Enhanced O2/N2 Separation of Mixed-Matrix Membrane Filled with Pluronic-Compatibilized Cobalt Phthalocyanine Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Fabrication
2.3. Characterization
2.4. Gas Adsorption Analysis
2.5. Gas Permeation Test
3. Results and Discussion
3.1. Characterization of Facilitated Carrier and Compatibilizer
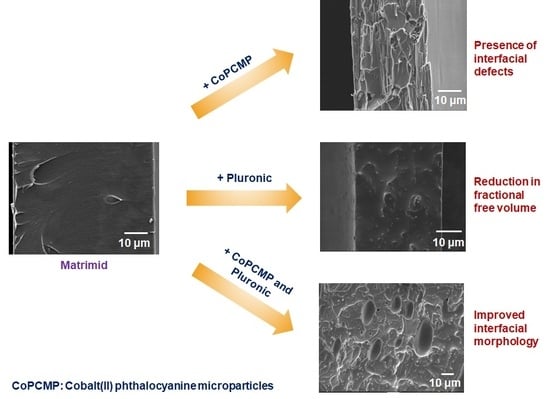
3.2. Characterization of Neat, Blended, and Composite Membranes
3.3. Gas Permeation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fernández-Barquín, A.; Casado-Coterillo, C.; Valencia, S.; Irabien, A. Mixed matrix membranes for O2/N2 separation: The influence of temperature. Membranes 2016, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Matsuura, T. Oxygen–Nitrogen Separation. In Encyclopedia of Membrane Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 1–26. [Google Scholar]
- Chong, K.; Lai, S.; Thiam, H.; Teoh, H.; Heng, S. Recent progress of oxygen/nitrogen separation using membrane technology. J. Eng. Sci. Technol. 2016, 11, 1016–1030. [Google Scholar]
- Stafford, T.M. Indoor air quality and academic performance. J. Environ. Econ. Manag. 2015, 70, 34–50. [Google Scholar] [CrossRef]
- Ismail, A.F. Separation. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1421–1422. [Google Scholar]
- Gong, H.; Chuah, C.Y.; Yang, Y.; Bae, T.-H. High performance composite membranes comprising Zn(pyrz)2(SiF6) nanocrystals for CO2/CH4 separation. J. Ind. Eng. Chem. 2018, 60, 279–285. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Li, W.; Samarasinghe, S.; Sethunga, G.; Bae, T.-H. Enhancing the CO2 separation performance of polymer membranes via the incorporation of amine-functionalized HKUST-1 nanocrystals. Micropor. Mesopor. Mater. 2019, 290, 109680. [Google Scholar] [CrossRef]
- Meyer, G.M. Method and Apparatus for the Production of Nitrogen for Use as an Inert Gas. U.S. Patent 3,891,411, 24 June 1975. [Google Scholar]
- Wellman, A.; Stewart, G. Storage of brewing yeasts by liquid nitrogen refrigeration. Appl. Environ. Microbiol. 1973, 26, 577–583. [Google Scholar] [CrossRef]
- Parfitt, D.E.; Almehdi, A.A. Cryogenic storage of grape pollen. Am. Soc. Enol Viticulture 1983, 34, 227–228. [Google Scholar]
- Ordonez, C.; Plummer, M. Cold thermal storage and cryogenic heat engines for energy storage applications. Energy Sources 1997, 19, 389–396. [Google Scholar] [CrossRef]
- Bonner, F.T. Storage of seeds: Potential and limitations for germplasm conservation. For. Ecol. Manag. 1990, 35, 35–43. [Google Scholar] [CrossRef]
- Smith, A.; Klosek, J. A review of air separation technologies and their integration with energy conversion processes. Fuel Process. Technol. 2001, 70, 115–134. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Kim, K.; Lee, J.; Koh, D.-Y.; Bae, T.-H. CO2 absorption using membrane contactors: Recent progress and future perspective. Ind. Eng. Chem. Res. 2020, 59, 6773–6794. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Li, W.; Goh, K.; Chuah, C.Y.; Bae, T.-H. Mixed-matrix carbon molecular sieve membranes using hierarchical zeolite: A simple approach towards high CO2 permeability enhancements. J. Membr. Sci. 2019, 588, 117220. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Goh, K.; Yang, Y.; Gong, H.; Li, W.; Karahan, H.E.; Guiver, M.D.; Wang, R.; Bae, T.-H. Harnessing filler materials for enhancing biogas separation membranes. Chem. Rev. 2018, 118, 8655–8769. [Google Scholar] [CrossRef]
- Yang, Y.; Chuah, C.Y.; Nie, L.; Bae, T.-H. Enhancing the mechanical strength and CO2/CH4 separation performance of polymeric membranes by incorporating amine-appended porous polymers. J. Membr. Sci. 2019, 569, 149–156. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Teplyakov, V.; Meares, P. Correlation aspects of the selective gas permeabilities of polymeric materials and membranes. Gas. Separ. Purif. 1990, 4, 66–74. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Bae, T.-H. Incorporation of Cu3BTC2 nanocrystals to increase the permeability of polymeric membranes in O2/N2 separation. BMC Chem. Eng. 2019, 1, 2. [Google Scholar] [CrossRef]
- Adam, J.; Michael, Z.; David, M.H.; Naomi, B.; Ryan, M.; Khetpakorn, C.; Jeffrey, A.R.; Jeffrey, R.L. Selective, High-Temperature O2 Adsorption in Chemically Reduced, Redox-Active Iron-Pyrazolate Metal–Organic Frameworks. ChemRxiv 2019. [Google Scholar] [CrossRef]
- Murray, L.J.; Dinca, M.; Yano, J.; Chavan, S.; Bordiga, S.; Brown, C.M.; Long, J.R. Highly-selective and reversible O2 binding in Cr3(1,3,5-benzenetricarboxylate)2. J. Am. Chem. Soc. 2010, 132, 7856–7857. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, R.T. Gas adsorption and storage in metal− organic framework MOF-177. Langmuir 2007, 23, 12937–12944. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, A.; Rizzuto, C.; Tocci, E.; Monteleone, M.; Esposito, E.; Budd, P.M.; Carta, M.; Comesaña-Gándara, B.; McKeown, N.B.; Jansen, J.C. The origin of size-selective gas transport through polymers of intrinsic microporosity. J. Mater. Chem. A 2019, 7, 20121–20126. [Google Scholar] [CrossRef]
- Kakuchi, R.; Theato, P. Efficient multicomponent postpolymerization modification based on Kabachnik-Fields reaction. ACS Macro Lett. 2014, 3, 329–332. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Pinnau, I. Fine-tuned intrinsically ultramicroporous polymers redefine the permeability/selectivity upper bounds of membrane-based air and hydrogen separations. ACS Macro Lett. 2015, 4, 947–951. [Google Scholar] [CrossRef]
- Alberto, M.; Bhavsar, R.; Luque-Alled, J.M.; Vijayaraghavan, A.; Budd, P.M.; Gorgojo, P. Impeded physical aging in PIM-1 membranes containing graphene-like fillers. J. Membr. Sci. 2018, 563, 513–520. [Google Scholar] [CrossRef]
- Bernardo, P.; Bazzarelli, F.; Tasselli, F.; Clarizia, G.; Mason, C.R.; Maynard-Atem, L.; Budd, P.M.; Lanč, M.; Pilnáček, K.; Vopička, O.; et al. Effect of physical aging on the gas transport and sorption in PIM-1 membranes. Polymer 2017, 113, 283–294. [Google Scholar] [CrossRef]
- Hou, R.; Smith, S.J.D.; Wood, C.D.; Mulder, R.J.; Lau, C.H.; Wang, H.; Hill, M.R. Solvation Effects on the Permeation and Aging Performance of PIM-1-Based MMMs for Gas Separation. ACS Appl. Mater. Interfaces 2019, 11, 6502–6511. [Google Scholar] [CrossRef]
- Harms, S.; Rätzke, K.; Faupel, F.; Chaukura, N.; Budd, P.M.; Egger, W.; Ravelli, L. Aging and Free Volume in a Polymer of Intrinsic Microporosity (PIM-1). J. Adhesion 2012, 88, 608–619. [Google Scholar] [CrossRef]
- Nagar, H.; Vadthya, P.; Prasad, N.S.; Sridhar, S. Air separation by facilitated transport of oxygen through a Pebax membrane incorporated with a cobalt complex. RSC Adv. 2015, 5, 76190–76201. [Google Scholar] [CrossRef]
- Preethi, N.; Shinohara, H.; Nishide, H. Reversible oxygen-binding and facilitated oxygen transport in membranes of polyvinylimidazole complexed with cobalt-phthalocyanine. React. Funct. Polym. 2006, 66, 851–855. [Google Scholar] [CrossRef]
- Li, H.; Choi, W.; Ingole, P.G.; Lee, H.K.; Baek, I.H. Oxygen separation membrane based on facilitated transport using cobalt tetraphenylporphyrin-coated hollow fiber composites. Fuel 2016, 185, 133–141. [Google Scholar] [CrossRef]
- Zheng, Q.; Thompson, S.J.; Zhou, S.; Lail, M.; Amato, K.; Rayer, A.V.; Mecham, J.; Mobley, P.; Shen, J.; Fletcher, B. Task-specific ionic liquids functionalized by Cobalt (II) salen for room temperature biomimetic dioxygen binding. Ind. Eng. Chem. Res. 2018, 58, 334–341. [Google Scholar] [CrossRef]
- Samarasinghe, S.; Chuah, C.Y.; Li, W.; Sethunga, G.; Wang, R.; Bae, T.-H. Incorporation of CoIII acetylacetonate and SNW-1 nanoparticles to tailor O2/N2 separation performance of mixed-matrix membrane. Sep. Purif. Technol. 2019, 223, 133–141. [Google Scholar] [CrossRef]
- Midda, M.O.; Suresh, A.K. Some mechanistic insights into the action of facilitating agents on gas permeation through glassy polymeric membranes. AIChE J. 2018, 64, 186–199. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Amooghin, A.E.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Emplit, A.; Tao, F.F.; Lipnik, P.; Heunen, G.; Bailly, C.; Huynen, I. Polypropylene Carbon Nanotubes Nanocomposites: Combined Influence of Block Copolymer Compatibilizer and Melt Annealing on Electrical Properties. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef]
- Patel, R.; Park, J.T.; Hong, H.P.; Kim, J.H.; Min, B.R. Use of block copolymer as compatibilizer in polyimide/zeolite composite membranes. Polym. Advan. Technol. 2011, 22, 768–772. [Google Scholar] [CrossRef]
- Bushell, A.F.; Budd, P.M.; Attfield, M.P.; Jones, J.T.A.; Hasell, T.; Cooper, A.I.; Bernardo, P.; Bazzarelli, F.; Clarizia, G.; Jansen, J.C. Nanoporous Organic Polymer/Cage Composite Membranes. Angew. Chem. Int. Ed. 2013, 52, 1253–1256. [Google Scholar] [CrossRef]
- Samarasinghe, S.; Chuah, C.Y.; Yang, Y.; Bae, T.-H. Tailoring CO2/CH4 separation properties of mixed-matrix membranes via combined use of two-and three-dimensional metal-organic frameworks. J. Membr. Sci. 2018, 557, 30–37. [Google Scholar] [CrossRef]
- Li, W.; Chuah, C.Y.; Nie, L.; Bae, T.-H. Enhanced CO2/CH4 selectivity and mechanical strength of mixed-matrix membrane incorporated with NiDOBDC/GO composite. J. Ind. Eng. Chem. 2019, 74, 118–125. [Google Scholar] [CrossRef]
- Al Kayal, T.; Panetta, D.; Canciani, B.; Losi, P.; Tripodi, M.; Burchielli, S.; Ottoni, P.; Salvadori, P.A.; Soldani, G. Evaluation of the effect of a gamma irradiated DBM-pluronic F127 composite on bone regeneration in Wistar rat. PLoS ONE 2015, 10, e0125110. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Daintree, L.S.; Ding, S.; Ledger, D.M.; Wang, B.; Zhao, W.; Qi, J.; Wu, W. Itraconazole solid dispersion prepared by a supercritical fluid technique: Preparation, in vitro characterization, and bioavailability in beagle dogs. Drug Des. Devel. Ther. 2015, 9, 2801. [Google Scholar] [PubMed]
- Ji, X.; Zou, T.; Gong, H.; Wu, Q.; Qiao, Z.; Wu, W.; Wang, H. Cobalt phthalocyanine nanowires: Growth, crystal structure, and optical properties. Crys. Res. Technol. 2016, 51, 154–159. [Google Scholar] [CrossRef]
- Karolewicz, B.; Górniak, A.; Owczarek, A.; Żurawska-Płaksej, E.; Piwowar, A.; Pluta, J. Thermal, spectroscopic, and dissolution studies of ketoconazole–Pluronic F127 system. J. Therm. Anal. Calorim. 2014, 115, 2487–2493. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Yang, Y.; Bae, T.-H. Hierarchically porous polymers containing triphenylamine for enhanced SF6 separation. Micropor. Mesopor. Mater. 2018, 272, 232–240. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Wu, X.; Tian, Z.; Wang, S.; Peng, D.; Yang, L.; Wu, Y.; Xin, Q.; Wu, H.; Jiang, Z. Mixed matrix membranes comprising polymers of intrinsic microporosity and covalent organic framework for gas separation. J. Membr. Sci. 2017, 528, 273–283. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Zhang, M.; Wang, B.; Wang, S. Miscibility, free volume behavior and properties of blends from cellulose acetate and castor oil-based polyurethane. Polymer 2003, 44, 1733–1739. [Google Scholar] [CrossRef]
- Yong, W.F.; Li, F.; Xiao, Y.; Li, P.; Pramoda, K.; Tong, Y.; Chung, T. Molecular engineering of PIM-1/Matrimid blend membranes for gas separation. J. Membr. Sci. 2012, 407, 47–57. [Google Scholar] [CrossRef]
- Yong, W.F.; Li, F.Y.; Xiao, Y.C.; Chung, T.S.; Tong, Y.W. High performance PIM-1/Matrimid hollow fiber membranes for CO2/CH4, O2/N2 and CO2/N2 separation. J. Membr. Sci. 2013, 443, 156–169. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Jia, C.; Wang, Y.; Zhai, L.; Wang, Q.; Zhao, D. Ultrathin mixed matrix membranes containing two-dimensional metal-organic framework nanosheets for efficient CO2/CH4 separation. J. Membr. Sci. 2017, 539, 213–223. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Barry, N.P. Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef]





| Membrane Composition (wt.%) a,b | O2 Permeability (Barrer) | % Change (with Respect to Matrimid) | O2/N2 Selectivity | % Change (with Respect to Matrimid) | ||
|---|---|---|---|---|---|---|
| Matrimid | CoPCMP | Pluronic | ||||
| 100 | 0 | 0 | 1.72 ± 0.29 | - | 5.79 ± 0.12 | - |
| 97 | 3 | 0 | 1.02 ± 0.22 | −40.7 | 6.63 ± 0.08 | 14.5 |
| 95 | 5 | 0 | 1.32 ± 0.32 | −23.3 | 7.62 ± 0.54 | 31.6 |
| 95 | 0 | 5 | 0.93 ± 0.32 | −45.9 | 7.09 ± 1.09 | 22.5 |
| 90 | 0 | 10 | 0.77 ± 0.07 | −55.2 | 6.19 ± 0.64 | 6.9 |
| 90 | 5 | 5 | 1.66 ± 0.15 | −3.4 | 3.82 ± 0.18 | −34.0 |
| 85 | 5 | 10 | 2.82 ± 0.24 | 64.0 | 7.75 ± 1.44 | 33.9 |
| Membrane Composition | Density (g cm−3) | O2 Solubility (mol m−3 bar−1) | N2 Solubility (mol m−3 bar−1) | O2 Diffusivity (m2 s−1), ×10−12 | N2 Diffusivity (m2 s−1), ×10−12 | O2/N2 Solubility Selectivity a | O2/N2 Diffusivity Selectivity a |
|---|---|---|---|---|---|---|---|
| Matrimid | 1.24 | 31.3 | 24.3 | 1.87 | 0.415 | 1.29 | 4.50 |
| 5 wt.% CoPCMP | 1.25 | 27.9 | 19.4 | 1.60 | 0.302 | 1.44 | 5.30 |
| 10 wt.% Pluronic | 1.13 | 12.4 | 7.0 | 2.11 | 0.603 | 1.77 | 3.50 |
| 5 wt.% CoPCMP, 5 wt.% Pluronic | 1.20 | 23.2 | 13.6 | 2.42 | 1.08 | 1.70 | 2.24 |
| 5 wt.% CoPCMP, 10 wt.% Pluronic | 1.24 | 20.6 | 7.1 | 4.65 | 1.74 | 2.90 | 2.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samarasinghe, S.A.S.C.; Chuah, C.Y.; Karahan, H.E.; Sethunga, G.S.M.D.P.; Bae, T.-H. Enhanced O2/N2 Separation of Mixed-Matrix Membrane Filled with Pluronic-Compatibilized Cobalt Phthalocyanine Particles. Membranes 2020, 10, 75. https://doi.org/10.3390/membranes10040075
Samarasinghe SASC, Chuah CY, Karahan HE, Sethunga GSMDP, Bae T-H. Enhanced O2/N2 Separation of Mixed-Matrix Membrane Filled with Pluronic-Compatibilized Cobalt Phthalocyanine Particles. Membranes. 2020; 10(4):75. https://doi.org/10.3390/membranes10040075
Chicago/Turabian StyleSamarasinghe, S. A. S. C., Chong Yang Chuah, H. Enis Karahan, G. S. M. D. P. Sethunga, and Tae-Hyun Bae. 2020. "Enhanced O2/N2 Separation of Mixed-Matrix Membrane Filled with Pluronic-Compatibilized Cobalt Phthalocyanine Particles" Membranes 10, no. 4: 75. https://doi.org/10.3390/membranes10040075
APA StyleSamarasinghe, S. A. S. C., Chuah, C. Y., Karahan, H. E., Sethunga, G. S. M. D. P., & Bae, T.-H. (2020). Enhanced O2/N2 Separation of Mixed-Matrix Membrane Filled with Pluronic-Compatibilized Cobalt Phthalocyanine Particles. Membranes, 10(4), 75. https://doi.org/10.3390/membranes10040075