Effect of Chitosan’s Degree of Deacetylation on the Performance of PES Membrane Infused with Chitosan during AMD Treatment
Abstract
:1. Introduction
2. Experimental Setup
2.1. Materials and Chemicals
2.2. Production of Chitosan from Chitin
- (i)
- Deproteinization: The crushed and milled seashells (chitin) were treated with an alkaline NaOH (6 w/w%) solution in a 500 mL Erlenmeyer flask at 60 °C. The concoction was stirred on a heating plate fitted with a magnetic stirrer for 2 h. After 2 h of stirring, the chitin was separated from the solution by decanting the alkaline solution. The collected chitin was rinsed with deionized water until the pH was measured neutral.
- (ii)
- Demineralization: After deproteinization, the resulting chitin was mixed for 2 h with 6% HCl solution in a 500 mL Erlenmeyer flask at 60 °C. After 2 h of mixing on heating plate equipped with a magnetic stirrer, the demineralized chitin was separated from the acidic solution by decanting the supernatant solution. The demineralized chitin was then washed with deionized water until neutral pH.
- (iii)
- Deacetylation: The deproteinized and demineralized chitin was treated with various NaOH concentration (20, 40 and 60 wt%) and temperature (80, 100 and 120 °C) to manipulate the degree of deacetylation (DD) of chitosan. Nine chitosan samples were synthesized and stored inside airtight containers. The solid to liquid ratio for all processes was set at 1:20. Nine chitosan samples were obtained and Table 1 shows synthesis process conditions and corresponding chitosan sample No.
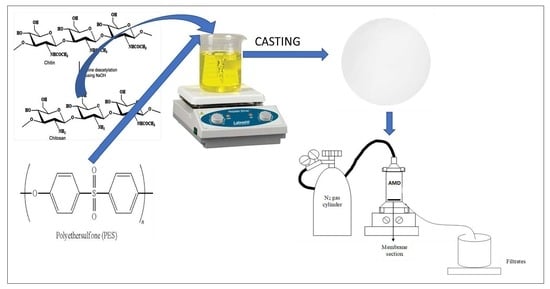
2.3. Fabrication of PES and Modified PES Membranes
2.4. Characterization of Chitosan and Membranes
2.5. Performance Evaluation of Fabricated Membranes Using Synthetic AMD
3. Results and Discussion
3.1. Membrane and Chitosan Characterization
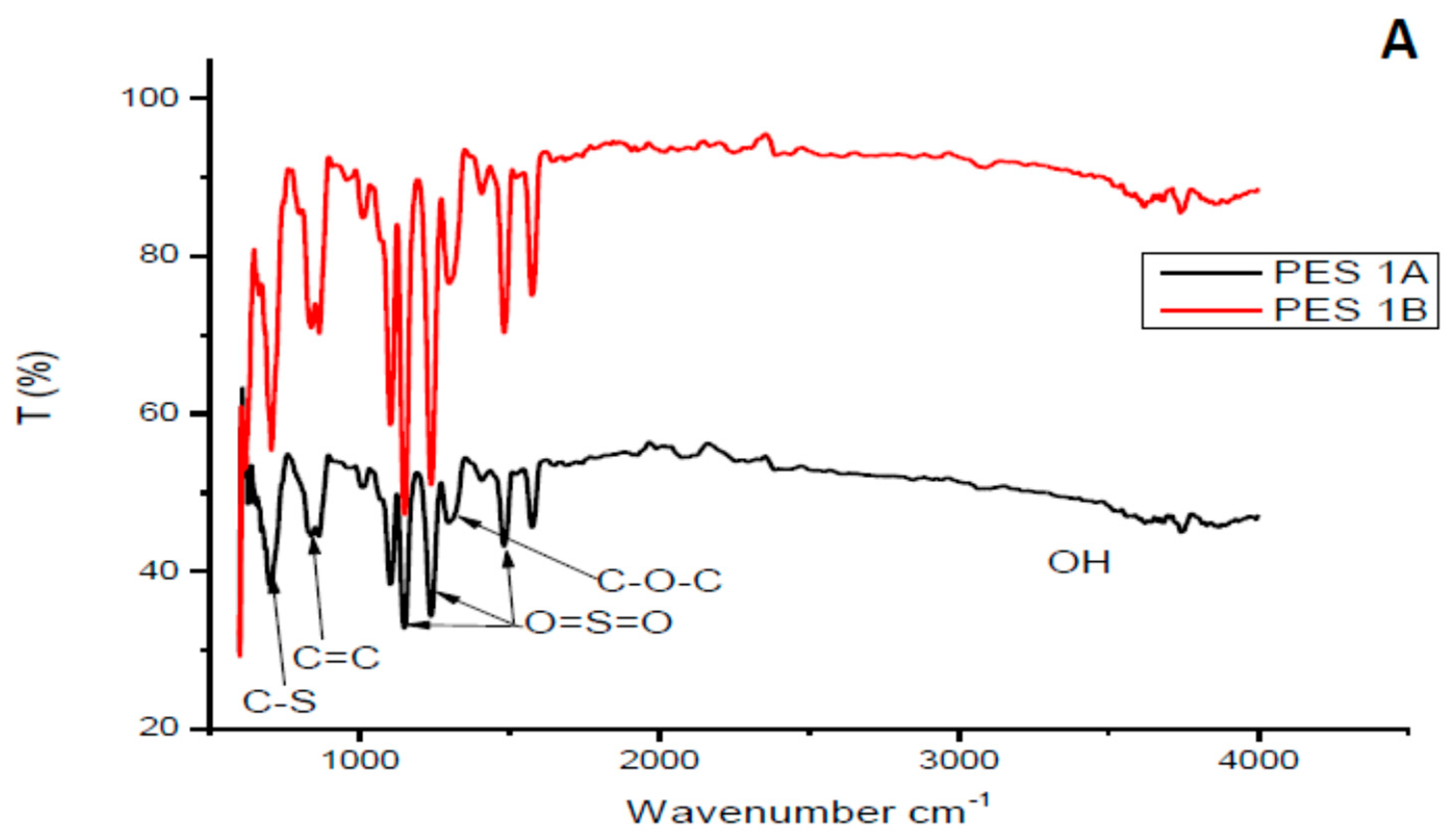
3.1.1. Fourier Transform Infrared Analysis of the Chitosan and Membranes
3.1.2. Chitosan Yield
3.1.3. Degree of Deacetylation of Chitosan
3.1.4. Degree of Hydrophilicity (Contact Angle Measurement) and Porosity Analysis
3.1.5. Thermal Stability of Membranes
3.2. Membrane Performance Evaluation
4. Conclusions
- Chitosan with the highest degree of deacetylation of 95.97% was reported with 40% NaOH concentration and temperature of 100 °C.
- Percentage yield of chitosan from chitin decreased as the concentration of NaOH and temperature increased, respectively. Higher temperature and NaOH concentration showed to have high capabilities to remove proteins from the chitin structure, thus, the low yield was expected.
- PES 5 was blended with chitosan having the highest degree of deacetylation of 95.975% and both reported the lowest contact angle values of 59.28°. Similarly, PES 1 was modified with chitosan having the lowest degree of deacetylation and showed to be hydrophobic.
- Pure water flux of the membranes showed to be enhanced with increasing degree of deacetylation.
- Similar behavior was observed for rejection investigations of the membranes. PES 5 reported high rejection and PES 1 had the least rejection. PES 5 with chitosan having the highest degree of deacetylation (95.97%) reported maximum rejection of 98.05, 97.39, 96.25, 95.24 and 80.34% for Mn2+, Fe2+, Mg2+ and Ca2+ and SO42−, respectively.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salahi, A.; Mohammadi, T.; Behbahani, R.M.; Hemmati, M. Asymmetric polyethersulfone ultrafiltration membranes for oily wastewater treatment: Synthesis, characterization, ANFIS modeling, and performance. J. Environ. Chem. Eng. 2015, 3, 170–178. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Chemical modification of polyethersulphone nanofiltration membranes: A review. J. Appl. Polym. Sci. 2009, 114, 630–642. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Feng, C.; Matsuura, T. The art of surface modification of synthetic polymeric membranes. J. Appl. Polym. Sci. 2010, 115, 855–895. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulphone membranes–a review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Garni, M.; Thamboo, S.; Schoenenberger, C.A.; Palivan, C.G. Biopores/membrane proteins in synthetic polymer membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 619–638. [Google Scholar] [CrossRef]
- Shockravi, A.; Vatanpour, V.; Najjar, Z.; Bahadori, S.; Javadi, A. A new high-performance polyamide as an effective additive for modification of antifouling properties and morphology of asymmetric PES blend ultrafiltration membranes. Microporous Mesoporous Mater. 2017, 246, 24–36. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Kuzmenko, D.; Gitis, V. Impact of chemical cleaning on properties and functioning of polyethersulphone membranes. Membr. Sci. 2007, 305, 176–184. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhang, H.; Liu, J.; Song, C. Biofouling control of halloysite nanotubes-decorated polyethersulfone ultrafiltration membrane modified with chitosan-silver nanoparticles. Chem. Eng. J. 2013, 228, 12–20. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Juang, R.S.; Shao, H.J. A simplified equilibrium model for sorption of heavy metal ions from aqueous solutions on chitosan. Water Res. 2002, 36, 2999–3008. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Rijith, S. Synthesis and characterization of carboxyl terminated poly (methacrylic acid) grafted chitosan/bentonite composite and its application for the recovery of uranium (VI) from aqueous media. J. Environ. Radioact. 2012, 106, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Reiad, N.A.; Salam, O.E.A.; Abadir, E.F.; Harraz, F.A. Adsorptive removal of iron and manganese ions from aqueous solutions with microporous chitosan/polyethylene glycol blend membrane. J. Environ. Sci. 2012, 24, 1425–1432. [Google Scholar] [CrossRef]
- Boricha, A.G.; Murthy, Z.V.P. Acrylonitrile butadiene styrene/chitosan blend membranes: Preparation, characterization and performance for the separation of heavy metals. J. Membr. Sci. 2009, 339, 239–249. [Google Scholar] [CrossRef]
- Hussain, M.R.; Iman, M.; Maji, T.K. Determination of degree of deacetylation of chitosan and their effect on the release behavior of essential oil from chitosan and chitosan-gelatin complex microcapsules. Int. J. Adv. Eng. Appl. 2013, 6, 4–12. [Google Scholar]
- Li, J.; Revol, J.F.; Marchessault, R.H. Effect of degree of deacetylation of chitin on the properties of chitin crystallites. J. Appl. Polym. Sci. 1997, 65, 373–380. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Tanhaei, B.; Ayati, A.; Bamoharram, F.F.; Lahtinen, M.; Sillanpää, M. A novel magnetic Preyssler acid grafted chitosan nano adsorbent: Synthesis, characterization and adsorption activity. J. Chem. Technol. Biotechnol. 2016, 91, 1452–1460. [Google Scholar] [CrossRef]
- Tutu, H.; McCarthy, T.S.; Cukrowska, E. The Chemical Characteristics of Acid Mine Drainage with Particular Reference to Sources, Distribution and Remediation: The Witwatersrand Basin, South Africa as a Case Study. Appl. Geochem. 2008, 23, 3666–3684. [Google Scholar] [CrossRef]
- Habiba, U.; Joo, T.C.; Siddique, T.A.; Salleh, A.; Ang, B.C.; Afifi, A.M. Effect of degree of deacetylation of chitosan on adsorption capacity and reusability of chitosan/polyvinyl alcohol/TiO2 nano composite. Int. J. Biol. Macromol. 2017, 104, 1133–1142. [Google Scholar] [CrossRef]
- Marei, N.H.; El-Samie, E.A.; Salah, T.; Saad, G.R.; Elwahy, A.H. Isolation and characterization of chitosan from different local insects in Egypt. Int. J. Biol. Macromol. 2016, 82, 871–877. [Google Scholar] [CrossRef]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Annamareddy, S.H.K.; Abanti, S.; Rath, P.K. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Liu, J.; Zhang, H.; Wang, K. Preparation and antibacterial property of polyethersulfone ultrafiltration hybrid membrane containing halloysite nanotubes loaded with copper ions. Chem. Eng. J. 2012, 210, 298–308. [Google Scholar] [CrossRef]
- Soon, C.Y.; Tee, Y.B.; Tan, C.H.; Rosnita, A.T.; Khalina, A. Extraction and physicochemical characterization of chitin and chitosan from Zophobas morio larvae in varying sodium hydroxide concentration. Int. J. Biol. Macromol. 2018, 108, 135–142. [Google Scholar] [CrossRef]
- Rinaudo, M.; Pavlov, G.; Desbrieres, J. Influence of acetic acid concentration on the solubilization of chitosan. Polymer 1999, 40, 7029–7032. [Google Scholar] [CrossRef]
- Palpandi, C.; Shanmugam, V.; Shanmugam, A. Extraction of chitin and chitosan from shell and operculum of mangrove gastropod Nerita (Dostia) crepidularia Lamarck. Int. J. Med. Med. Sci. 2009, 1, 198–205. [Google Scholar]
- Wang, F.J.; Li, W.; Xue, M.S.; Yao, J.P.; Lu, J.S. BaTiO3–polyethersulfone nanocomposites with high dielectric constant and excellent thermal stability. Compos. Part B Eng. 2011, 42, 87–91. [Google Scholar] [CrossRef]
- Akbari, A.; Derikvandi, Z.; Rostami, S.M.M. Influence of chitosan coating on the separation performance, morphology and anti-fouling properties of the polyamide nanofiltration membranes. J. Ind. Eng. Chem. 2015, 28, 268–276. [Google Scholar] [CrossRef]
- Tanninen, J.; Nyström, M. Separation of ions in acidic conditions using NF. Desalination 2002, 147, 295–299. [Google Scholar] [CrossRef]
- Mthethwa, V. Investigation of Polyethersulphone (PES) Hollow Fiber Membrane for The Treatment of Acid Mine Drainage; Research Report Master of Science; Engineering, Faculty of Engineering and the Built Environment, University of The Witwatersrand: Johannesburg, South Africa, 2014. [Google Scholar]






| Chitosan Sample No. | Synthesis Process | Conditions |
|---|---|---|
| Temperature( °C) | NaOH Conc (wt%) | |
| 1 | 80 | 20 |
| 2 | 80 | 40 |
| 3 | 80 | 60 |
| 4 | 100 | 20 |
| 5 | 100 | 40 |
| 6 | 100 | 60 |
| 7 | 120 | 20 |
| 8 | 120 | 40 |
| 9 | 120 | 60 |
| Species | Concentration (mg/L) pH = 3.2 | Salt Dissolved |
|---|---|---|
| Fe2+ | 933 | FeSO4·7H2O |
| Ca2+ | 461 | CaSO4·2H2O |
| Mg2+ | 345 | MgSO4·7H2O |
| Mn2+ | 321 | MnSO4·H2O |
| SO42− | 4556 | Na2SO4 |
| Metal | Lamp Current | Wavelength | Flame Used |
|---|---|---|---|
| (mA) | (nm) | ||
| Al | 10 | 309.27 | Air/Acetylene/N2O |
| Ca | 18 | 422.67 | Air/Acetylene |
| Co | 10 | 240.73 | Air/Acetylene |
| Cu | 4 | 324.75 | Air/Acetylene |
| Fe | 15 | 248.30 | Air/Acetylene |
| Mg | 18 | 285.21 | Air/Acetylene |
| Mn | 25 | 279.50 | Air/Acetylene |
| Na | 5 | 589.00 | Air/Acetylene |
| Ni | 5 | 232.00 | Air/Acetylene |
| Chitosan Sample No. | %Degree of Deacetylation | PES/Chitosan Membranes Coded Names |
|---|---|---|
| 1 | 33.93 | PES 1 |
| 2 | 73.05 | PES 2 |
| 3 | 60.82 | PES 3 |
| 4 | 61.94 | PES 4 |
| 5 | 95.97 | PES 5 |
| 6 | 82.92 | PES 6 |
| 7 | 79.92 | PES 7 |
| 8 | 90.17 | PES 8 |
| 9 | 85.55 | PES 9 |
| Chitosan Sample No. | %Yield | Standard Deviation |
|---|---|---|
| 1 | 28.8 | 5.4 |
| 2 | 25.3 | 1.9 |
| 3 | 18.2 | 2.6 |
| 4 | 23.8 | 4.0 |
| 5 | 19.8 | 3.5 |
| 6 | 14.2 | 1.6 |
| 7 | 13.7 | 4.2 |
| 8 | 12.7 | 0.9 |
| 9 | 10.8 | 1.6 |
| Sample No. | Experimental Conditions | DD% | Standard Deviation | |
|---|---|---|---|---|
| Temperature (°C) | NaOH Conc (wt%) | |||
| 1 | 80 | 20 | 33.93 | 1.41 |
| 2 | 80 | 40 | 73.05 | 5.20 |
| 3 | 80 | 60 | 60.82 | 1.67 |
| 4 | 100 | 20 | 61.94 | 0.10 |
| 5 | 100 | 40 | 95.97 | 1.49 |
| 6 | 100 | 60 | 82.92 | 2.65 |
| 7 | 120 | 20 | 79.92 | 3.59 |
| 8 | 120 | 40 | 90.17 | 0.82 |
| 9 | 120 | 60 | 85.55 | 5.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathaba, M.; Daramola, M.O. Effect of Chitosan’s Degree of Deacetylation on the Performance of PES Membrane Infused with Chitosan during AMD Treatment. Membranes 2020, 10, 52. https://doi.org/10.3390/membranes10030052
Mathaba M, Daramola MO. Effect of Chitosan’s Degree of Deacetylation on the Performance of PES Membrane Infused with Chitosan during AMD Treatment. Membranes. 2020; 10(3):52. https://doi.org/10.3390/membranes10030052
Chicago/Turabian StyleMathaba, Machodi, and Michael Olawale Daramola. 2020. "Effect of Chitosan’s Degree of Deacetylation on the Performance of PES Membrane Infused with Chitosan during AMD Treatment" Membranes 10, no. 3: 52. https://doi.org/10.3390/membranes10030052
APA StyleMathaba, M., & Daramola, M. O. (2020). Effect of Chitosan’s Degree of Deacetylation on the Performance of PES Membrane Infused with Chitosan during AMD Treatment. Membranes, 10(3), 52. https://doi.org/10.3390/membranes10030052