Enhancing the Separation Performance of Glassy PPO with the Addition of a Molecular Sieve (ZIF-8): Gas Transport at Various Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Mixed-Matrix Membrane Preparation
Membrane Casting
Thermal Annealing
2.2.2. Density Measurement
2.2.3. Morphological Characterization
2.2.4. Thermal and Calorimetric Properties
2.2.5. Gas Permeability Experiments
3. Results and Discussion
3.1. Aspect of the MMMs
3.2. Density
3.3. SEM Analysis
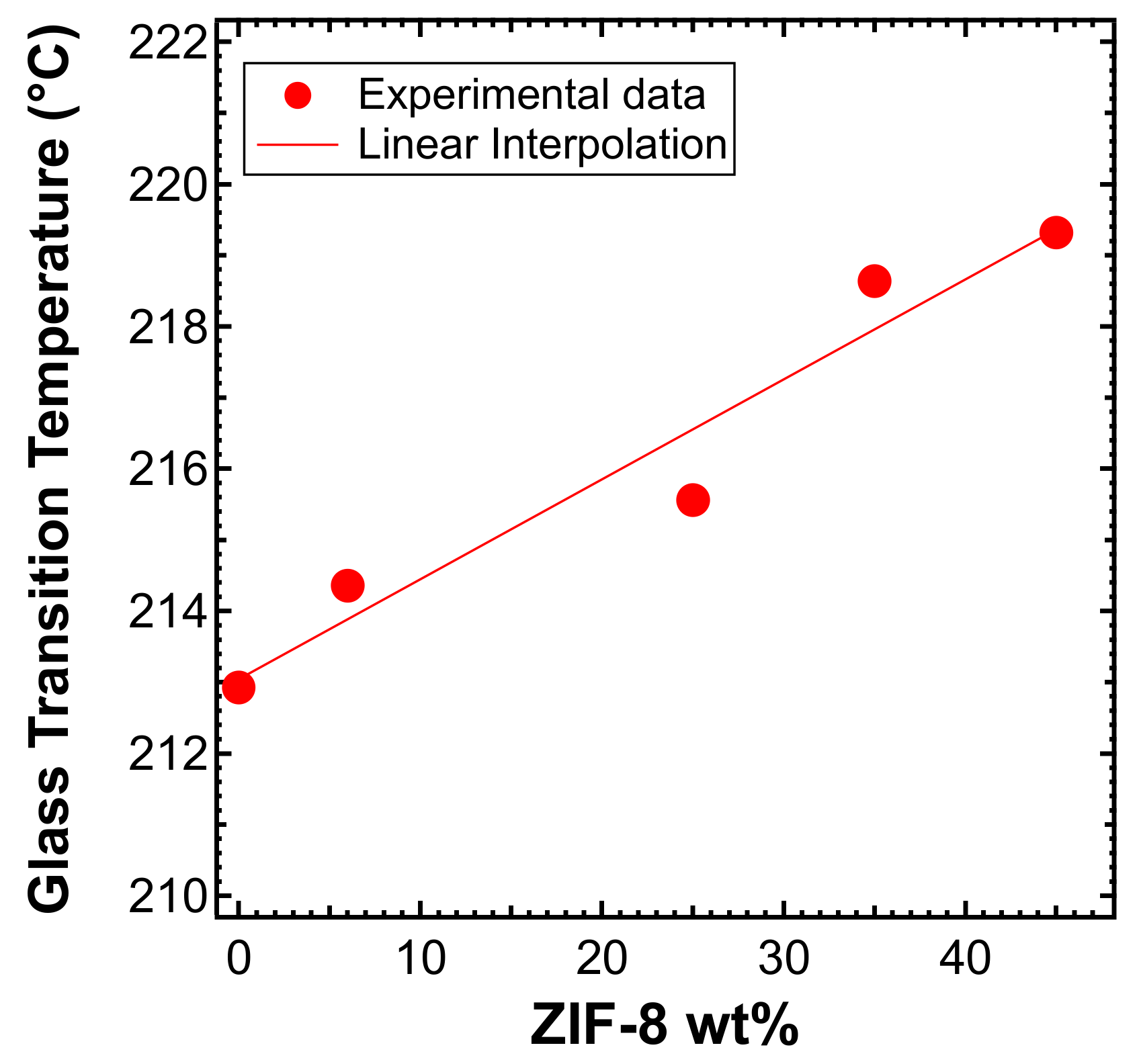
3.4. DSC Tests
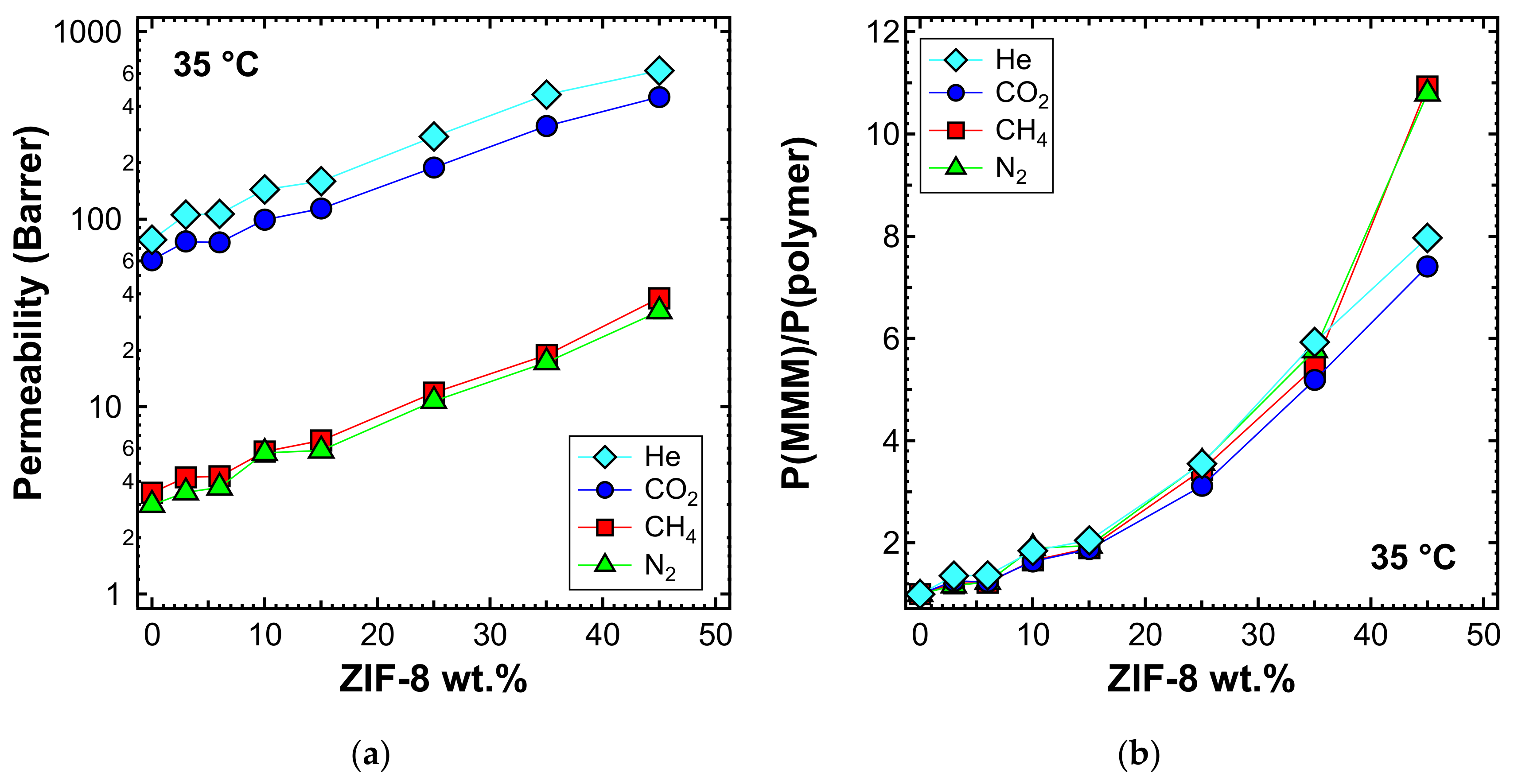
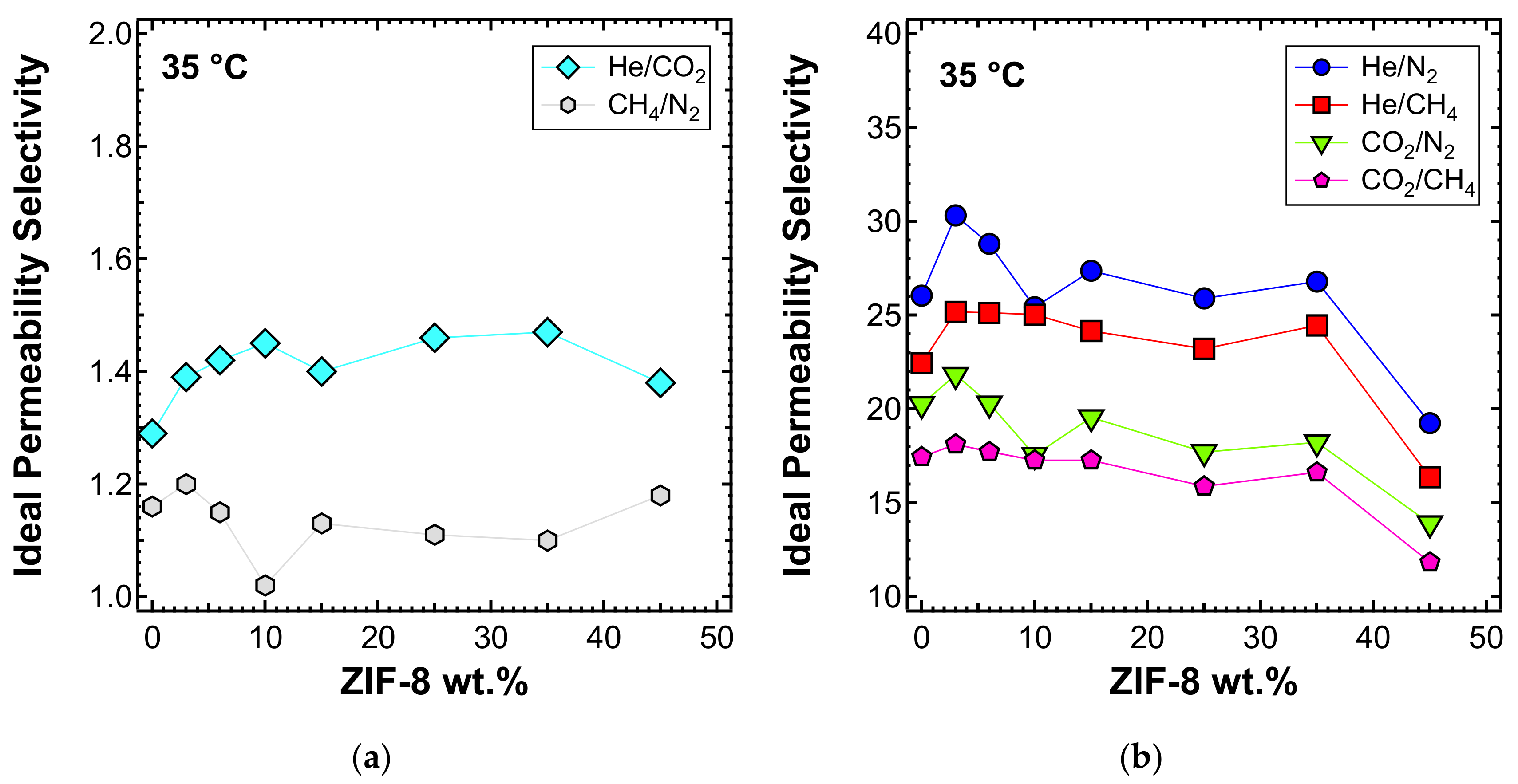
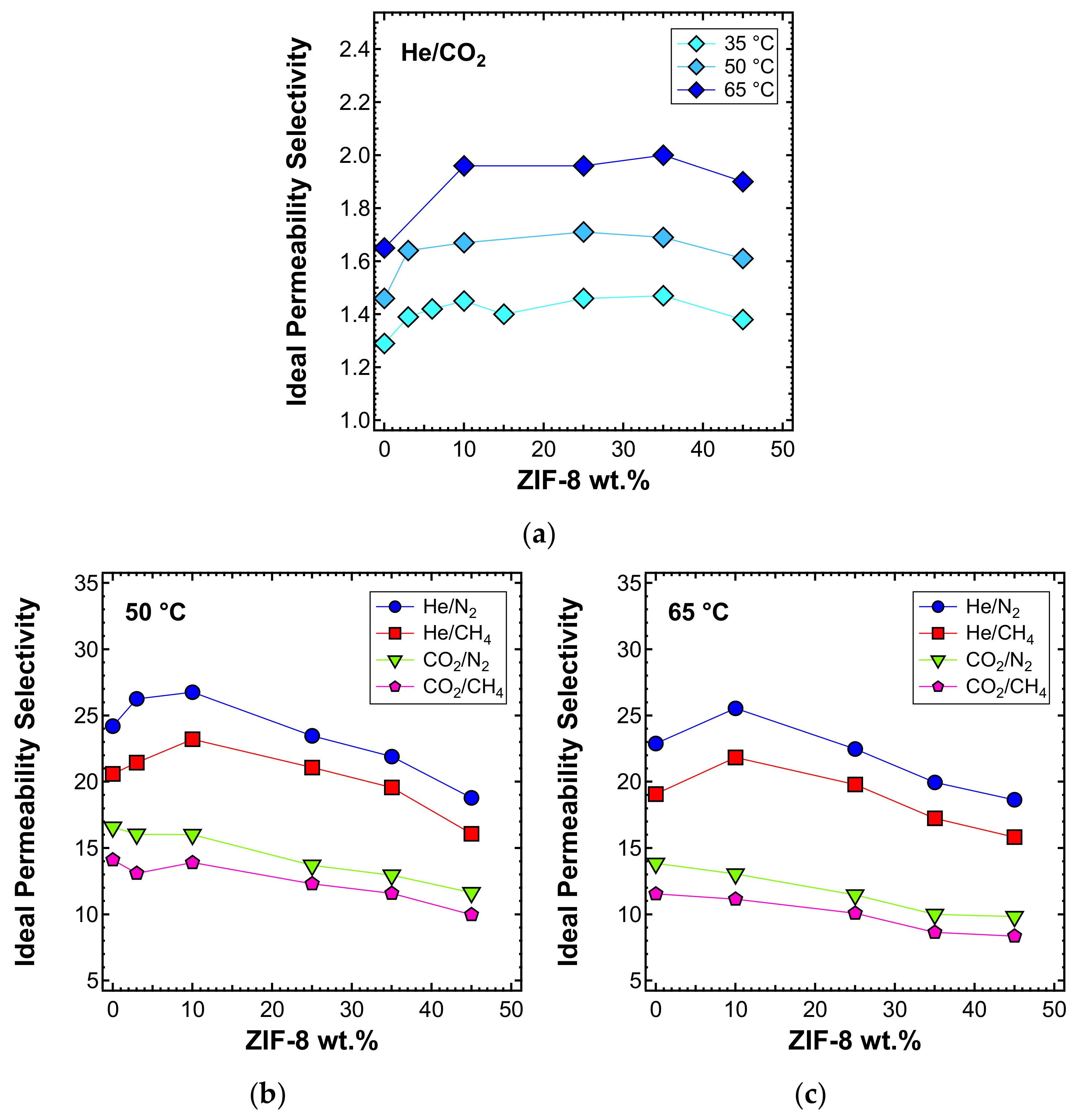
3.5. Permeability and Permselectivity
3.5.1. Gas Transport in PPO and ZIF-8
3.5.2. Thermal Annealing
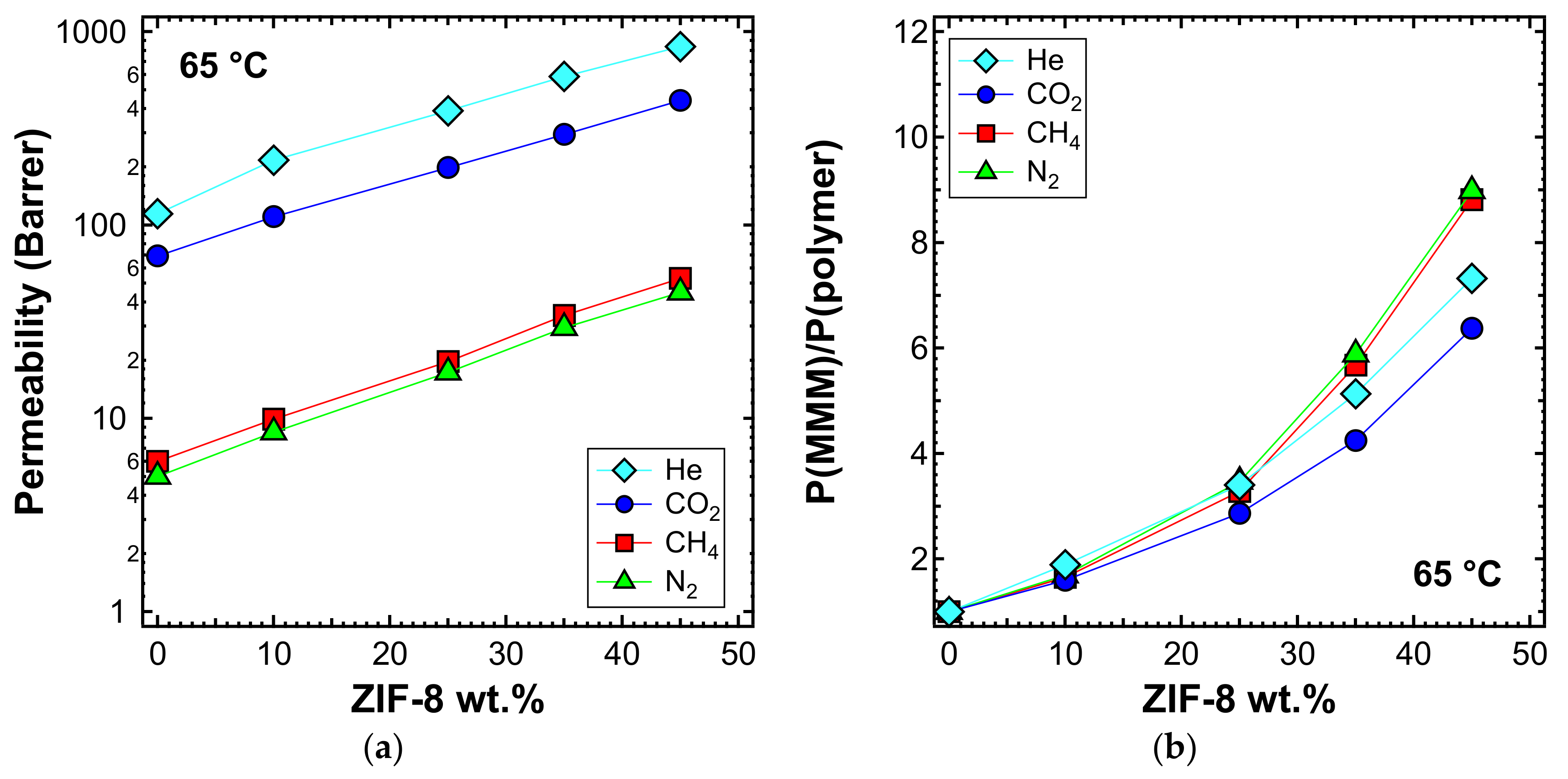
3.5.3. Effect of the Filler Loading
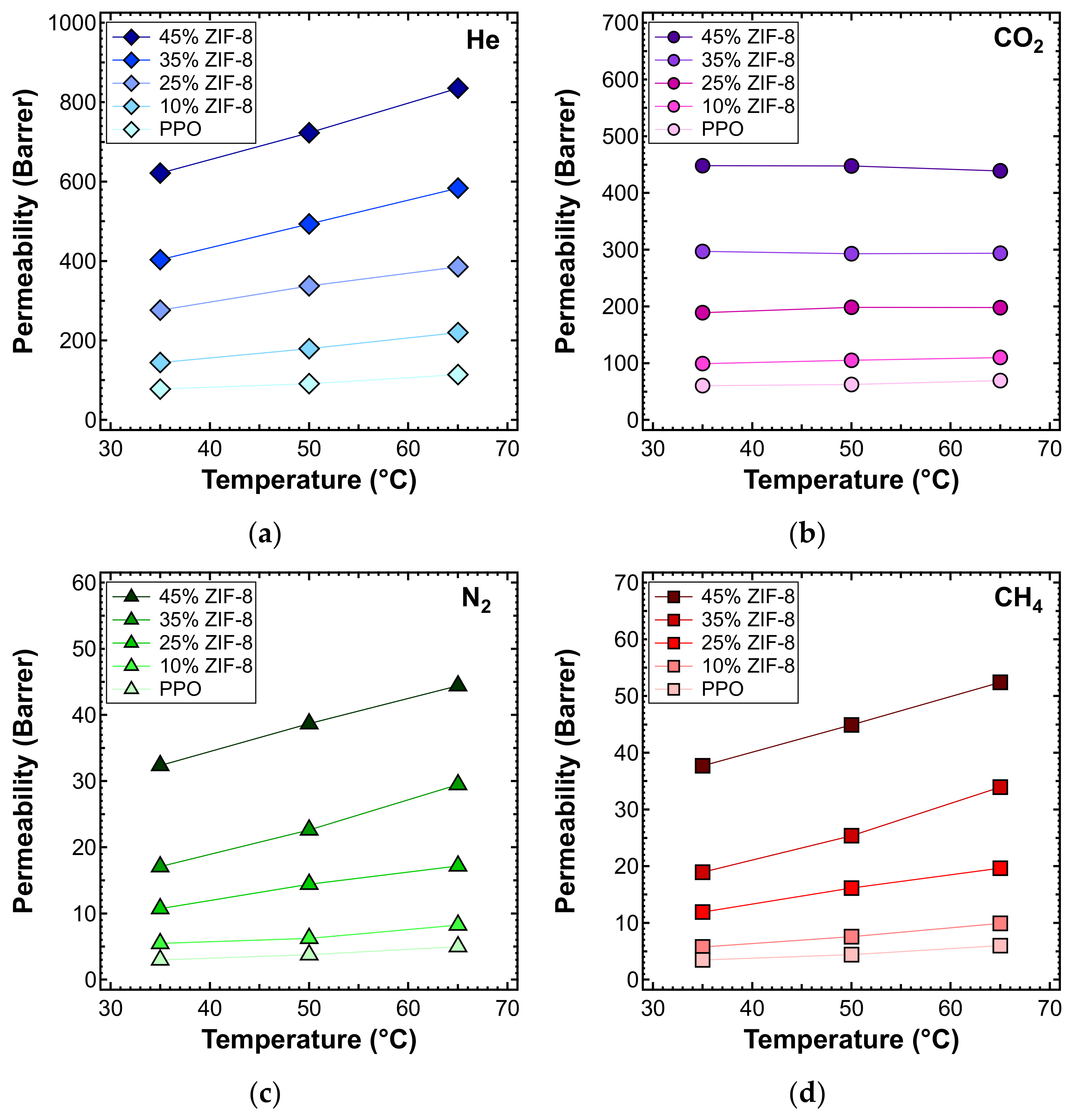
3.5.4. Effect of Temperature
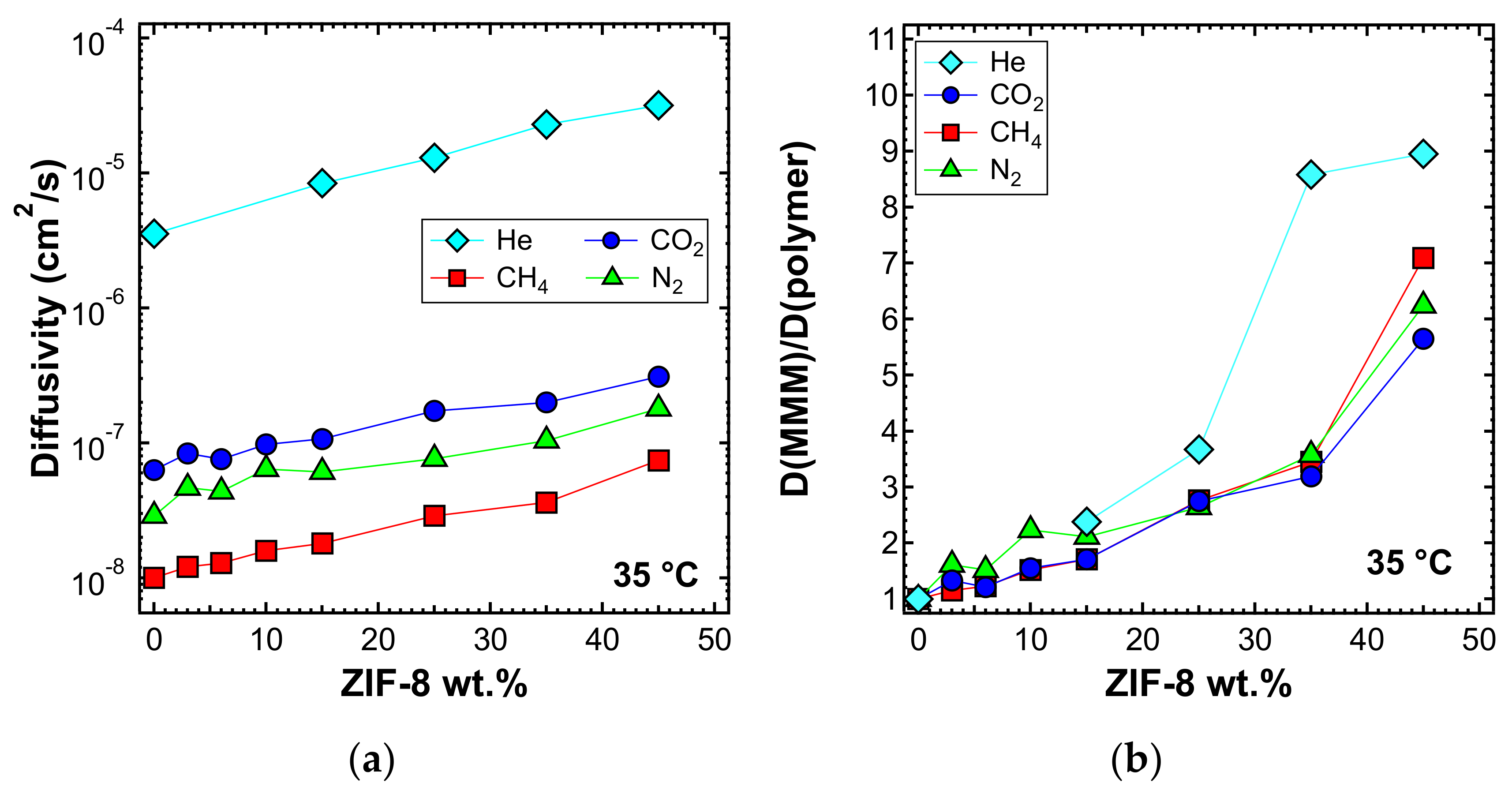
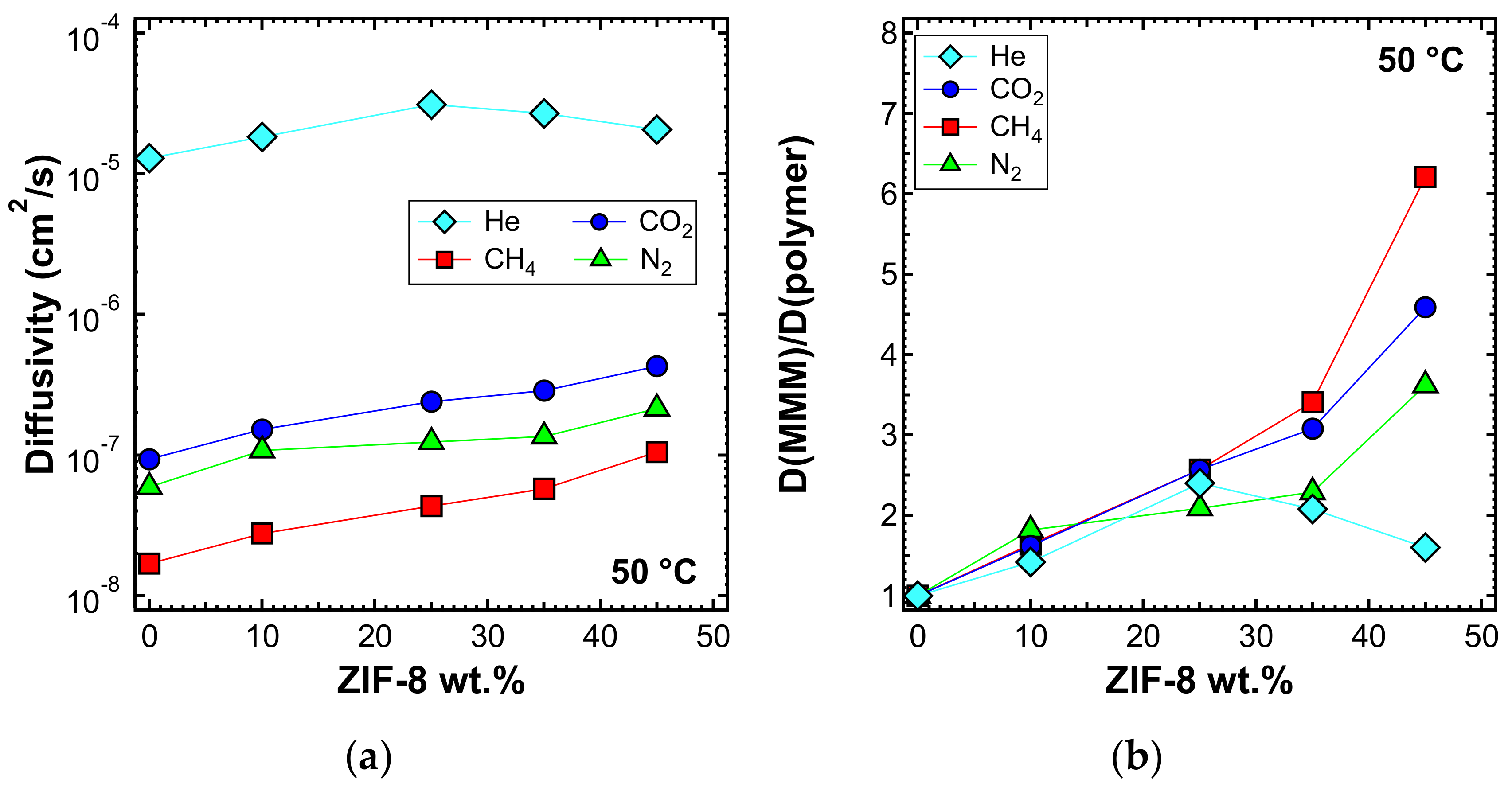
3.6. Diffusivity
3.6.1. Effect of Filler Loading
3.6.2. Effect of Temperature
3.7. Estimated Solubility
Effect of Temperature: Sorption Enthalpy ()
3.8. Performance Evaluation Using a Robeson Upper Bound Plot
3.9. Comparison with Other MMMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ockwig, N.W.; Nenoff, T.M. Membranes for hydrogen separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef] [PubMed]
- Koros, W.J.; Mahajan, R. Pushing the limits on possibilities for large scale gas separation: Which strategies? J. Membr. Sci. 2000, 175, 181–196. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Henis, J.M.S.; Tripodi, M.K. A Novel Approach to Gas Separations Using Composite Hollow Fiber Membranes. Sep. Sci. Technol. 1980, 15, 1059–1068. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Rose, I.; Bezzu, C.G.; Carta, M.; Comesanã-Gándara, B.; Lasseuguette, E.; Ferrari, M.C.; Bernardo, P.; Clarizia, G.; Fuoco, A.; Jansen, J.C.; et al. Polymer ultrapermeability from the inefficient packing of 2D chains. Nat. Mater. 2017, 16, 932–937. [Google Scholar] [CrossRef]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. An efficient polymer molecular sieve for membrane gas separations. Science 2013, 339, 303–307. [Google Scholar] [CrossRef]
- Budd, P.M.; Elabas, E.S.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E.; Wang, D. Solution-Processed, Organophilic Membrane Derived from a Polymer of Intrinsic Microporosity. Adv. Mater. 2004, 16, 456–459. [Google Scholar] [CrossRef]
- Lai, H.W.H.; Benedetti, F.M.; Jin, Z.; Teo, Y.C.; Wu, A.X.; De Angelis, M.G.; Smith, Z.P.; Xia, Y. Tuning the Molecular Weights, Chain Packing, and Gas-Transport Properties of CANAL Ladder Polymers by Short Alkyl Substitutions. Macromolecules 2019, 52, 6294–6302. [Google Scholar] [CrossRef]
- He, Y.; Benedetti, F.M.; Lin, S.; Liu, C.; Zhao, Y.; Ye, H.; Van Voorhis, T.; De Angelis, M.G.; Swager, T.M.; Smith, Z.P. Polymers with Side Chain Porosity for Ultrapermeable and Plasticization Resistant Materials for Gas Separations. Adv. Mater. 2019, 31, 1807871. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Peng, Y.; Qian, Y.; Yuan, D.; Addicoat, M.A.; Heine, T.; Hu, Z.; Tee, L.; Guo, Z.; Zhao, D. Mixed Matrix Membranes (MMMs) Comprising Exfoliated 2D Covalent Organic Frameworks (COFs) for Efficient CO2 Separation. Chem. Mater. 2016, 28, 1277–1285. [Google Scholar] [CrossRef]
- Biswal, B.P.; Chaudhari, H.D.; Banerjee, R.; Kharul, U.K. Chemically Stable Covalent Organic Framework (COF)-Polybenzimidazole Hybrid Membranes: Enhanced Gas Separation through Pore Modulation. Chem. A Eur. J. 2016, 22, 4695–4699. [Google Scholar] [CrossRef] [PubMed]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-Matrix Membranes. Angew. Chem. Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Challenges and opportunities for mixed-matrix membranes for gas separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Zornoza, B.; Tellez, C.; Coronas, J.; Gascon, J.; Kapteijn, F. Metal organic framework based mixed matrix membranes: An increasingly important field of research with a large application potential. Microporous Mesoporous Mater. 2013, 166, 67–78. [Google Scholar] [CrossRef]
- Chung, T.S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Olivieri, L.; Ligi, S.; De Angelis, M.G.; Cucca, G.; Pettinau, A. Effect of Graphene and Graphene Oxide Nanoplatelets on the Gas Permselectivity and Aging Behavior of Poly(trimethylsilyl propyne) (PTMSP). Ind. Eng. Chem. Res. 2015, 54, 11199–11211. [Google Scholar] [CrossRef]
- Althumayri, K.; Harrison, W.J.; Shin, Y.; Gardiner, J.M.; Casiraghi, C.; Budd, P.M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. The influence of few-layer graphene on the gas permeability of the high-free-volume polymer PIM-1. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374. [Google Scholar] [CrossRef] [Green Version]
- Merkel, T.C.; He, Z.; Pinnau, I.; Freeman, B.D.; Meakin, P.; Hill, A.J. Sorption and Transport in Poly(2,2-bis(trifluoromethyl)-4,5-difluoro-1,3-dioxole-co-tetrafluoroethylene) Containing Nanoscale Fumed Silica. Macromolecules 2003, 36, 8406–8414. [Google Scholar] [CrossRef]
- Chi, W.S.; Sundell, B.J.; Zhang, K.; Harrigan, D.J.; Hayden, S.C.; Smith, Z.P. Mixed-Matrix Membranes Formed from Multi-Dimensional Metal–Organic Frameworks for Enhanced Gas Transport and Plasticization Resistance. ChemSusChem 2019, 12, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Li, H.; Chen, V. Plasticization mechanisms and effects of thermal annealing of Matrimid hollow fiber membranes for CO2 removal. J. Membr. Sci. 2011, 369, 206–220. [Google Scholar] [CrossRef]
- Li, J.; Sculley, J.; Zhou, H. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal–organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Yehia, H.; Pisklak, T.J.; Balkus, K.J.; Musselman, I.H. Methane Facilitated Transport Using Copper(II) Biphenyl Dicarboxylate-Triethylenediamine/Poly (3-Acetoxyethylthiophene) Mixed Matrix Membranes. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2004. [Google Scholar]
- Mahajan, R.; Koros, W.J. Factors Controlling Successful Formation of Mixed-Matrix Gas Separation Materials. Ind. Eng. Chem. Res. 2000, 39, 2692–2696. [Google Scholar] [CrossRef]
- Mahajan, R.; Koros, W.J. Mixed matrix membrane materials with glassy polymers. Part 1. Polym. Eng. Sci. 2002, 42, 1420–1431. [Google Scholar] [CrossRef]
- Caro, J. Are MOF membranes better in gas separation than those made of zeolites? Curr. Opin. Chem. Eng. 2011, 1, 77–83. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Zhang, S.; Hu, L.; Jin, J. Interfacial Design of Mixed Matrix Membranes for Improved Gas Separation Performance. Adv. Mater. 2016, 28, 3399–3405. [Google Scholar] [CrossRef]
- Xin, Q.; Ouyang, J.; Liu, T.; Li, Z.; Li, Z.; Liu, Y.; Wang, S.; Wu, H.; Jiang, Z.; Cao, X. Enhanced Interfacial Interaction and CO2 Separation Performance of Mixed Matrix Membrane by Incorporating Polyethylenimine-Decorated Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2015, 7, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Wu, A.X.; Chi, W.S.; Asinger, P.A.; Lin, S.; Hypsher, A.; Smith, Z.P. Mixed-Matrix Membranes Formed from Imide-Functionalized UiO-66-NH 2 for Improved Interfacial Compatibility. ACS Appl. Mater. Interfaces 2019, 11, 31257–31269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, F.; Bux, H.; Yang, W.; Caro, J. Zeolitic imidazolate framework ZIF-7 based molecular sieve membrane for hydrogen separation. J. Membr. Sci. 2010, 354, 48–54. [Google Scholar] [CrossRef]
- Melgar, V.M.A.; Kim, J.; Othman, M.R. Zeolitic imidazolate framework membranes for gas separation: A review of synthesis methods and gas separation performance. J. Ind. Eng. Chem. 2015, 28, 1–15. [Google Scholar] [CrossRef]
- Parent, L.R.; Pham, C.H.; Patterson, J.P.; Denny, M.S.; Cohen, S.M.; Gianneschi, N.C.; Paesani, F. Pore Breathing of Metal-Organic Frameworks by Environmental Transmission Electron Microscopy. J. Am. Chem. Soc. 2017, 139, 13973–13976. [Google Scholar] [CrossRef]
- Hyun, S.; Lee, J.H.; Jung, G.Y.; Kim, Y.K.; Kim, T.K.; Jeoung, S.; Kwak, S.K.; Moon, D.; Moon, H.R. Exploration of Gate-Opening and Breathing Phenomena in a Tailored Flexible Metal–Organic Framework. Inorg. Chem. 2016, 55, 1920–1925. [Google Scholar] [CrossRef]
- Shieh, J.-J.; Chung, T.S. Gas permeability, diffusivity, and solubility of poly(4-vinylpyridine) film. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2851–2861. [Google Scholar] [CrossRef]
- Bux, H.; Liang, F.; Li, Y.; Cravillon, J.; Wiebcke, M.; Caro, J. Zeolitic Imidazolate Framework Membrane with Molecular Sieving Properties by Microwave-Assisted Solvothermal Synthesis. J. Am. Chem. Soc. 2009, 131, 16000–16001. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Varela-Guerrero, V.; Barnett, G.V.; Jeong, H.K. Synthesis of zeolitic imidazolate framework films and membranes with controlled microstructures. Langmuir 2010, 26, 14636–14641. [Google Scholar] [CrossRef]
- Bux, H.; Feldhoff, A.; Cravillon, J.; Wiebcke, M.; Li, Y.-S.; Caro, J. Oriented Zeolitic Imidazolate Framework-8 Membrane with Sharp H2/C3H8 Molecular Sieve Separation. Chem. Mater. 2011, 23, 2262–2269. [Google Scholar] [CrossRef]
- Melgar, V.M.A.; Ahn, H.; Kim, J.; Othman, M.R. Highly selective micro-porous ZIF-8 membranes prepared by rapid electrospray deposition. J. Ind. Eng. Chem. 2015, 21, 575–579. [Google Scholar] [CrossRef]
- Hara, N.; Yoshimune, M.; Negishi, H.; Haraya, K.; Hara, S.; Yamaguchi, T. Diffusive separation of propylene/propane with ZIF-8 membranes. J. Membr. Sci. 2014, 450, 215–223. [Google Scholar] [CrossRef]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Merkel, T.C.; Zhou, M.; Baker, R.W. Carbon dioxide capture with membranes at an IGCC power plant. J. Membr. Sci. 2012, 389, 441–450. [Google Scholar] [CrossRef]
- Marano, J.J.; Ciferino, J.P. Integration of Gas Separation Membranes with IGCC Identifying the right membrane for the right job. Energy Procedia 2009, 1, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Ordoñez, M.J.C.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Molecular sieving realized with ZIF-8/Matrimid® mixed-matrix membranes. J. Membr. Sci. 2010, 361, 28–37. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Vega, M.; Paul, D.R. Gas transport properties of polyphenylene ethers. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1577–1589. [Google Scholar] [CrossRef]
- Alentiev, A.; Drioli, E.; Gokzhaev, M.; Golemme, G.; Ilinich, O.; Lapkin, A.; Volkov, V.; Yampolskii, Y. Gas permeation properties of phenylene oxide polymers. J. Membr. Sci. 1998, 138, 99–107. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Effect of Molecular Weight and Temperature on Physical Aging of ThinGlassy Poly(2,6-dimethyl-1,4-phenylene oxide) Films. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 1390–1398. [Google Scholar] [CrossRef]
- Galizia, M.; Daniel, C.; Fasano, G.; Guerra, G.; Mensitieri, G. Gas Sorption and Diffusion in Amorphous and Semicrystalline Nanoporous Poly(2,6-dimethyl-1,4-phenylene)oxide. Macromolecules 2012, 45, 3604–3615. [Google Scholar] [CrossRef]
- Khan, A.L.; Cano-Odena, A.; Gutiérrez, B.; Minguillón, C.; Vankelecom, I.F.J. Hydrogen separation and purification using polysulfone acrylate–zeolite mixed matrix membranes. J. Membr. Sci. 2010, 350, 340–346. [Google Scholar] [CrossRef]
- Song, Q.; Nataraj, S.K.; Roussenova, M.V.; Tan, J.C.; Hughes, D.J.; Li, W.; Bourgoin, P.; Alam, M.A.; Cheetham, A.K.; Al-Muhtaseb, S.A.; et al. Zeolitic imidazolate framework (ZIF-8) based polymer nanocomposite membranes for gas separation. Energy Environ. Sci. 2012, 5, 8359. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, Y.; Chung, T.-S. Poly-/metal-benzimidazole nano-composite membranes for hydrogen purification. Energy Environ. Sci. 2011, 4, 4171. [Google Scholar] [CrossRef]
- Yang, T.; Chung, T.-S. High performance ZIF-8/PBI nano-composite membranes for high temperature hydrogen separation consisting of carbon monoxide and water vapor. Int. J. Hydrog. Energy 2013, 38, 229–239. [Google Scholar] [CrossRef]
- Yang, T.; Shi, G.M.; Chung, T.-S. Symmetric and Asymmetric Zeolitic Imidazolate Frameworks (ZIFs)/Polybenzimidazole (PBI) Nanocomposite Membranes for Hydrogen Purification at High Temperatures. Adv. Energy Mater. 2012, 2, 1358–1367. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, Y.; Johnson, J.R.; Karvan, O.; Koros, W.J. High performance ZIF-8/6FDA-DAM mixed matrix membrane for propylene/propane separations. J. Membr. Sci. 2012, 389, 34–42. [Google Scholar] [CrossRef]
- Díaz, K.; López-González, M.; Del Castillo, L.F.; Riande, E. Effect of zeolitic imidazolate frameworks on the gas transport performance of ZIF8-poly(1,4-phenylene ether-ether-sulfone) hybrid membranes. J. Membr. Sci. 2011, 383, 206–213. [Google Scholar] [CrossRef]
- Bushell, A.F.; Attfield, M.P.; Mason, C.R.; Budd, P.M.; Yampolskii, Y.; Starannikova, L.; Rebrov, A.; Bazzarelli, F.; Bernardo, P.; Jansen, J.C.; et al. Gas permeation parameters of mixed matrix membranes based on the polymer of intrinsic microporosity PIM-1 and the zeolitic imidazolate framework ZIF-8. J. Membr. Sci. 2013, 427, 48–62. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Hou, J.; Li, H.; Zhang, Y.; Chen, V. Improved operational stability of Pebax-based gas separation membranes with ZIF-8: A comparative study of flat sheet and composite hollow fibre membranes. J. Membr. Sci. 2017, 524, 266–279. [Google Scholar] [CrossRef]
- Ma, X.; Swaidan, R.J.; Wang, Y.; Hsiung, C.; Han, Y.; Pinnau, I. Highly Compatible Hydroxyl-Functionalized Microporous Polyimide-ZIF-8 Mixed Matrix Membranes for Energy Efficient Propylene/Propane Separation. ACS Appl. Nano Mater. 2018, 1, 3541–3547. [Google Scholar] [CrossRef]
- Küsgens, P.; Rose, M.; Senkovska, I.; Fröde, H.; Henschel, A.; Siegle, S.; Kaskel, S. Characterization of metal-organic frameworks by water adsorption. Microporous Mesoporous Mater. 2009, 120, 325–330. [Google Scholar] [CrossRef]
- Ortiz, A.U.; Freitas, A.P.; Boutin, A.; Fuchs, A.H.; Coudert, F.X. What makes zeolitic imidazolate frameworks hydrophobic or hydrophilic? the impact of geometry and functionalization on water adsorption. Phys. Chem. Chem. Phys. 2014, 16, 9940–9949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khulbe, K.C.; Matsuura, T.; Lamarche, G.; Kim, H.J. The morphology characterisation and performance of dense PPO membranes for gas separation. J. Membr. Sci. 1997, 135, 211–223. [Google Scholar] [CrossRef]
- Khayet, M.; Villaluenga, J.P.G.; Godino, M.P.; Mengual, J.I.; Seoane, B.; Khulbe, K.C.; Matsuura, T. Preparation and application of dense poly(phenylene oxide) membranes in pervaporation. J. Colloid Interface Sci. 2004, 278, 410–422. [Google Scholar] [CrossRef]
- Das, M.; Perry, J.D.; Koros, W.J. Gas-Transport-Property Performance of Hybrid Carbon Molecular Sieve−Polymer Materials. Ind. Eng. Chem. Res. 2010, 49, 9310–9321. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineers’ Handbook, 7th ed.; McGraw-Hill: New York, NY, USA, 1999; pp. 291–293. [Google Scholar]
- Horn, N.R.; Paul, D.R. Carbon dioxide plasticization and conditioning effects in thick vs. thin glassy polymer films. Polymer 2011, 52, 1619–1627. [Google Scholar] [CrossRef]
- Bos, A.; Pünt, I.G.M.; Wessling, M.; Strathmann, H. CO2-induced plasticization phenomena in glassy polymers. J. Membr. Sci. 1999, 155, 67–78. [Google Scholar] [CrossRef]
- Handa, Y.P.; Lampron, S.; O’neill, M.L. On the plasticization of poly(2,6-dimethyl phenylene oxide) by CO2. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 2549–2553. [Google Scholar] [CrossRef] [Green Version]
- Minelli, M.; Deangelis, M.; Doghieri, F.; Marini, M.; Toselli, M.; Pilati, F. Oxygen permeability of novel organic–inorganic coatings: I. Effects of organic–inorganic ratio and molecular weight of the organic component. Eur. Polym. J. 2008, 44, 2581–2588. [Google Scholar] [CrossRef]
- Bevington, P.R.; Robinson, D.K. Error Data Reduction and Error Analysis for the Physical Sciences, 3rd ed.; AIP Publishing LLC: Melville, NY, USA, 1992. [Google Scholar] [CrossRef]
- Daynes, H.A. The Process of Diffusion through a Rubber Membrane. Proc. R. Soc. A Math. Phys. Eng. Sci. 1920, 97, 286–307. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- Moaddeb, M.; Koros, W.J. Gas transport properties of thin polymeric membranes in the presence of silicon dioxide particles. J. Membr. Sci. 1997, 125, 143–163. [Google Scholar] [CrossRef]
- Muller, J.C.M.; Hakvoort, G.; Jansen, J.C. DSC and TG study of water adsorption and desorption on zeolite NaA: Powder and attached as layer on metal. J. Therm. Anal. Calorim. 1998, 53, 449–466. [Google Scholar] [CrossRef]
- Story, B.J.; Koros, W.J. Sorption of CO2/CH4 mixtures in poly(phenylene oxide) and a carboxylated derivative. J. Appl. Polym. Sci. 1991, 42, 2613–2626. [Google Scholar] [CrossRef]
- Toi, K.; Morel, G.; Paul, D.R. Gas sorption and transport in poly(phenylene oxide) and comparisons with other glassy polymers. J. Appl. Polym. Sci. 1982, 27, 2997–3005. [Google Scholar] [CrossRef]
- Le Roux, J.D.; Paul, D.R.; Kampa, J.; Lagow, R.J. Surface fluorination of poly (phenylene oxide) composite membranes Part I. Transport properties. J. Membr. Sci. 1994, 90, 21–35. [Google Scholar] [CrossRef]
- Yasuda, H.; Rosengren, K. Isobaric measurement of gas permeability of polymers. J. Appl. Polym. Sci. 1970, 14, 2839–2877. [Google Scholar] [CrossRef]
- Ansaloni, L.; Minelli, M.; Baschetti, M.G.; Sarti, G.C. Effects of Thermal Treatment and Physical Aging on the Gas Transport Properties in Matrimid®. Oil Gas Sci. Technol. Rev. 2015, 70, 367–379. [Google Scholar] [CrossRef]
- Savoca, A.C.; Surnamer, A.D.; Tien, C. Gas Transport in Poly(sily1propynes): The Chemical Structure Point. Macromolecules 1993, 26, 6211–6216. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Physical aging of thin glassy polymer films monitored by gas permeability. Polymer 2004, 45, 8377–8393. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Effect of Temperature on Physical Aging of Thin Glassy Polymer Films. Macromolecules 2005, 38, 10148–10154. [Google Scholar] [CrossRef]
- Perez, E.V.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Mixed-matrix membranes containing MOF-5 for gas separations. J. Membr. Sci. 2009, 328, 165–173. [Google Scholar] [CrossRef]
- Rea, R.; Ligi, S.; Christian, M.; Morandi, V.; Baschetti, M.G.; De Angelis, M. Permeability and Selectivity of PPO/Graphene Composites as Mixed Matrix Membranes for CO2 Capture and Gas Separation. Polymers 2018, 10, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, B.W.; Robeson, L.M.; Freeman, B.D.; Paul, D.R. Influence of temperature on the upper bound: Theoretical considerations and comparison with experimental results. J. Membr. Sci. 2010, 360, 58–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Musselman, I.H.; Ferraris, J.P.; Balkus, K.J. Gas permeability properties of Matrimid® membranes containing the metal-organic framework Cu–BPY–HFS. J. Membr. Sci. 2008, 313, 170–181. [Google Scholar] [CrossRef]
- Hu, J.; Cai, H.; Ren, H.; Wei, Y.; Xu, Z.; Liu, H.; Hu, Y. Mixed-Matrix Membrane Hollow Fibers of Cu3(BTC)2 MOF and Polyimide for Gas Separation and Adsorption. Ind. Eng. Chem. Res. 2010, 49, 12605–12612. [Google Scholar] [CrossRef]


















| Polymer | (25 °C) | %FFV [52] | Refractive Index [52] | Average Molecular Weight [53] | |
|---|---|---|---|---|---|
| PPO | g/cm3 | °C | g/mol | ||
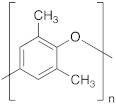 | 1.06 | 213 | 19 | 1.573 | 59,000 |
| Filler | Composition [49] | Net | da [34] | dp [34] | Surface Area (BET) [49] | Theoretical Density | Thermal Stability [49] | Hydrophilicity [49,64] |
|---|---|---|---|---|---|---|---|---|
| Å | Å | m2/g | g/cm3 | °C | ||||
| ZIF-8 | Zn(MeIM)2 | sod | 3.4 | 11.6 | 1630 | 0.95 [55] 0.93 [61] | 550 | Hydrophobic |
| Thickness (μm) | ~30 | ~20 | ~20 |
| Ref. | [40] | [41] | [42] |
| Permeance (10−8 mol m−2 s−1 Pa−1) | |||
| H2 | 6.04 | 17.3 | 8.23 |
| N2 | 0.52 | 1.49 | 0.69 |
| CH4 | 0.48 | 1.33 | 0.63 |
| CO2 | 1.33 | 4.45 | / |
| Permeability | |||
| (Barrer) | |||
| H2 | 5411 | 10,333 | 4916 |
| N2 | 466 | 890 | 412 |
| CH4 | 430 | 794 | 376 |
| CO2 | 1192 | 2658 | / |
| Ideal Selectivity | |||
| H2/CO2 | 4.54 | 3.89 | / |
| CO2/N2 | 2.56 | 2.99 | / |
| CO2/CH4 | 2.77 | 3.35 | / |
| H2/CH4 | 12.6 | 13.0 | 13.1 |
| H2/N2 | 11.6 | 11.6 | 11.9 |
| ZIF-8 Loading (wt%) | Pure Gas Permeability (Barrera) | Ideal Selectivity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| He | N2 | CH4 | CO2 | He/CO2 | CO2/N2 | CO2/CH4 | He/CH4 | He/N2 | |
| 0 (PPO) [88] | 77.9 ± 2.3 | 2.99 ± 0.07 | 3.47 ± 0.09 | 60.6 ± 1.5 | 1.29 | 20.2 | 17.4 | 22.3 | 26.0 |
| 3 | 105.8 ± 2.5 | 3.49 ± 0.08 | 4.20 ± 0.10 | 76.1 ± 1.9 | 1.39 | 21.8 | 18.1 | 25.2 | 30.3 |
| 6 | 106.7 ± 2.4 | 3.71 ± 0.08 | 4.25 ± 0.10 | 75.3 ± 1.7 | 1.42 | 20.3 | 17.7 | 25.4 | 28.8 |
| 10 | 144.3 ± 3.6 | 5.67 ± 0.13 | 5.76 ± 0.14 | 99.5 ± 2.4 | 1.45 | 17.5 | 17.3 | 24.9 | 25.4 |
| 15 | 159.7 ± 1.5 | 5.83 ± 0.06 | 6.61 ± 0.07 | 114.1 ± 1.1 | 1.40 | 19.6 | 17.3 | 24.2 | 27.4 |
| 25 | 276.4 ± 10.4 | 10.7 ± 0.4 | 11.9 ± 0.5 | 189.0 ± 7.2 | 1.46 | 17.7 | 15.9 | 23.2 | 25.9 |
| 35 | 462.0 ± 31.0 | 17.2 ± 1.1 | 18.9 ± 1.3 | 314.2 ± 21.0 | 1.47 | 18.2 | 16.6 | 24.4 | 26.8 |
| 45 | 620.9 ± 54.0 | 32.3 ± 2.8 | 37.9 ± 3.3 | 448.7 ± 38.9 | 1.38 | 13.9 | 11.8 | 16.4 | 19.2 |
| ZIF-8 Loading (wt%) | ||||
|---|---|---|---|---|
| He | N2 | CH4 | CO2 | |
| 0 [50] | 9.7 | 9.8 | 12.1 | 1.5 |
| 0 | 11.07 | 14.81 | 15.78 | 3.84 |
| 10 | 12.11 | 11.74 | 15.59 | 2.99 |
| 25 | 9.64 | 13.68 | 14.46 | 1.34 |
| 35 | 10.66 | 15.76 | 16.85 | 0.33 |
| 45 | 8.53 | 9.15 | 9.52 | 0.59 |
| ZIF-8 Loading (wt%) | ED (kJ/mol) | ||
|---|---|---|---|
| N2 | CH4 | CO2 | |
| 0 [50] | 22.4 | 29.4 | 23.4 |
| 0 | 39.10 | 32.87 | 23.33 |
| 10 | 28.62 * | 29.99 | 24.78 |
| 25 | 26.73 * | 25.37 | 19.82 |
| 35 | 22.32 | 29.28 | 19.73 |
| 45 | 16.42 | 18.47 | 12.35 |
| ZIF-8 Loading (wt%) | ∆HS (kJ/mol) | ||
|---|---|---|---|
| N2 | CH4 | CO2 | |
| 0 [50] | −12.6 | −17.3 | −21.9 |
| 0 | −24.32 | −17.10 | −19.49 |
| 10 | −16.88 * | −14.40 | −21.79 |
| 25 | −13.05 * | −10.91 | −18.48 |
| 35 | −6.56 | −12.43 | −20.06 |
| 45 | −7.27 | −8.95 | −12.94 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetti, F.M.; De Angelis, M.G.; Degli Esposti, M.; Fabbri, P.; Masili, A.; Orsini, A.; Pettinau, A. Enhancing the Separation Performance of Glassy PPO with the Addition of a Molecular Sieve (ZIF-8): Gas Transport at Various Temperatures. Membranes 2020, 10, 56. https://doi.org/10.3390/membranes10040056
Benedetti FM, De Angelis MG, Degli Esposti M, Fabbri P, Masili A, Orsini A, Pettinau A. Enhancing the Separation Performance of Glassy PPO with the Addition of a Molecular Sieve (ZIF-8): Gas Transport at Various Temperatures. Membranes. 2020; 10(4):56. https://doi.org/10.3390/membranes10040056
Chicago/Turabian StyleBenedetti, Francesco M., Maria Grazia De Angelis, Micaela Degli Esposti, Paola Fabbri, Alice Masili, Alessandro Orsini, and Alberto Pettinau. 2020. "Enhancing the Separation Performance of Glassy PPO with the Addition of a Molecular Sieve (ZIF-8): Gas Transport at Various Temperatures" Membranes 10, no. 4: 56. https://doi.org/10.3390/membranes10040056