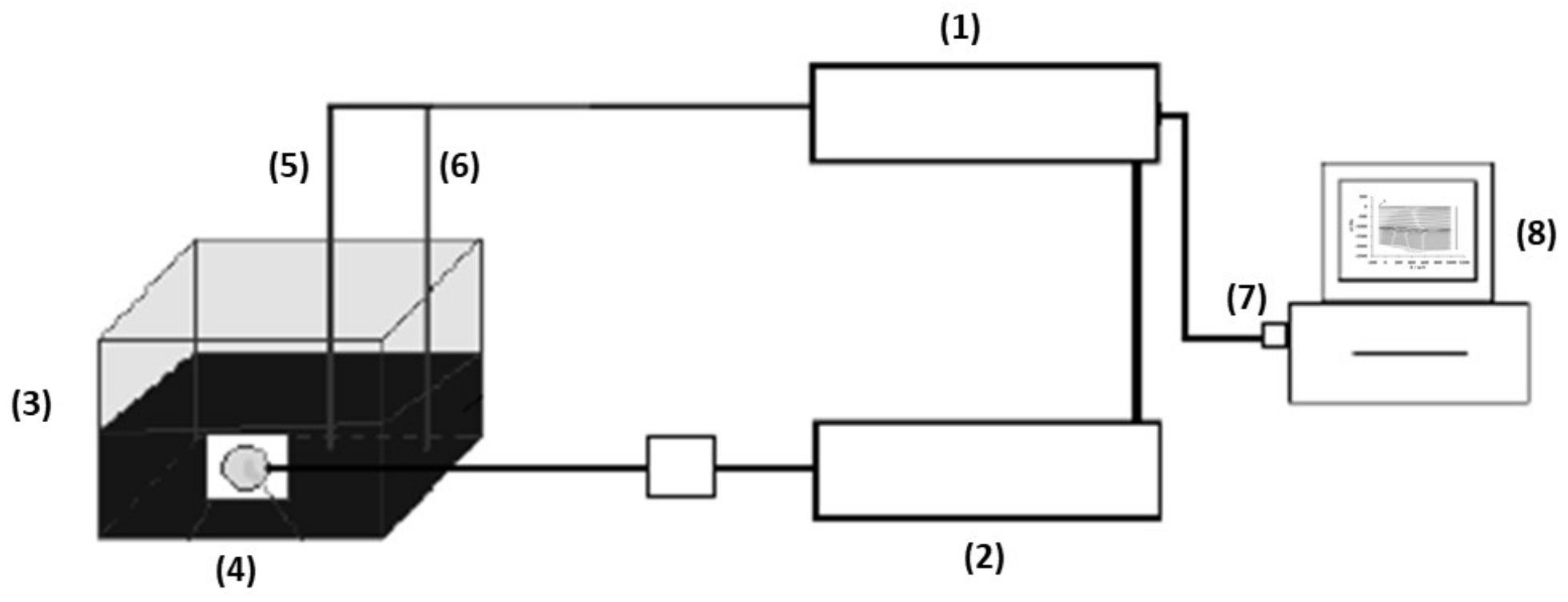
2.1.2. Experimental Setup
2.1.3. Characterization of the PAni/PSS Composite Films Using Scanning Electron Microscopy
2.2. Modification of the Commercial Anion Exchange Membrane
2.2.1. The Commercial Membrane
2.2.2. Procedure for the Modification of Membranes
2.3. Characterization of the Modified Membranes
2.3.1. Electrochemical Characterization of Modified Membranes
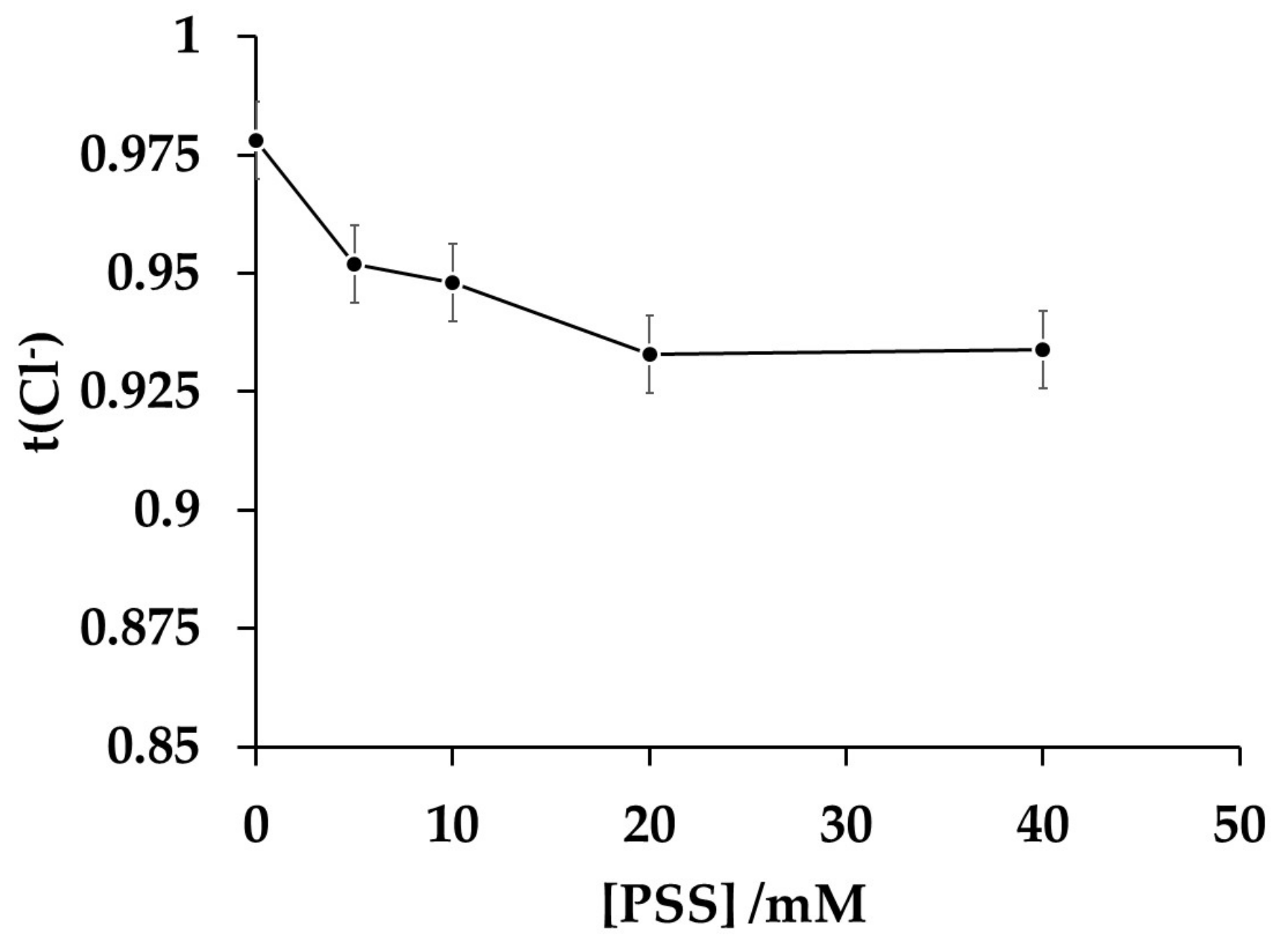
2.3.2. Determination of the Transport Numbers Using the Modified Membranes
3. Results
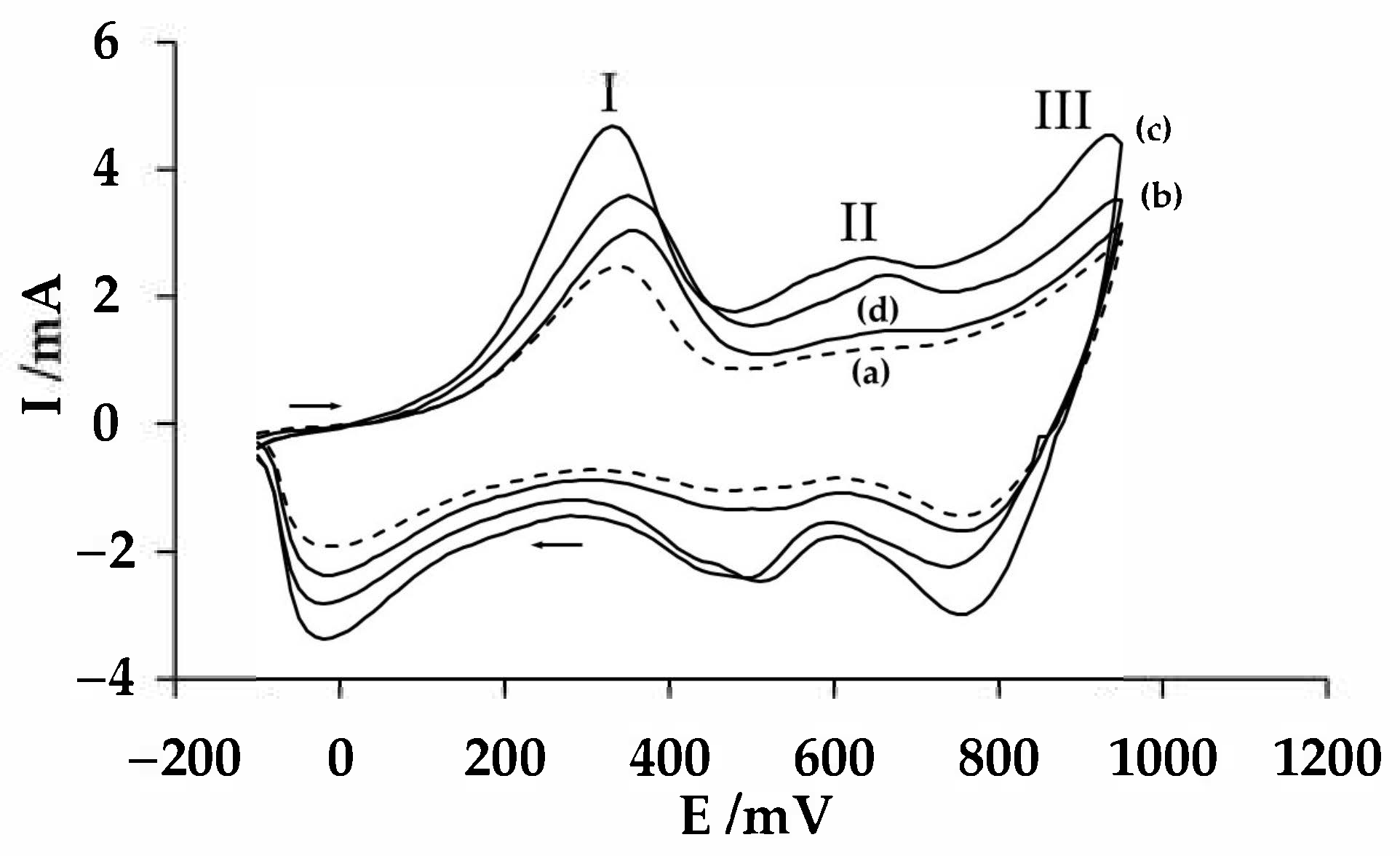
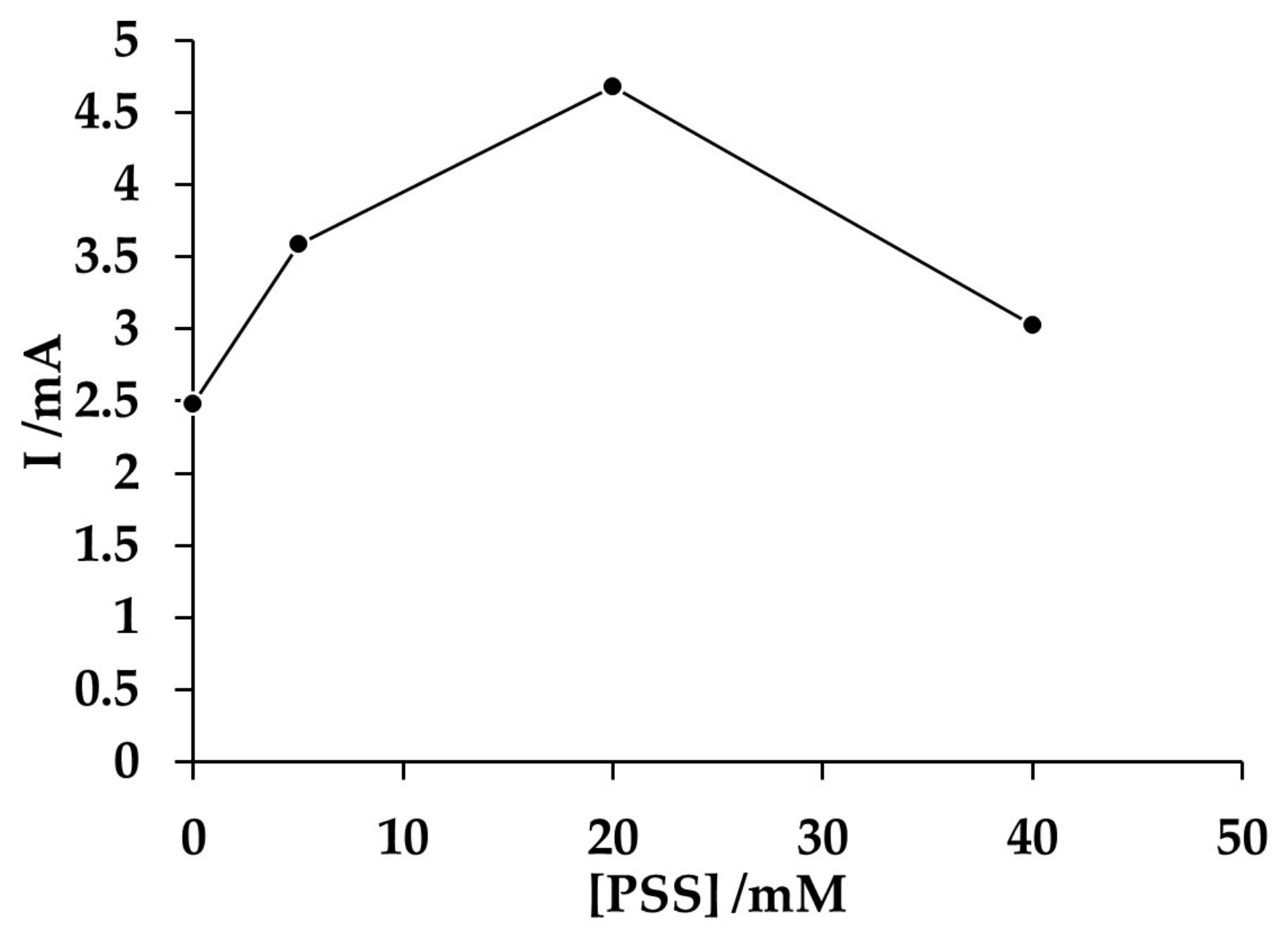
3.1. Potentiodynamic Behavior of the PAni/PSS Composites on the Gold Electrode
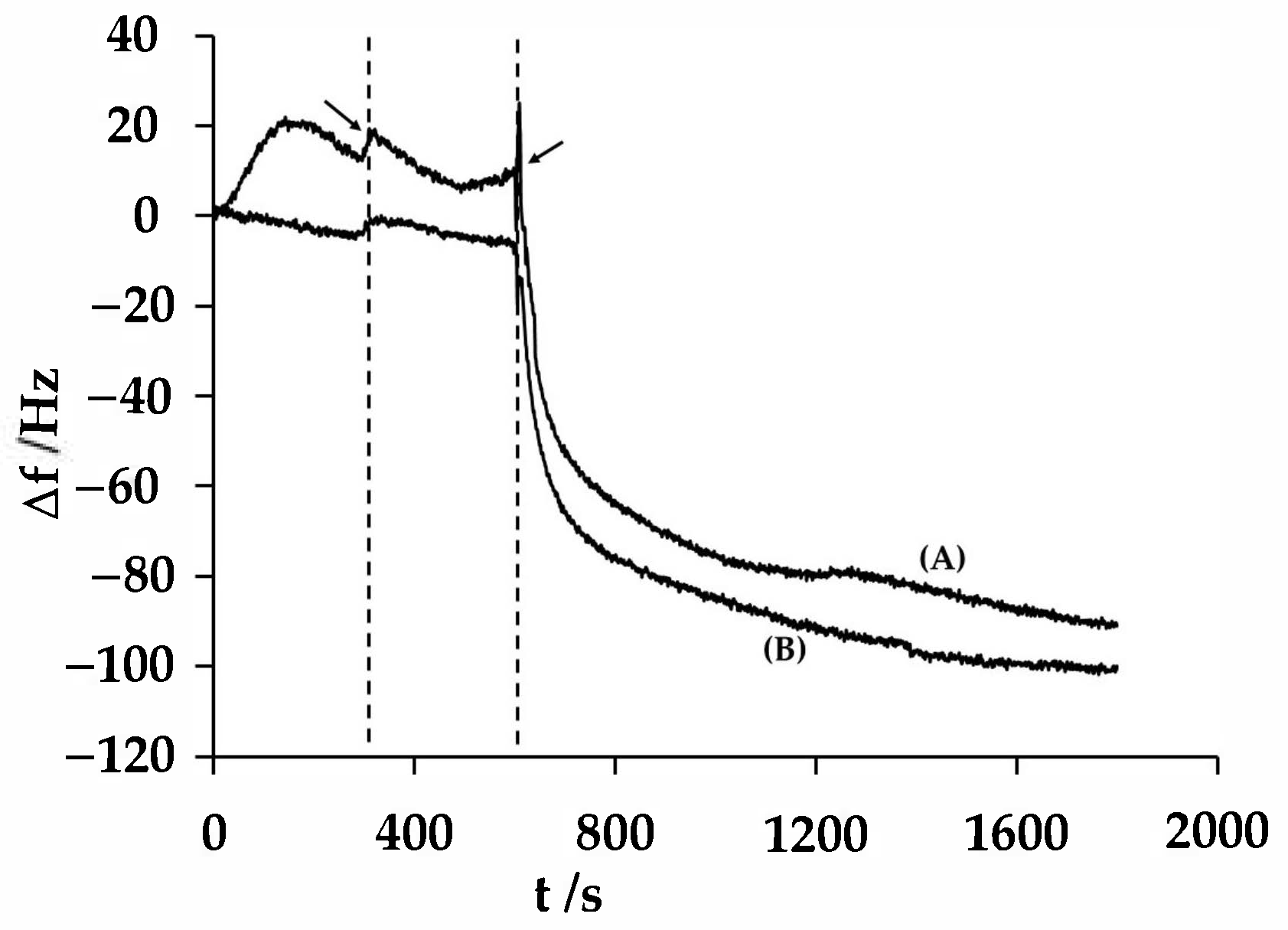
3.2. Electromicrogravimetric Behavior of PAni/PSS Composites on a Gold Electrode
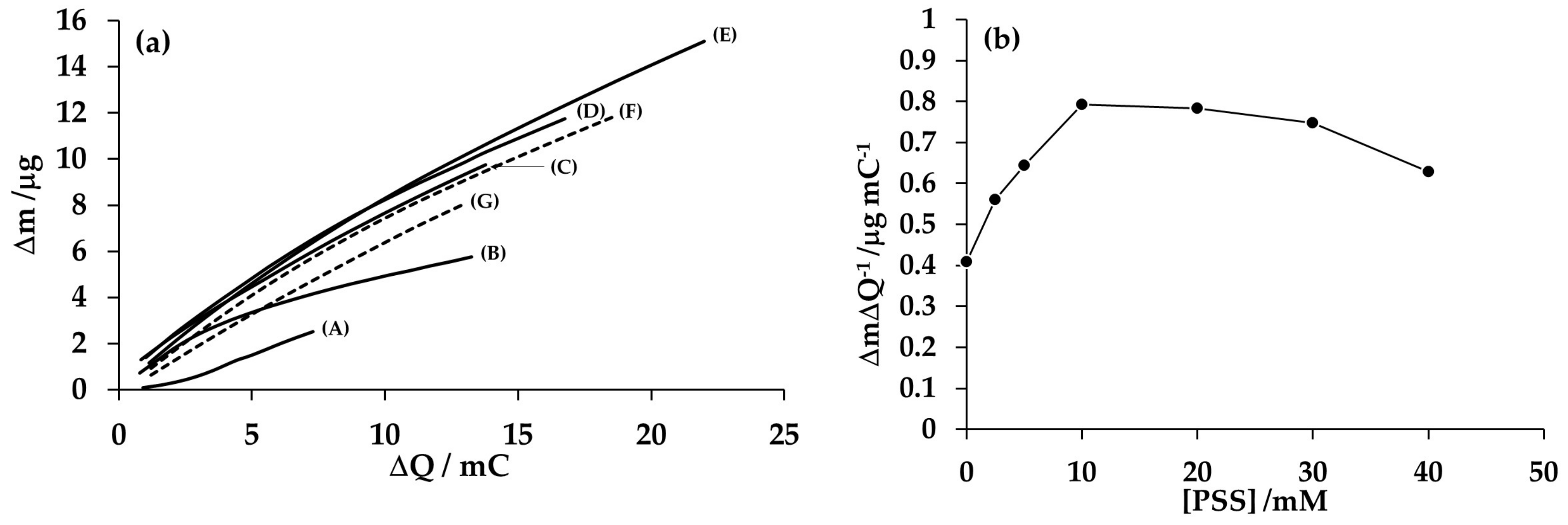
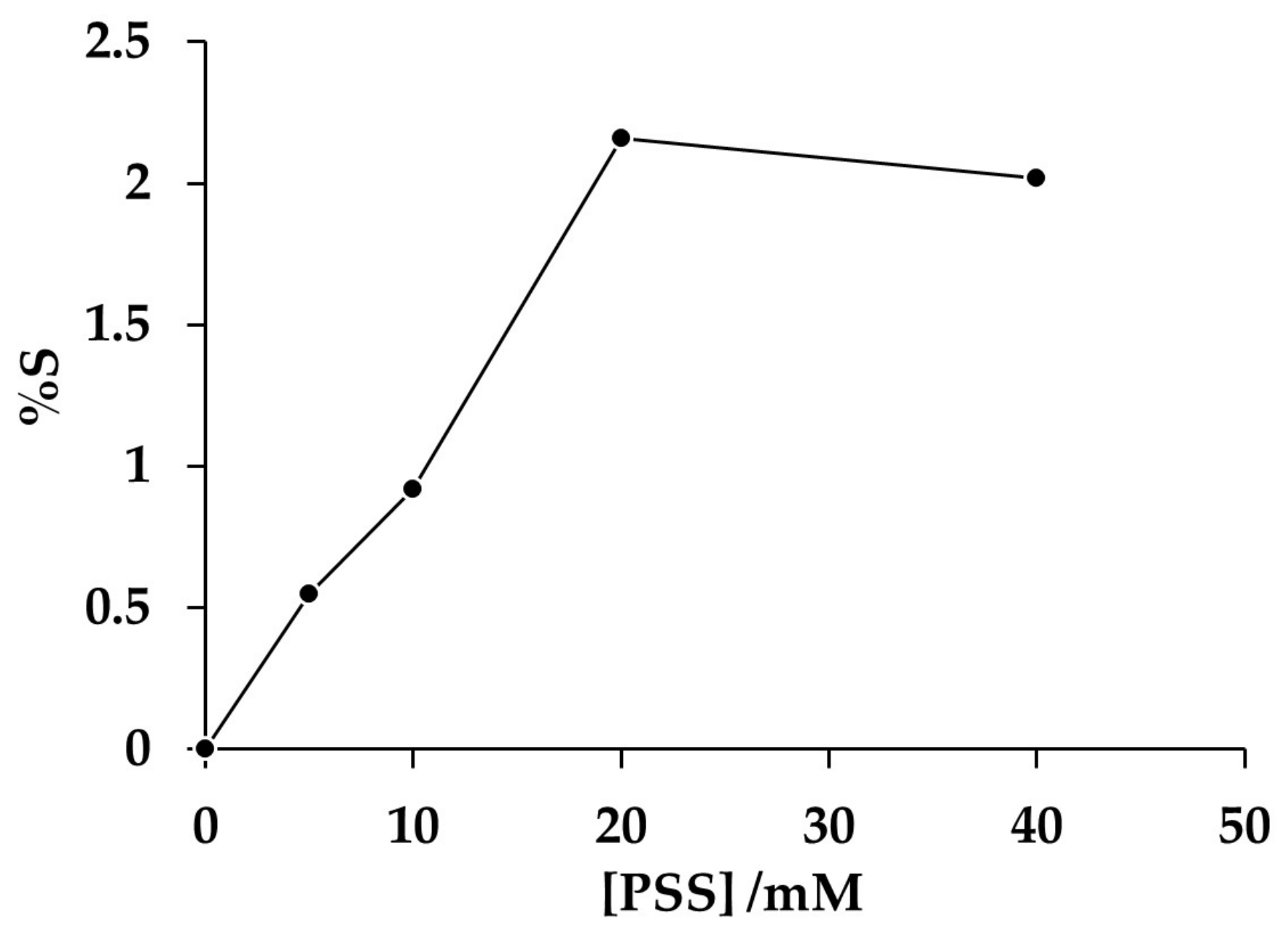
3.3. Electromicrogravimetric Analysis of the Effect of Polyelectrolyte Concentration on the Electrosynthesis of Composite PAni/PSS Deposits
Analysis of the Mass/Charge Ratio at Different Concentrations of PSS
3.4. Study of the Charge Compensation Process of the Composites
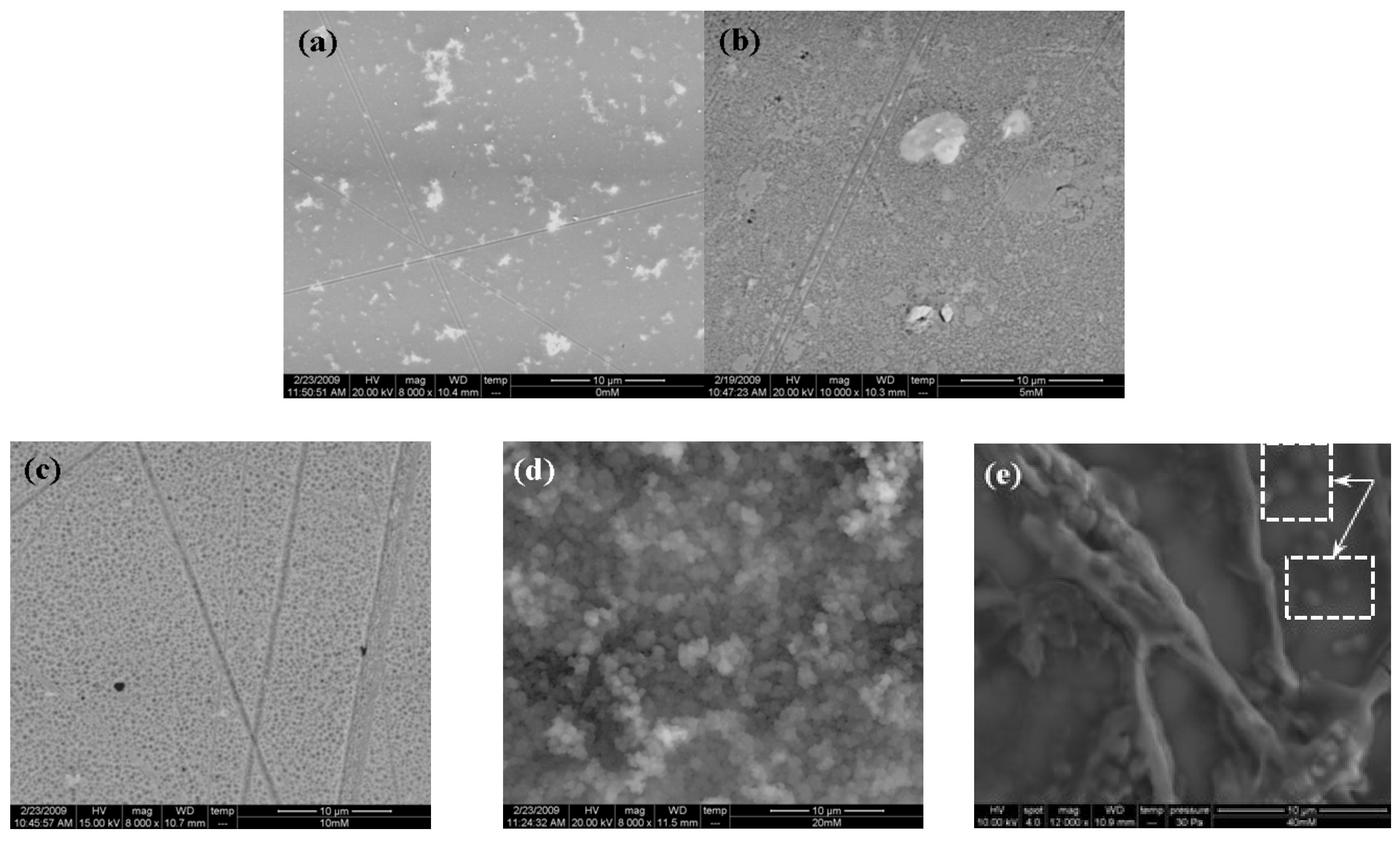
3.5. Analysis of the Composite Deposits by Scanning Electron Microscopy
3.6. Modifications of the Commercial Anion Exchange Membranes with PAni/PSS Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van der Bruggen, B. Chemical Modification of Polyethersulfone Nanofiltration Membranes: A Review. J. Appl. Polym. 2009, 114, 630–642. [Google Scholar] [CrossRef]
- Khan, M.A.; Arsalan, M.; Khan, M.R. Membrane Applications of Polyaniline based Nanocomposite Ion-exchanger and its Electrochemical Properties for Desalination. J. Membr. Sci. Technol. 2016, 6, 1. [Google Scholar]
- Largier, T.D.; Wang, D.; Mueller, J.; Cornelius, C.J. Improving electrodialysis based water desalination using a sulfonated Diels-Alder poly(phenylene). J. Membr. Sci. 2017, 531, 103–110. [Google Scholar] [CrossRef]
- Louati, I.; Guesmi, F.; Hannachi, C.; Hamrouni, B. Effect of ionic strength on the ion exchange equilibrium between AMX membrane and electrolyte solutions. Water Qual. Res. J. Can. 2016, 51, 60–68. [Google Scholar] [CrossRef]
- Tan, S.; Laforgue, A.; Bélanger, D. Characterization of a Cation-Exchange/Polyaniline Composite Membrane. Langmuir 2003, 19, 744–751. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Koninckx, A.; Vandecasteele, C. Separation of monovalent and divalent ions from aqueous solution by electrodialysis and nanofiltration. Water Res. 2004, 38, 1347–1353. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Lai, A.N.; Zhou, K.; Zhuo, Y.Z.; Zhang, Q.G.; Zhu, A.M.; Ye, M.L.; Liu, Q.L. Anion exchange membranes based on carbazole-containing polyolefin for direct methanol fuel cells. J. Membr. Sci. 2016, 497, 99–107. [Google Scholar] [CrossRef]
- Zhu, L.; Pan, J.; Wang, Y.; Han, J.; Zhuang, L.; Hickner, M.A. Multication Side Chain Anion Exchange Membranes. Macromolecules 2016, 49, 815–824. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. 2016, 1, 225–255. [Google Scholar] [CrossRef] [Green Version]
- Farrokhzad, H.; Moghbeli, M.R.; Van Gerven, T.; Van der Bruggen, B. Surface modification of composite ion exchange membranes by polyaniline. React. Funct. Polym. 2015, 86, 161–167. [Google Scholar] [CrossRef]
- Shkirskaya, S.A.; Senchikhin, I.N.; Kononenko, N.A.; Roldugin, V.I. Effect of Polyaniline on the Stability of Electrotransport Characteristics and Thermochemical Properties of Sulfocationite Membranes with Different Polymer Matrices. Russ. J. Electrochem. 2017, 53, 78–85. [Google Scholar] [CrossRef]
- Berezina, N.P.; Kubaisi, A.A.-R.; Alpatova, N.M.; Andreev, V.N.; Griga, E.I. Composite Polyaniline/MF-4SK Membranes: A Chemical Template Synthesis and the Sorption and Conduction Properties. Russ. J. Electrochem. 2004, 40, 286–293. [Google Scholar] [CrossRef]
- Berezina, N.P.; Gnusin, N.P.; Demina, O.A.; Annikova, L.A. Effect of Polyaniline on the Current Passing through Structural Fragments of Ion-Exchange Sulfonic-Cationite Resins and Membranes. Russ. J. Electrochem. 2009, 45, 1226–1233. [Google Scholar] [CrossRef]
- Compañ, V.; Riande, E.; Fernandez-Carretero, F.J.; Berezina, N.P.; Sytcheva, A.A.-R. Influence of polyaniline intercalations on the conductivity and permselectivity of perfluorinated cationexchange membranes. J. Membr. Sci. 2008, 318, 255–263. [Google Scholar] [CrossRef]
- Schwenzer, B.; Kim, S.; Vijayakumar, M.; Yang, Z.; Liu, J. Correlation of structural differences between Nafion/polyaniline and Nafion/polypyrrole composite membranes and observed transport properties. J. Membr. Sci. 2011, 372, 11–19. [Google Scholar] [CrossRef]
- Berezina, N.P.; Shkirskaya, S.A.; Kolechko, M.V.; Popova, O.V.; Senchikhin, I.N.; Roldugin, V.I. Barrier Effects of Polyaniline Layer in Surface Modified MF4SK/Polyaniline Membranes. Russ. J. Electrochem. 2011, 47, 995–1005. [Google Scholar] [CrossRef]
- Berezina, N.P.; Kononenko, N.A.; Sytcheva, A.A.-R.; Loza, N.V.; Shkirskaya, S.A.; Hegman, N.; Pungor, A. Perfluorinated nanocomposite membranes modified by polyaniline: Electrotransport phenomena and morphology. Electrochim. Acta 2009, 54, 2342–2352. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K.; Trivedi, G.S.; Rangarajan, R. Preparation and electrochemical characterization of cation- and anion-exchange/polyaniline composite membranes. J. Colloid Interface Sci. 2004, 277, 162–171. [Google Scholar] [CrossRef]
- Baker, C.K.; Qiu, Y.-J.; Reynolds, J.R. Electrochemically-induced charge and mass transport in polypyrrole/poly(styrene sulfonate) molecular composites. J. Phys. Chem. 1991, 95, 4446–4452. [Google Scholar] [CrossRef]
- Pickup, P.G. Electrochemistry of Electronically Conducting Polymer Films. In Modern Aspects of Electrochemistry; White, R.E., Bockris, J.O.M., Conway, B.E., Eds.; Kluwer/Plenum: New York, NY, USA, 1999; No. 33; pp. 549–597. [Google Scholar]
- Inzelt, G. Mechanism of charge transport in polymer-modified electrodes. In Electroanalytical Chemistry; Bard, A.J., Ed.; Marcel Dekker: New York, NY, USA, 1994; Volume 18, pp. 89–241. [Google Scholar]
- Jang, J.; Ha, J.; Kim, K. Organic light-emitting diode with polyaniline-poly(styrene sulfonate) as a hole injection layer. Thin Solid Films 2008, 516, 3152–3156. [Google Scholar] [CrossRef]
- Hyodo, K.; Omae, M.; Kagami, Y. Effect of molecular weight on the ion selective electrochemical polymerization of aniline. Electrochim. Acta 1991, 36, 357–360. [Google Scholar] [CrossRef]
- Hyodo, K.; Nokazi, M. High ion selective electrochemical synthesis of polyaniline. Electrochim. Acta 1988, 33, 165–166. [Google Scholar] [CrossRef]
- Hwang, J.H.; Yang, S.C. Morphological modification of polyaniline using polyelectrolyte template molecules. Synth. Met. 1989, 29, 271–276. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M. Toward Genolelectronics: Nucleic Acid Doped Conducting Polymers. Langmuir 2000, 16, 2269–2274. [Google Scholar] [CrossRef]
- Buttry, D.A.; Ward, M. Measurement of Interfacial Processes at Electrode Surfaces with the Electrochemical Quartz Crystal Microbalance. Chem. Rev. 1992, 92, 1355–1379. [Google Scholar] [CrossRef]
- Ward, M. Principles and applications of the electrochemical quartz crystal microbalance. In Monographs in Electroanalytical Chemistry and Electrochemistry: Physical Electrochemistry, Principles, Methods, and Applications; Rubinstein, I., Ed.; Marcel Dekker: New York, NY, USA, 1995; pp. 293–338. [Google Scholar]
- Orata, D.; Buttry, D.A. Determination of ion populations and solvent content as functions of redox state and pH in polyaniline. J. Am. Chem. Soc. 1987, 109, 3574–3581. [Google Scholar] [CrossRef]
- Ding, H.; Park, S.-M. Electrochemistry of conductive polymers XXVII. Effects of polystyrene sulfonate on electrochemical behavior of polyaniline. J. Electrochem. Soc. 2003, 150, E33–E38. [Google Scholar] [CrossRef]
- Iyoda, T.; Ohtani, A.; Honda, K.; Shimidzu, T. Diaphragmatic Chemical Polymerization of Pyrrole in the Nafion Film. Macromolecules 1990, 23, 1971–1976. [Google Scholar] [CrossRef]
- Shimidzu, T.; Ohtani, A.; Honda, K. Charge-controllable polypyrrole/polyelectrolyte composite membranes: Part II. Effect of incorporated anion size on the electrochemical oxidation-reduction process. J. Electroanal. Chem. Interfacial Electrochem. 1987, 224, 123–135. [Google Scholar] [CrossRef]
- Shimidzu, T.; Ohtani, A.; Honda, K. Charge-controllable poly pyrrole/poly electrolyte composite membranes: Part III. Electrochemical deionization system constructed by anionexchangeable and cation-exchangeable polypyrrole electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1998, 251, 323–337. [Google Scholar] [CrossRef]
- Pile, D.L.; Zhang, Y.; Hillier, A.C. Electrochemically Modulated Permeability of Poly(aniline) and Composite Poly(aniline)−Poly(styrenesulfonate) Membranes. Langmuir 2006, 22, 5925–5931. [Google Scholar] [CrossRef] [Green Version]
- ASTOM Corporation. Available online: http://www.astom-corp.jp/en/product/10.html (accessed on 8 October 2020).
- Tongwen, X.; Weihua, Y. Fundamental studies of a new series of anion exchange membranes: Membrane preparation and characterization. J. Membr. Sci. 2001, 190, 159–166. [Google Scholar] [CrossRef]
- Montes Rojas, A.; Olivares Maldonado, Y.; Torres Rodríguez, L.M. An easy method to modify the exchange membranes of electrodialysis with electrosynthetized polyaniline. J. Membr. Sci. 2007, 300, 2–5. [Google Scholar] [CrossRef]
- Lteif, R.; Dammak, L.; Larchet, C.; Auclair, B. Détermination du nombre de transport d’un contreion dans une membrane échangeuse d’ions en utilisant la méthode de la pile de concentration. Eur. Polym. J. 2001, 37, 627–639. [Google Scholar] [CrossRef]
- Robson Wright, M. An Introduction to Aqueous Electrolyte Solutions; John Wiley & Sons Ltd.: Chichester, UK, 2007; p. 381. [Google Scholar]
- Genies, E.M.; Tsintavis, C. Electrochemical behaviour, chronocoulometric and kinetic study of the redox mechanism of polyaniline deposits. J. Electroanal. Chem. 1986, 200, 127–145. [Google Scholar] [CrossRef]
- Mazeikiene, R.; Malinauskas, A. Kinetic study of the electrochemical degradation of polyaniline. Synth. Met. 2001, 123, 349–354. [Google Scholar] [CrossRef]
- Mathebe, N.G.R.; Morrin, A.; Owuoha, E.I. Electrochemistry and scanning electron microscopy of polyaniline/peroxidase-based biosensor. Talanta 2004, 64, 115–120. [Google Scholar] [CrossRef]
- Prasad, K.R.; Munichandraiah, N. Potentiodynamic deposition of polyaniline on non-platinum metals and characterization. Synth. Met. 2001, 123, 459–468. [Google Scholar] [CrossRef]
- Donjuan-Medrano, A.-L.; Montes-Rojas, A. Comparison of sensitivity constants of an electrochemical quartz crystal microbalance determined by potentiostatic deposition of Tl, Pb, Ag and Cu films. Curr. Top. Electrochem. 2012, 17, 75–85. [Google Scholar]
- Bruckeinstein, S.; Swathirajam, S. Potential dependence of lead and silver underpotential coverages in acetonitrile using a piezoelectric crystal oscillator method. Electrochim. Acta 1985, 30, 851–855. [Google Scholar] [CrossRef]
- Gabrielli, C.; Keddam, M.; Torresi, R. Calibration of the Electrochemical Quartz Crystal Microbalance. J. Electrochem. Soc. 1991, 138, 2657–2660. [Google Scholar] [CrossRef]
- Mondal, S.K.; Prasad, K.R.; Munichandraiah, N. Analysis of electrochemical impedance of polyaniline films prepared by galvanostatic, potentiostatic and potentiodynamic methods. Synth. Met. 2005, 148, 275–286. [Google Scholar] [CrossRef]
- Li, L.; Ferng, L.; Wei, Y.; Yang, C.; Ji, H.-F. Effects of acidity on the size of polyanilinepoly(sodium 4-styrenesulfonate) composite particles and the stability of corresponding colloids in water. J. Colloid Interface Sci. 2012, 381, 11–16. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Tsukruk, T.; Slocik, J.M.; Ko, H.; Poulsen, N.; Naik, R.R.; Kröger, N.; Tsukruk, V.V. Bioenabled Surface-Mediated Growth of Titania Nanoparticles. Adv. Mater. 2008, 20, 3274–3279. [Google Scholar] [CrossRef]
- Garratt-Reed, A.J.; Bell, D.C. Energy-Dispersive X-ray Analysis in the Electron Microscope; BIOS Scientific Publishers Limited: Oxford, UK, 2003; Chapter 5. [Google Scholar]





| Parameter | Value |
|---|---|
| Type | Permeable at anions, strongly basic |
| Electric resistance (Ω cm2) | 0.4−1.5 |
| Thickness (μm) | 160 |
| Ionic exchange capacity (meq g−1) | 2.0−3.5 |
| Water uptake (g H2O g−1) | 0.40−0.55 |
| Specifications | Resistance to fouling by organic particles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Rojas, A.; Ramírez-Orizaga, M.; Ávila-Rodríguez, J.G.; Torres-Rodríguez, L.M. Study of Polyaniline/Poly(Sodium 4-Styrenesulfonate) Composite Deposits Using an Electrochemical Quartz Crystal Microbalance for the Modification of a Commercial Anion Exchange Membrane. Membranes 2020, 10, 387. https://doi.org/10.3390/membranes10120387
Montes-Rojas A, Ramírez-Orizaga M, Ávila-Rodríguez JG, Torres-Rodríguez LM. Study of Polyaniline/Poly(Sodium 4-Styrenesulfonate) Composite Deposits Using an Electrochemical Quartz Crystal Microbalance for the Modification of a Commercial Anion Exchange Membrane. Membranes. 2020; 10(12):387. https://doi.org/10.3390/membranes10120387
Chicago/Turabian StyleMontes-Rojas, Antonio, Marlen Ramírez-Orizaga, Jesús Gerardo Ávila-Rodríguez, and Luz María Torres-Rodríguez. 2020. "Study of Polyaniline/Poly(Sodium 4-Styrenesulfonate) Composite Deposits Using an Electrochemical Quartz Crystal Microbalance for the Modification of a Commercial Anion Exchange Membrane" Membranes 10, no. 12: 387. https://doi.org/10.3390/membranes10120387
APA StyleMontes-Rojas, A., Ramírez-Orizaga, M., Ávila-Rodríguez, J. G., & Torres-Rodríguez, L. M. (2020). Study of Polyaniline/Poly(Sodium 4-Styrenesulfonate) Composite Deposits Using an Electrochemical Quartz Crystal Microbalance for the Modification of a Commercial Anion Exchange Membrane. Membranes, 10(12), 387. https://doi.org/10.3390/membranes10120387




