Structural Characterisation of Deposit Layer during Milk Protein Microfiltration by Means of In-Situ MRI and Compositional Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reconstituted Skim Milk Solution
2.2. Ceramic Hollow Fibre Membranes
2.3. MRI Contrast Agent
2.4. In-situ MRI
2.5. MR Image Data Processing
2.6. Analysis of the Protein Composition of the Deposit Layer
3. Results and Discussion
3.1. MRI Measurements of Deposit Layer
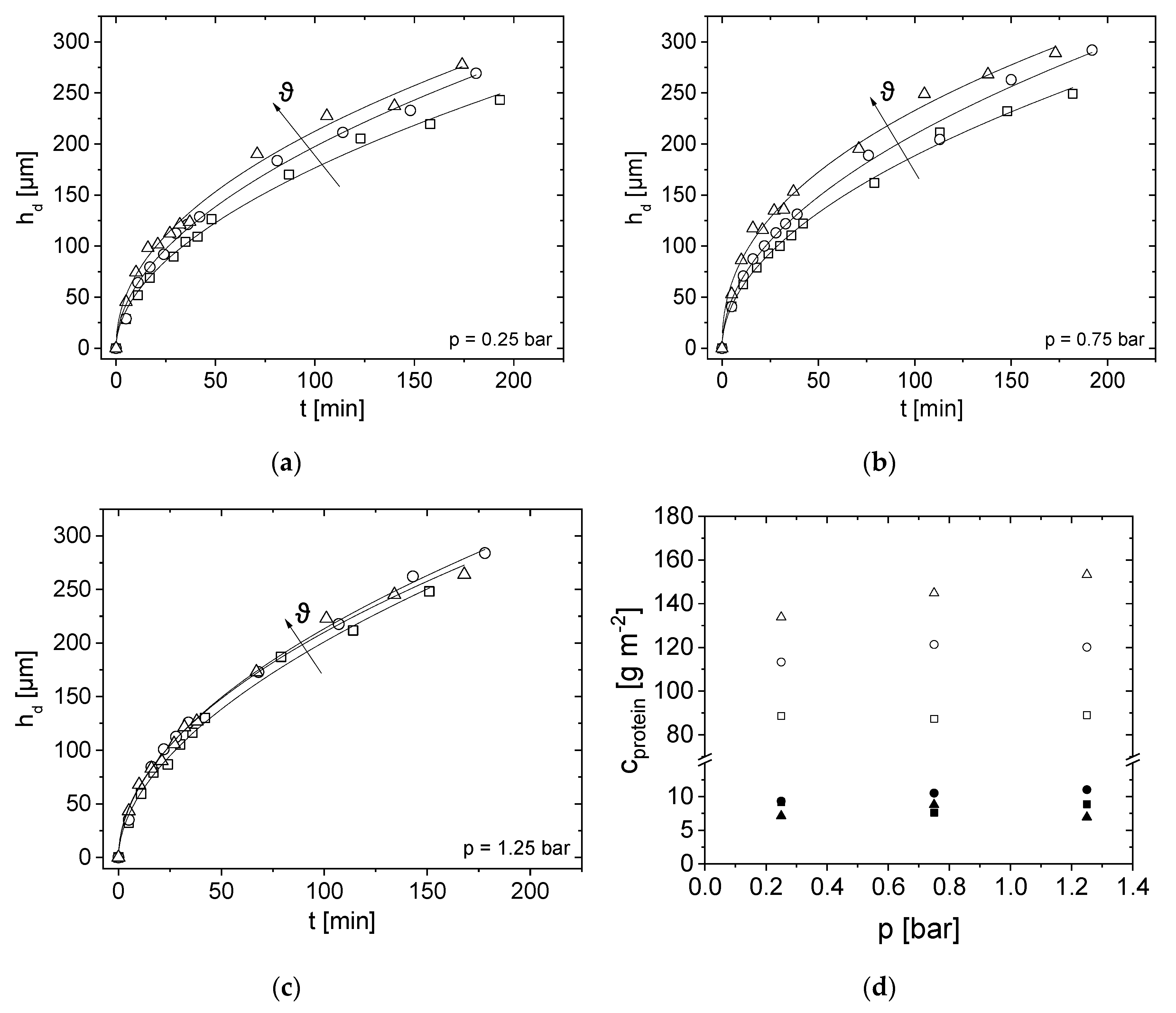
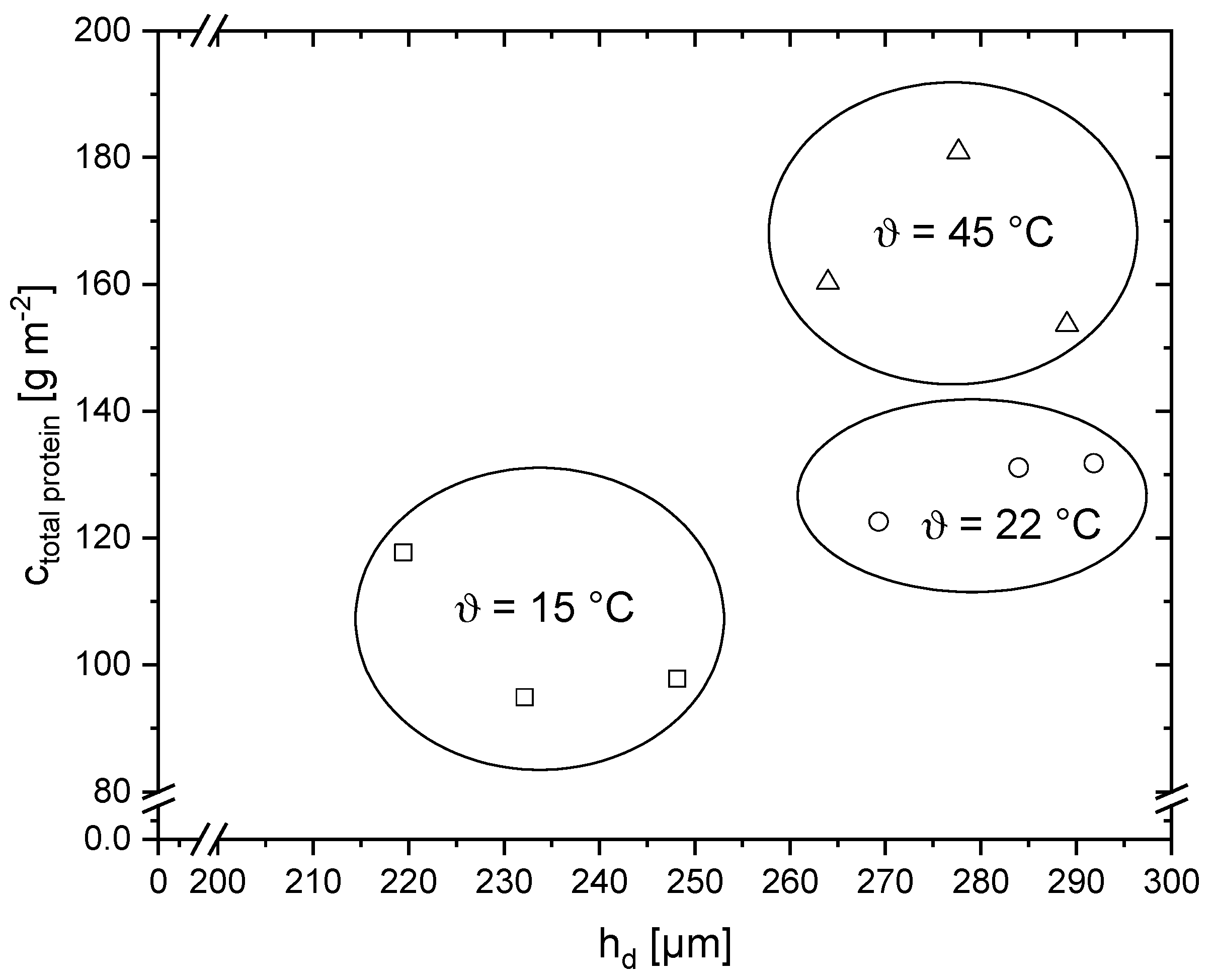
3.2. Compositional Analysis of the Deposit Layer
3.3. Influence of Pressure on Flux
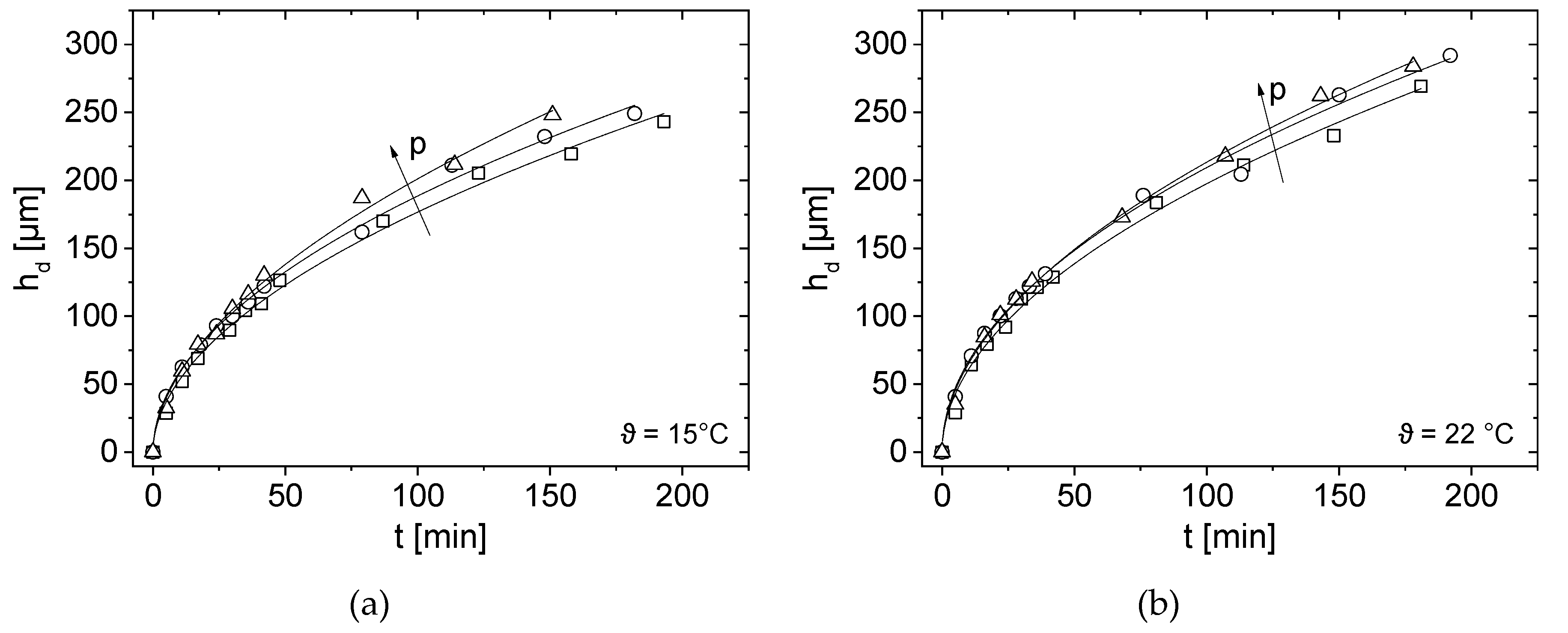
3.4. Effect of Pressure and Temperature on Deposit Layer Height
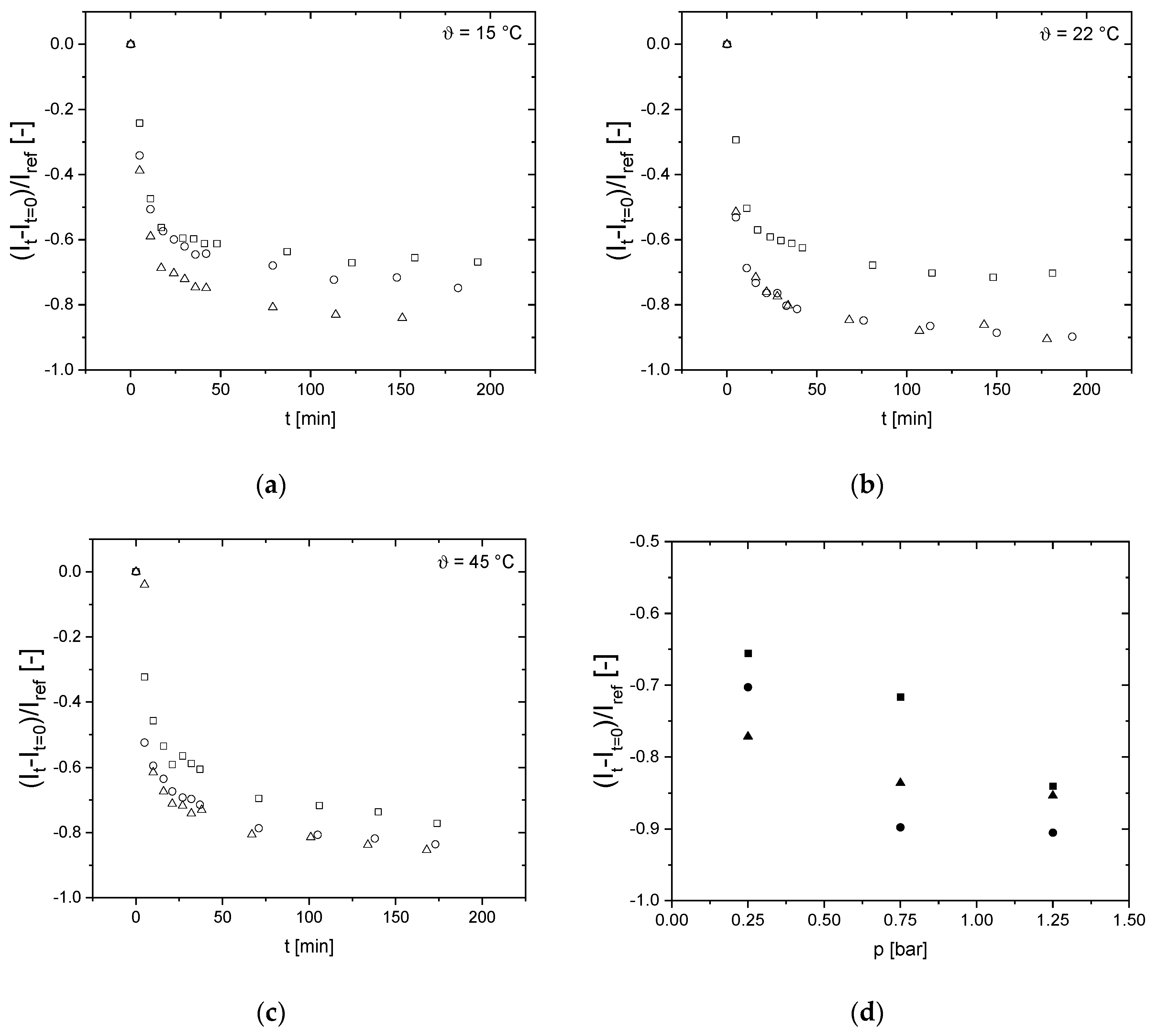
3.5. Effects on Signal Intensity of the Last Voxel Right before the Selective Membrane Layer
3.6. Impact of Milk Protein Concentration on the Height of the Deposit Layer
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kersten, M. Proteinfraktionierung Mittels Membrantrennverfahren. Ph.D. Thesis, Technische Universität München, Freising-Weihenstephan, Germany, 2000. [Google Scholar]
- Schiffer, S.; Schopf, R.; Hartinger, M.; Kulozik, U. Fractionation of complex foods through the use of membrane separation technology using milk as an example. In Global Guide 2018–2020—Filtration and Separation, Global Guide of the Filtration and Seperation Industry; VDL-Verlag GmbH: Rödermark, Germany, 2018; ISBN 978-3-00-059320-8. [Google Scholar]
- Panglisch, S. Formation and prevention of hardly removable particle layers in inside-out capillary membranes operating in dead-end mode. Water Sci. Technol. Water Supply 2003, 3, 117–124. [Google Scholar] [CrossRef]
- Kulozik, U.; Kersten, M. Membrane fractionation of dairy proteins by means of microfiltration. Eng. Life Sci. 2002, 2, 275–278. [Google Scholar] [CrossRef]
- Hartinger, M.; Heidebrecht, H.-J.; Schiffer, S.; Dumpler, J.; Kulozik, U. Milk protein fractionation by means of spiral-wound microfiltration membranes: Effect of the pressure adjustment mode and temperature on flux and protein permeation. Foods 2019, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, M.; Schiffer, S.; Heidebrecht, H.-J.; Dumpler, J.; Kulozik, U. Investigation on the spatial filtration performance in spiral-wound membranes—Influence and length-dependent adjustment of the transmembrane pressure. J. Membr. Sci. 2019, 591, 117311. [Google Scholar] [CrossRef]
- Le Berre, O.; Daufin, G. Skimmilk crossflow microfiltration performance versus permeation flux to wall shear stress ratio. J. Membr. Sci. 1996, 117, 261–270. [Google Scholar] [CrossRef]
- Gésan, G.; Daufin, G.; Merin, U.; Labbé, J.-P.; Quémerais, A. Fouling during constant flux crossflow microfiltration of pretreated whey. Influence of transmembrane pressure gradient. J. Membr. Sci. 1993, 80, 131–145. [Google Scholar] [CrossRef]
- Heidebrecht, H.-J.; Kulozik, U. Fractionation of casein micelles and minor proteins by microfiltration in diafiltration mode. Study of the transmission and yield of the immunoglobulins IgG, IgA and IgM. Int. Dairy J. 2019, 93, 1–10. [Google Scholar] [CrossRef]
- Heidebrecht, H.-J.; Toro-Sierra, J.; Kulozik, U. Concentration of immunoglobulins in microfiltration permeates of skim milk: Impact of transmembrane pressure and temperature on the IgG transmission using different ceramic membrane types and pore sizes. Foods 2018, 7, 101. [Google Scholar] [CrossRef]
- Jimenez-Lopez, A.J.E.; Leconte, N.; Dehainault, O.; Geneste, C.; Fromont, L.; Gesanguiziou, G. Role of milk constituents on critical conditions and deposit structure in skimmilk microfiltration (0.1 μm). Sep. Purif. Technol. 2018, 61, 33–43. [Google Scholar] [CrossRef]
- Jimenez-Lopez, A.J.E.; Leconte, N.; Garnier-Lambrouin, F.; Bouchoux, A.; Rousseau, F.; Gésan-Guiziou, G. Ionic strength dependence of skimmed milk microfiltration: Relations between filtration performance, deposit layer characteristics and colloidal properties of casein micelles. J. Membr. Sci. 2011, 369, 404–413. [Google Scholar] [CrossRef]
- Kühnl, W. Kollodiale Wechselwirkung zwischen Proteinen im Zusammenspiel mit fluiddynamischen Kräften an der Grenzfläche von Filtrationsmembranen-gezeigt am Beispiel der Fraktionierung von Milchproteinen mittels Mikrofiltration. Ph.D. Thesis, Technische Universität München, Freising-Weihen-Stephan, Germany, 2010. [Google Scholar]
- Steinhauer, T.; Kühnl, W.; Kulozik, U. Impact of protein interactions and transmembrane pressure on physical properties of filter cakes formed during filtrations of skim milk. Procedia Food Sci. 2011, 1, 886–892. [Google Scholar] [CrossRef]
- Bacchin, P. A possible link between critical and limiting flux for colloidal systems: Consideration of critical deposit formation along a membrane. J. Membr. Sci. 2004, 228, 237–241. [Google Scholar] [CrossRef]
- Kühnl, W.; Piry, A.; Kaufmann, V.; Grein, T.; Ripperger, S.; Kulozik, U. Impact of colloidal interactions on the flux in cross-flow microfiltration of milk at different pH values: A surface energy approach. J. Membr. Sci. 2010, 352, 107–115. [Google Scholar] [CrossRef]
- Field, R.W.; Pearce, G.K. Critical, sustainable and threshold fluxes for membrane filtration with water industry applications. Adv. Colloid Interface Sci. 2011, 164, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, M.; Kulozik, U. Milk protein fractionation by spiral-wound microfiltration membranes in diafiltration mode—Influence of feed protein concentration and composition on the filtration performance. Int. Dairy J. 2020, 102, 104606. [Google Scholar] [CrossRef]
- Piry, A.; Heino, A.; Kühnl, W.; Grein, T.; Ripperger, S.; Kulozik, U. Effect of membrane length, membrane resistance, and filtration conditions on the fractionation of milk proteins by microfiltration. J. Dairy Sci. 2012, 95, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, T.; Lonfat, J.; Hager, I.; Gebhardt, R.; Kulozik, U. Effect of pH, transmembrane pressure and whey proteins on the properties of casein micelle deposit layers. J. Membr. Sci. 2015, 493, 452–459. [Google Scholar] [CrossRef]
- Arndt, F.; Heidebrecht, H.J.; Schork, N.; Schuhmann, S.; Kulozik, U.; Schütz, S.; Nirschl, H.; Guthausen, G. Deposit layer formation during skim milk dead-end filtration with ceramic hollow fiber membranes using magnetic resonance imaging. In Proceedings of the XIII International Conference on the Applications of Magnetic Resonance in Food Science 2016, Karlsruhe, Germany, 7–10 June 2016. [Google Scholar] [CrossRef]
- Arndt, F.; Schuhmann, S.; Guthausen, G.; Schütz, S.; Nirschl, H. In situ MRI of alginate fouling and flow in ceramic hollow fiber membranes. J. Membr. Sci. 2017, 524, 691–699. [Google Scholar] [CrossRef]
- Schuhmann, S.; Simkins, J.W.; Schork, N.; Codd, S.L.; Seymour, J.D.; Heijnen, M.; Saravia, F.; Horn, H.; Nirschl, H.; Guthausen, G. Characterization and quantification of structure and flow in multichannel polymer membranes by MRI. J. Membr. Sci. 2019, 570–571, 472–480. [Google Scholar] [CrossRef]
- Schuhmann, S.; Schork, N.; Beller, K.; Nirschl, H.; Oerther, T.; Guthausen, G. In-situ characterization of deposits in ceramic hollow fiber membranes by compressed sensing RARE-MRI. AICHE J. 2018, 64, 4039–4046. [Google Scholar] [CrossRef]
- Schork, N.; Schuhmann, S.; Nirschl, H.; Guthausen, G. In situ measurement of deposit layer formation during skim milk filtration by MRI. Magn. Reson. Chem. 2019, 57, 738–748. [Google Scholar] [CrossRef]
- Çulfaz, P.Z.; Buetehorn, S.; Utiu, L.; Kueppers, M.; Bluemich, B.; Melin, T.; Wessling, M.; Lammertink, R.G.H. Fouling behavior of microstructured hollow fiber membranes in dead-end filtrations: Critical flux determination and NMR imaging of particle deposition. Langmuir 2011, 27, 1643–1652. [Google Scholar] [CrossRef]
- Arndt, F.; Roth, U.; Nirschl, H.; Schütz, S.; Guthausen, G. New insights into sodium alginate fouling of ceramic hollow fiber membranes by NMR imaging. AICHE J. 2016, 62, 2459–2467. [Google Scholar] [CrossRef]
- Schork, N.; Schuhmann, S.; Arndt, F.; Schütz, S.; Guthausen, G.; Nirschl, H. MRI investigations of filtration: Fouling and cleaning processes. Microporous Mesoporous Mater. 2018, 269, 60–64. [Google Scholar] [CrossRef]
- Tiller, F.M.; Lu, R.; Kwon, J.H.; Lee, D.J. Variable flow rate in compactible filter cakes. Water Res. 1999, 33, 15–22. [Google Scholar] [CrossRef]
- Steinhauer, T.; Marx, M.; Bogendörfer, K.; Kulozik, U. Membrane fouling during ultra- and microfiltration of whey and whey proteins at different environmental conditions: The role of aggregated whey proteins as fouling initiators. J. Membr. Sci. 2015, 489, 20–27. [Google Scholar] [CrossRef]
- Chen, V. Non-invasive observation of synthetic membrane processes—A review of methods. J. Membr. Sci. 2004, 241, 23–44. [Google Scholar] [CrossRef]
- Delaunay, D.; Rabiller-Baudry, M.; Gozálvez-Zafrilla, J.M.; Balannec, B.; Frappart, M.; Paugam, L. Mapping of protein fouling by FTIR-ATR as experimental tool to study membrane fouling and fluid velocity profile in various geometries and validation by CFD simulation. Chem. Eng. Process. Process Intensif. 2008, 47, 1106–1117. [Google Scholar] [CrossRef]
- Suwal, S.; Doyen, A.; Bazinet, L. Characterization of protein, peptide and amino acid fouling on ion-exchange and filtration membranes: Review of current and recently developed methods. J. Membr. Sci. 2015, 496, 267–283. [Google Scholar] [CrossRef]
- Li, H.; Hsu, Y.-C.; Zhang, Z.; Dharsana, N.; Ye, Y.; Chen, V. The influence of milk components on the performance of ultrafiltration/diafiltration of concentrated skim milk. Sep. Sci. Technol. 2016, 52, 381–391. [Google Scholar] [CrossRef]
- Li, X.; Mo, Y.; Li, J.; Guo, W.; Ngo, H.H. In-situ monitoring techniques for membrane fouling and local filtration characteristics in hollow fiber membrane processes: A critical review. J. Membr. Sci. 2017, 528, 187–200. [Google Scholar] [CrossRef]
- Ng, K.S.Y.; Dunstan, D.E.; Martin, G.J.O. Influence of processing temperature on flux decline during skim milk ultrafiltration. Sep. Purif. Technol. 2018, 195, 322–331. [Google Scholar] [CrossRef]
- Piry, A. Untersuchung zur Längenabhängigkeit der Filtrationsleistung bei der Fraktionierung von Milchproteinen mittels Mikrofiltration. Ph.D. Thesis, Technische Universität München, Freising-Weihen-Stephan, Germany, 2011. [Google Scholar]
- Hartinger, M.; Heidebrecht, H.-J.; Schiffer, S.; Dumpler, J.; Kulozik, U. Technical concepts for the investigation of spatial effects in spiral-wound microfiltration membranes. Membranes 2019, 9, 80. [Google Scholar] [CrossRef]
- Hurt, E. Production of Micellar Casein Concentrates Using Ceramic Microfiltration Membranes: Op- Timal Process Design And System Operation. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 2015. [Google Scholar]
- Van Hekken, D.L.; Holsinger, V.H. Use of cold microfiltration to produce unique β-casein enriched milk gels. Le Lait 2000, 80, 69–76. [Google Scholar] [CrossRef]
- Crowley, S.V.; Caldeo, V.; McCarthy, N.A.; Fenelon, M.A.; Kelly, A.L.; O’Mahony, J.A. Processing and protein-fractionation characteristics of different polymeric membranes during filtration of skim milk at refrigeration temperatures. Int. Dairy J. 2015, 48, 23–30. [Google Scholar] [CrossRef]
- Bolton, G.R.; Apostolidis, A.J. Mechanistic modeling of the loss of protein sieving due to internal and external fouling of microfilters. Biotechnol. Prog. 2017, 33, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Iritani, E.; Katagiri, N. Developments of blocking filtration model in membrane filtration. KONA 2016, 33, 179–202. [Google Scholar] [CrossRef]
- Walstra, P.; Jenness, R. Dairy Chemistry and Physics; Wiley: New York, NY, USA, 1984; ISBN 0471097799. [Google Scholar]
- Jenness, R. 2 d protein composition of milk. In Milk Proteins V1: Chemistry and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2012; p. 17. ISBN 9780323146647. [Google Scholar]
- Bonizzi, I.; Buffoni, J.N.; Feligini, M. Quantification of bovine casein fractions by direct chromatographic analysis of milk. Approaching the application to a real production context. J. Chromatogr. A 2009, 1216, 165–168. [Google Scholar] [CrossRef]
- Dumpler, J.; Wohlschläger, H.; Kulozik, U. Dissociation and coagulation of caseins and whey proteins in concentrated skim milk heated by direct steam injection. Dairy Sci. Technol. 2017, 96, 807–826. [Google Scholar] [CrossRef]
- Callaghan, P.T. Principles of Nuclear Magnetic Resonance Microscopy; Univ. Press: Oxford, UK, 1991; ISBN 0198539975. [Google Scholar]
- Dumpler, J. Heat Stability of Concentrated Milk Systems; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2018; ISBN 978-3-658-19695-0. [Google Scholar]
- Reihanian, H.; Robertson, C.R.; Michaels, A.S. Mechanisms of polarization and fouling of ultrafiltration membranes by proteins. J. Membr. Sci. 1983, 16, 237–258. [Google Scholar] [CrossRef]
- Ng, K.S.Y.; Haribabu, M.; Harvie, D.J.E.; Dunstan, D.E.; Martin, G.J.O. Mechanisms of flux decline in skim milk ultrafiltration: A review. J. Membr. Sci. 2017, 523, 144–162. [Google Scholar] [CrossRef]


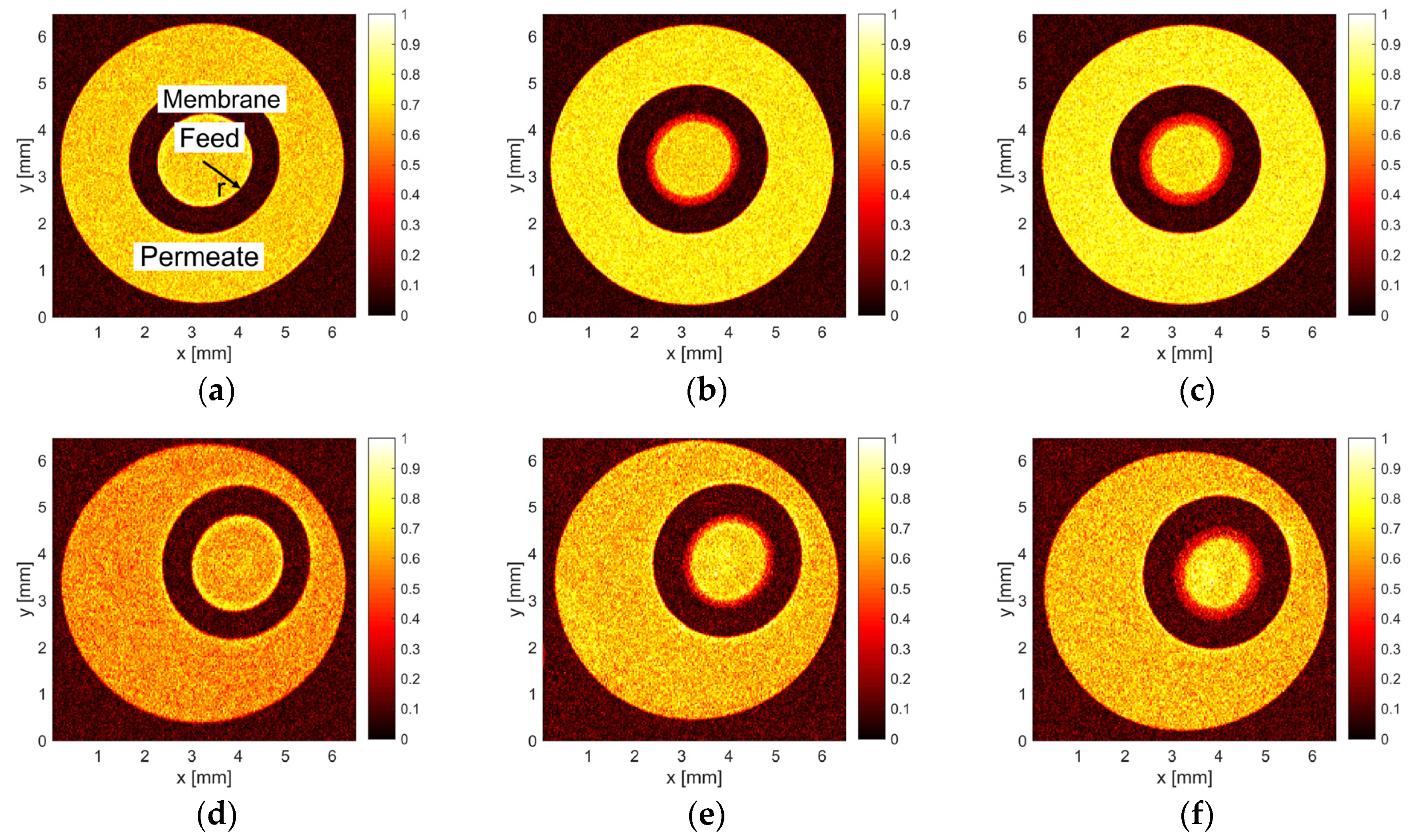



| Content or Property of the Redispersed Skim Milk | Value Determined by Manufacturer (%w/w) | Measured Value |
|---|---|---|
| Casein protein | 2.8 | |
| Whey protein | 0.7 | |
| Fat | 0.06 | |
| Lactose | 5.61 | |
| Ash | 0.8 | |
| pH | 6.6 | |
| ϑ | 22 °C | |
| ηWater | 0.992 mPa s | |
| ηMilk | 1.477 mPa s | |
| ηPermeate | 1.106 mPa s | |
| φMilk | 0.9977 kg dm−3 |
| RARE Parameter | Value |
|---|---|
| TR | 4 s |
| 5.5 ms | |
| pixel size x = y | 32.5 µm |
| RF | 2 |
| No. of averages | 1 |
| Slice thickness along z | 3 mm |
| Time for the measurement | 5 min 8 s |
| Encoding order | Centric |
| Partial Fourier Factor (Phase) | 1.3 |
| p [bar] | ϑ [°C] | aintegral [-] | bintegral [-] | alocal [-] | blocal [-] |
|---|---|---|---|---|---|
| 0.25 | 15 | 0.32 | 0.48 | 15.9 | 0.52 |
| 22 | 0.30 | 0.52 | 18.8 | 0.51 | |
| 45 | 0.28 | 0.56 | 24.3 | 0.47 | |
| 0.75 | 15 | 1.29 | 0.37 | 18.3 | 0.51 |
| 22 | 0.60 | 0.44 | 21.2 | 0.50 | |
| 45 | 0.80 | 0.44 | 31.8 | 0.43 | |
| 1.25 | 15 | 0.089 | 0.67 | 16.6 | 0.54 |
| 22 | 0.46 | 0.52 | 19.8 | 0.52 | |
| 45 | 0.50 | 0.52 | 20.6 | 0.51 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schopf, R.; Schork, N.; Amling, E.; Nirschl, H.; Guthausen, G.; Kulozik, U. Structural Characterisation of Deposit Layer during Milk Protein Microfiltration by Means of In-Situ MRI and Compositional Analysis. Membranes 2020, 10, 59. https://doi.org/10.3390/membranes10040059
Schopf R, Schork N, Amling E, Nirschl H, Guthausen G, Kulozik U. Structural Characterisation of Deposit Layer during Milk Protein Microfiltration by Means of In-Situ MRI and Compositional Analysis. Membranes. 2020; 10(4):59. https://doi.org/10.3390/membranes10040059
Chicago/Turabian StyleSchopf, Roland, Nicolas Schork, Estelle Amling, Hermann Nirschl, Gisela Guthausen, and Ulrich Kulozik. 2020. "Structural Characterisation of Deposit Layer during Milk Protein Microfiltration by Means of In-Situ MRI and Compositional Analysis" Membranes 10, no. 4: 59. https://doi.org/10.3390/membranes10040059
APA StyleSchopf, R., Schork, N., Amling, E., Nirschl, H., Guthausen, G., & Kulozik, U. (2020). Structural Characterisation of Deposit Layer during Milk Protein Microfiltration by Means of In-Situ MRI and Compositional Analysis. Membranes, 10(4), 59. https://doi.org/10.3390/membranes10040059






