Pertraction of Co(II) through Novel Ultrasound Prepared Supported Liquid Membranes Containing D2EHPA. Optimization and Transport Parameters
Abstract
:1. Introduction
2. Theoretical Background
3. Materials and Methods
3.1. Materials
3.2. Methods
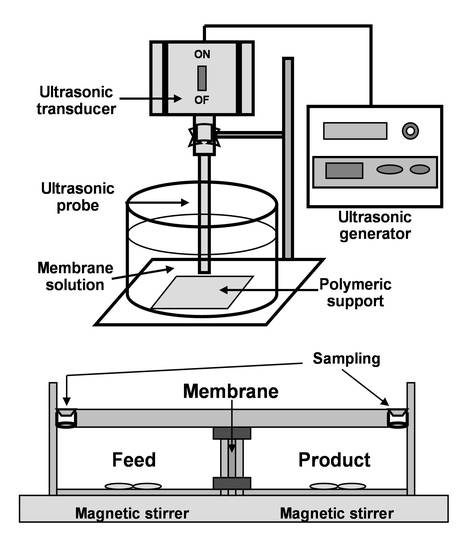
3.2.1. Preparation of Supported Liquid Membrane
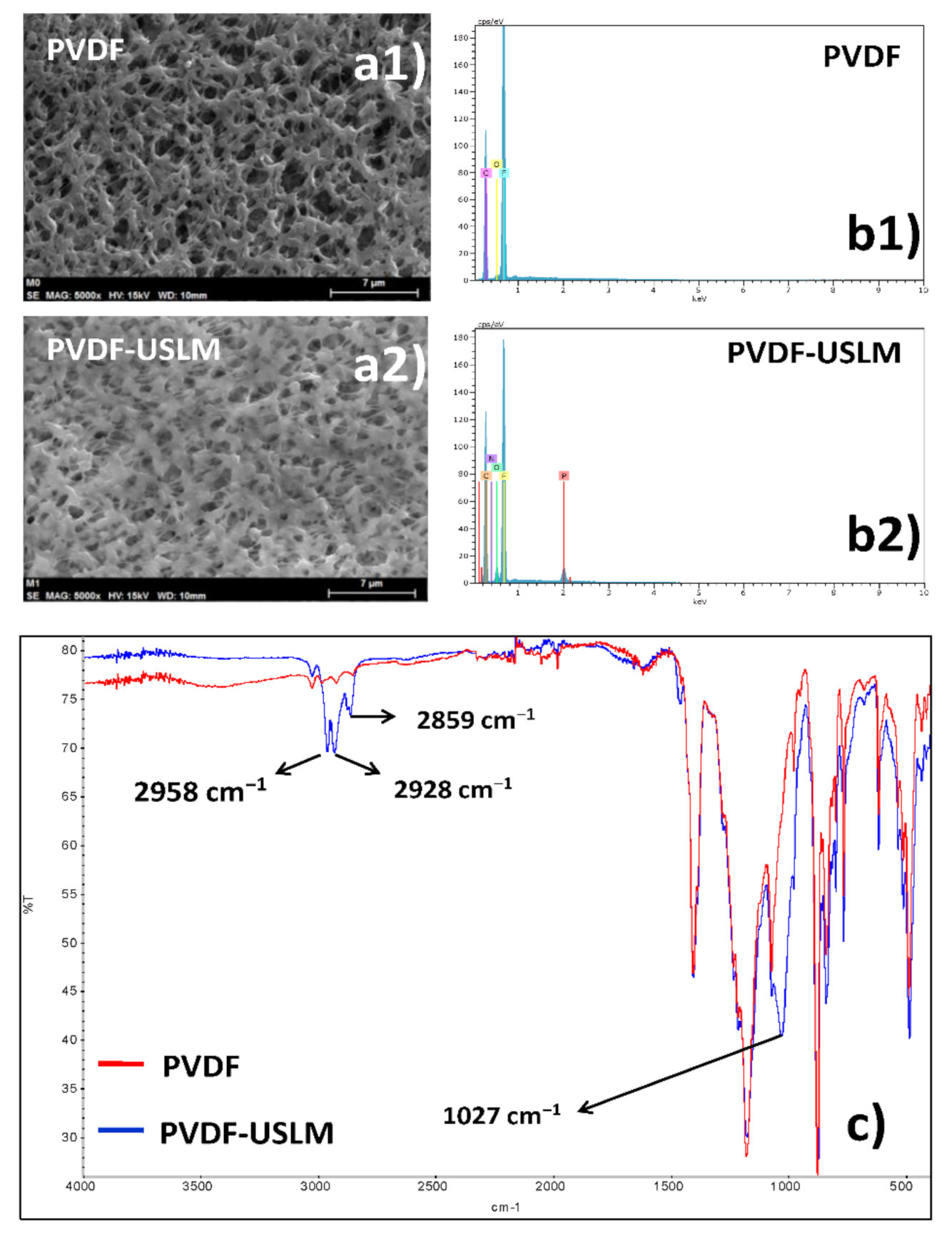
3.2.2. Supported Liquid Membrane Characterization
3.2.3. Transport Experiments
3.2.4. Analytical Methods and Calculations
4. Results
4.1. Membrane Characterization
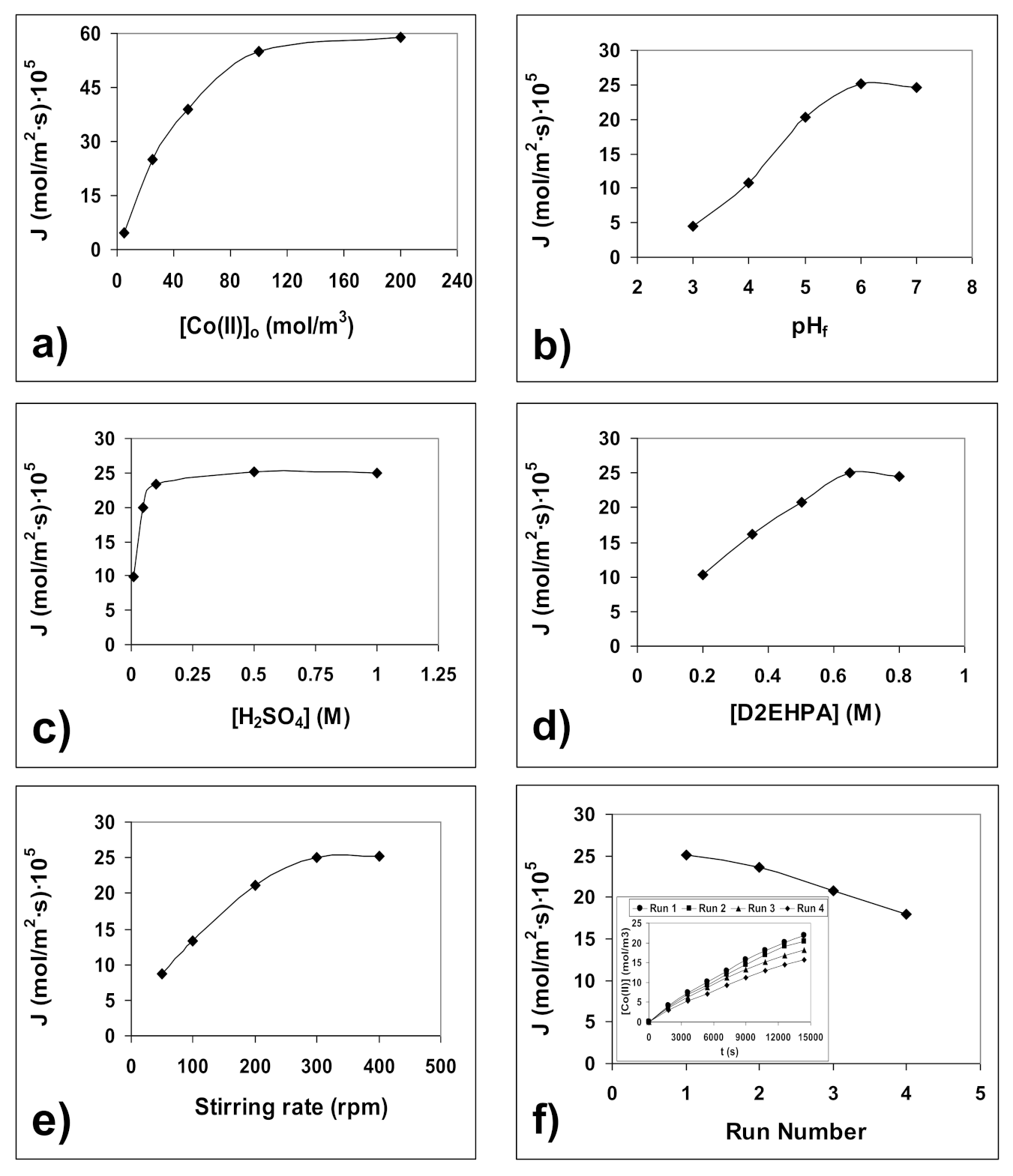
4.2. Optimization of Co(II) Transport Process
4.3. Determination of Transport Parameters
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cote, G. Hydrometallurgy of strategic materials. Solvent Extr. Ion. Exch. 2000, 18, 703–727. [Google Scholar] [CrossRef]
- Kapusca, J.P.T. Cobalt production and markets: A brief overview. Cobalt News 2007, 7, 9–12. [Google Scholar]
- Weight, D. Cobalt Production Statistics; Cobalt Development Institute: Surrey, UK, 2018; Available online: https://www.cobaltinstitute.org/statistics.html (accessed on 28 July 2020).
- Leyssensa, L.; Vincka, B.; Van Der Straeten, C.; Wuytse, F.; Maesa, L. Cobalt toxicity in humans. A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, Y.; Jiang, S.; Ma, X.; Wei, B. Removal and recovery of cobalt from Co(II)–Containing water samples by dithiocarboxyl polyethyleneimine. Sep. Purif. Technol. 2020, 251, 117338. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Chen, Z. Radioactive Cobalt(II) Removal from aqueous solutions using a reusable nanocomposite: Kinetic, isotherms, and mechanistic study. Int. J. Environ. Res. Public Health 2017, 14, 1453. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Pramanik, B.K.; Mainali, B.; Angove, M.J. Opportunities and constraints of using the innovative adsorbents for the removal of cobalt(II) from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2018, 10, 435–456. [Google Scholar] [CrossRef]
- Salmani, M.H.; Ehrampoush, M.H.; Eslami, H.; Eftekhar, B. Synthesis, characterization and application of mesoporous silica in removal of cobalt ions from contaminated water. Groundw. Sustain. Dev. 2020, 11, 100425. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Yetilmezsoy, K.; Salari, M.; Heidarinejad, Z.; Yousefi, M.; Sillanpää, M. Adsorptive removal of cobalt(II) from aqueous solutions using multiwalled carbon nanotubes and γ-alumina as novel adsorbents: Modelling and optimization based on response surface methodology and artificial neural network. J. Mol. Liq. 2020, 299, 112154. [Google Scholar]
- Vafajoo, L.; Cheraghi, R.; Dabbagh, R.; McKay, G. Removal of cobalt (II) ions from aqueous solutions utilizing the pre-treated 2-Hypnea Valentiae algae: Equilibrium, thermodynamic, and dynamic studies. Chem. Eng. J. 2018, 331, 39–47. [Google Scholar] [CrossRef]
- Qian Zhang, Q.; Zhuang, S.; Wang, J. Biosorptive removal of cobalt(II) from aqueous solutions using magnetic cyanoethyl chitosan beads. J. Environ. Chem. Eng. 2020, 8, 104531. [Google Scholar] [CrossRef]
- Iáñez-Rodríguez, I.; Calero, M.; Blázquez, G.; Martín-Lara, M.Á. Greenhouse crop residue and its derived biochar: Potential as adsorbent of cobalt from aqueous solutions. Water 2020, 12, 1282. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Boran, S. Adsorption, bioaccumulation and kinetics parameters of the phytoremediation of cobalt from wastewater using elodea canadensis. Bull. Environ. Contam. Toxicol. 2018, 100, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Almanza, D.; Gamiño-Arroyo, Z.; Sánchez-Cadena, L.E.; Gómez-Castro, F.I.; Uribe-Ramírez, A.R.; Aguilera-Alvarado, A.F.; Ocampo-Carmona, L.M. Recovery of cobalt from spent lithium-ion mobile phone batteries using liquid–liquid extraction. Batteries 2019, 5, 44. [Google Scholar] [CrossRef] [Green Version]
- Wiecka, Z.; Rzelewska-Piekut, M.; Cierpiszewski, R.; Staszak, K.; Regel-Rosocka, M. Hydrometallurgical recovery of Cobalt(II) from spent industrial catalysts. Catalysts 2020, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Jamiu, Z.A.; Saleh, T.A.; Ali, S.A. Biogenic glutamic acid-based resin: Its synthesis and application in the removal of cobalt(II). J. Hazard. Mater. 2017, 327, 44–54. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Wang, J. Electro-enhanced removal of cobalt ions from aqueous solution by capacitive deionization. Sci. Total Environ. 2019, 697, 134–144. [Google Scholar] [CrossRef]
- Lu, J.; Dreisinger, D.; Glück, T. Cobalt electrowinning. A systematic investigation for high quality electrolytic cobalt production. Hydrometallurgy 2018, 178, 19–29. [Google Scholar] [CrossRef]
- Kumar, V.A.; Marathe, K.V. Selective separation of copper (II) and cobalt (II) from wastewater by using continuous cross-flow micellar-enhanced ultrafiltration and surfactant recovery from metal micellar solutions. Can. J. Chem. Eng. 2011, 89, 292–298. [Google Scholar]
- Gherasim, C.V.; Hancková, K.; Palarčík, J.; Mikulášek, P. Investigation of cobalt (II) retention from aqueous solutions by a polyamide nanofiltration membrane. J. Membr. Sci. 2015, 490, 46–56. [Google Scholar] [CrossRef]
- Kryvoruchko, A.P.; Yurlova, L.Y. Influence of some organic and inorganic additives on pressure-driven purification of waters containing cobalt. J. Water Chem. Technol. 2015, 37, 271–276. [Google Scholar]
- Liu, X.; Wu, J.; Liu, C.; Wang, J. Removal of cobalt ions from aqueous solution by forward osmosis. Sep. Purif. Technol. 2017, 177, 8–20. [Google Scholar] [CrossRef]
- Jia, F.; Yin, Y.; Wang, J. Removal of cobalt ions from simulated radioactive wastewater by vacuum membrane distillation. Prog. Nucl. Energy 2018, 103, 20–27. [Google Scholar] [CrossRef]
- León, G.; Martínez, G.; Guzmán, M.A.; Moreno, J.I.; Miguel, B.; Fernández-López, J.A. Increasing stability and transport efficiency of supported liquid membranas through a novel ultrsound-assisted preparation method: Its application to cobalt(II) removal. Ultrason. Sonochem. 2013, 20, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Hachemaoui, A.; Belhamel, K. Simultaneous extraction and separation of cobalt and nickel from chloride solution through emulsion liquid membrane using Cyanex 301 as extractant. Int. J. Min. Process. 2017, 161, 7–12. [Google Scholar] [CrossRef]
- Vafaei, F.; Torkaman, R.; Moosavian, M.A.; Zaheri, P. Optimization of extraction conditions using centralcomposite design for the removal of Co(II) from chloride solution by supported liquid membrane. Chem. Eng. Res. Des. 2018, 133, 126–136. [Google Scholar] [CrossRef]
- Parbat, S.A.; Bhanvase, B.A.; Sonawane, S.H. Investigation on liquid emulsion membrane (LEM) prepared with hydrodynamic cavitation process for cobalt (II) extraction from wastewater. Sep. Purif. Technol. 2020, 237, 116385. [Google Scholar] [CrossRef]
- Sadyrbaeva, T.Z. Recovery of cobalt(II) by the hybrid liquid membrane—Electrodialysis—Electrolysis process. Electrochim. Acta 2014, 133, 161–168. [Google Scholar] [CrossRef]
- Sastre, A.M.; Kumar, A.; Shukla, J.P.; Singh, R.K. Improved techniques in liquid membrane separations: An overview. Sep. Purif. Meth. 1998, 27, 213–298. [Google Scholar] [CrossRef]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef]
- Masselin, I.; Chasseray, X.; Durand-Bourlier, L.; Lainé, J.M.; Syzaret, P.I.; Lemordant, D. Effect of sonication on polymeric membranes. J. Membr. Sci. 2001, 181, 213–220. [Google Scholar] [CrossRef]
- Kallioinen, M.; Mänttäri, M. Influence of ultrasonic treatment on various membrane materials: A Review. Sep. Sci. Technol. 2011, 46, 1388–1395. [Google Scholar] [CrossRef]
- León, G. Facilitated transport. In Encyclopedia of Membranes, 1st ed.; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 763–764. ISBN 978-3-642-40872-4. [Google Scholar]
- De Gyves, J.; Rodríguez, E. Metal ion separations by supported liquid membranes. Ind. Eng. Chem. Res. 1999, 38, 2182–2202. [Google Scholar] [CrossRef]
- Biswas, R.K.; Habib, M.A.; Islam, M.N. Some Physicochemical Properties of (D2EHPA). 1. Distribution, dimerization, and acid dissociation constants of D2EHPA in a Kerosene/0.10 kmol m−3 (Na+,H+)Cl− system and the extraction of Mn(II). Ind. Eng. Chem. Res. 2000, 39, 155–160. [Google Scholar] [CrossRef]
- Juang, R.S.; Su, J.Y. Thermodynamic equilibria of the extraction of cobalt (II) from sulfate solutions with bis(2-ethylhexyl)phosphoric acid. Ind. Eng. Chem. Res. 1992, 31, 2395–2400. [Google Scholar] [CrossRef]
- Miyake, Y.; Matsuyama, H.; Nishida, M.; Nakai, M.; Nagase, M.; Teramoto, M. Kinetics and mechanism of metal extraction with acidic organophosphorus extractants (I): Extraction rate limited by diffusion process. Hydrometallurgy 1990, 24, 19–35. [Google Scholar] [CrossRef]
- Sastre, A.M.; Madi, A.; Alguacil, F.J. Facilitated supported liquid-membrane transport of gold(I) using LIX 79 in cumene. J. Membr. Sci. 2000, 166, 213–219. [Google Scholar] [CrossRef]
- Huang, T.C.; Juang, R.S. Rate and mechanism of divalent metal transport through supported liquid membrane containing di(2-ethylhexyl)phosphoric acid as mobile carrier. J. Chem. Technol. Biotechnol. 1988, 42, 1–17. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Coedo, A.G.; Dorado, M.T. Transport of chromium(VI) through a Cyanex 923-xylene flat-sheet supported liquid membrane. Hydrometallurgy 2000, 57, 51–56. [Google Scholar] [CrossRef]
- Stolwijk, T.B.; Sudhoelter, E.J.R.; Reinhoudt, D.N. Crown ether mediated transport: A kinetic study of potassium perchlorate transport through a supported liquid membrane containing dibenzo-18-crown-6. J. Am. Chem. Soc. 1987, 109, 7042–7047. [Google Scholar] [CrossRef]
- Zaghbani, A.; Tayeb, R.; Dhahbi, M. Studies on the transport of chromium(III) through a supported liquid membrane containing D2EHPA as carrier. Desal. Water Treat. 2009, 12, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Ochromowicz, K.; Apostoluk, W. Modelling of carrier mediated transport of chromium(III) in the supported liquid membrane system with D2EHPA. Sep. Purif. Technol. 2010, 72, 112–117. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Alonso, M. Separation of zinc(II) from cobalt(II) solutions using supported liquid membranes with DP-8R (di(2-ethylhexyl) phosphoric acid) as carrier. Sep. Purif. Technol. 2005, 41, 179–184. [Google Scholar] [CrossRef]
- Van de Voorde, I.; Pinoy, L.; Courtijn, E.; Verpoort, F. Equilibrium Studies of Nickel(II), Copper(II), and Cobalt(II) Extraction with Aloxime 800, D2EHPA, and Cyanex Reagents. Solvent Extr. Ion. Exch. 2006, 24, 893–914. [Google Scholar] [CrossRef]
- Rubcumintara, T.; Han, K.N. The effect of concentration and temperature on diffusivity of metal compounds. Metall. Trans. B 1990, 21, 429–438. [Google Scholar] [CrossRef]



| ∆fbl (sm−1) | ∆m (sm−1) | δfbl (m) | Dbm (m2·s−1) |
|---|---|---|---|
| 3.7576 × 104 | 1.1434 × 1010 | 4.0206 × 10−6 | 4.0490 × 10−14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
León, G.; Hidalgo, A.M.; Miguel, B.; Guzmán, M.A. Pertraction of Co(II) through Novel Ultrasound Prepared Supported Liquid Membranes Containing D2EHPA. Optimization and Transport Parameters. Membranes 2020, 10, 436. https://doi.org/10.3390/membranes10120436
León G, Hidalgo AM, Miguel B, Guzmán MA. Pertraction of Co(II) through Novel Ultrasound Prepared Supported Liquid Membranes Containing D2EHPA. Optimization and Transport Parameters. Membranes. 2020; 10(12):436. https://doi.org/10.3390/membranes10120436
Chicago/Turabian StyleLeón, Gerardo, Asunción María Hidalgo, Beatriz Miguel, and María Amelia Guzmán. 2020. "Pertraction of Co(II) through Novel Ultrasound Prepared Supported Liquid Membranes Containing D2EHPA. Optimization and Transport Parameters" Membranes 10, no. 12: 436. https://doi.org/10.3390/membranes10120436
APA StyleLeón, G., Hidalgo, A. M., Miguel, B., & Guzmán, M. A. (2020). Pertraction of Co(II) through Novel Ultrasound Prepared Supported Liquid Membranes Containing D2EHPA. Optimization and Transport Parameters. Membranes, 10(12), 436. https://doi.org/10.3390/membranes10120436