ZrO2-TiO2 Incorporated PVDF Dual-Layer Hollow Fiber Membrane for Oily Wastewater Treatment: Effect of Air Gap
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nanoparticles
2.3. Preparation of PVDF DLHF
2.4. Characterizations
2.5. Membrane Performance Evaluation
2.6. Preparation of Synthetic Oily Wastewater
3. Results and Discussion
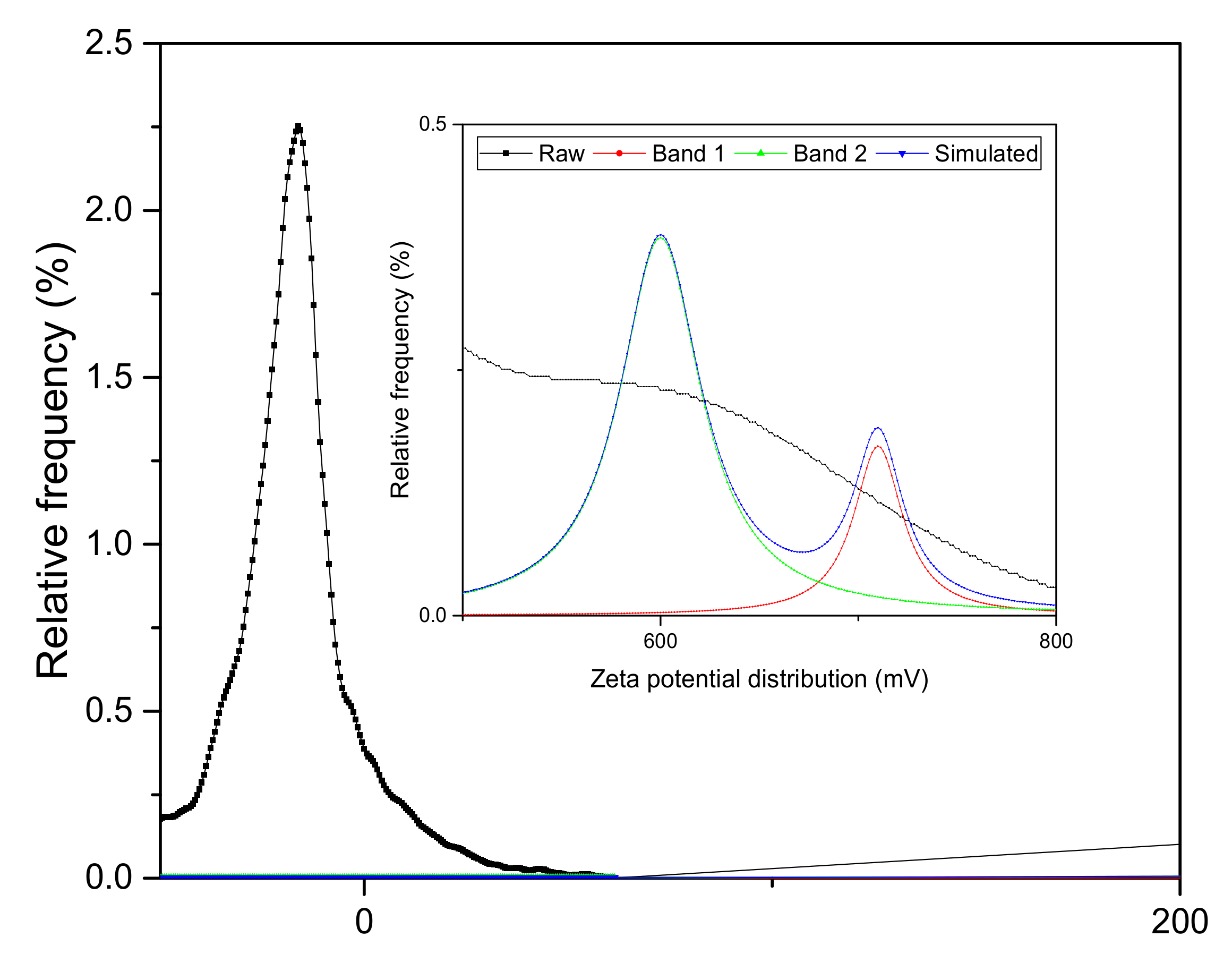
3.1. Surface Charge Analysis
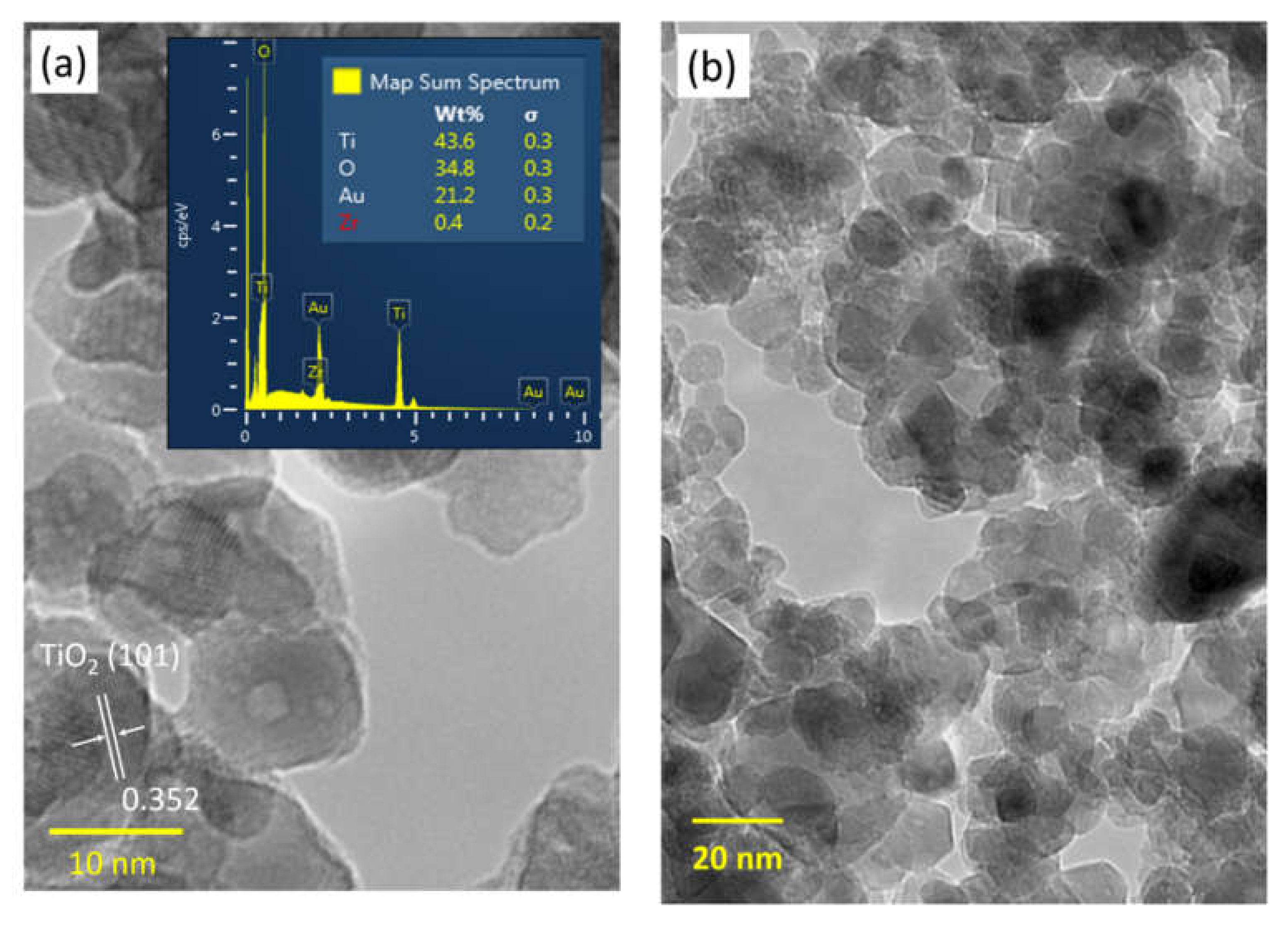
3.2. Nanostructure and Elemental Composition Analysis
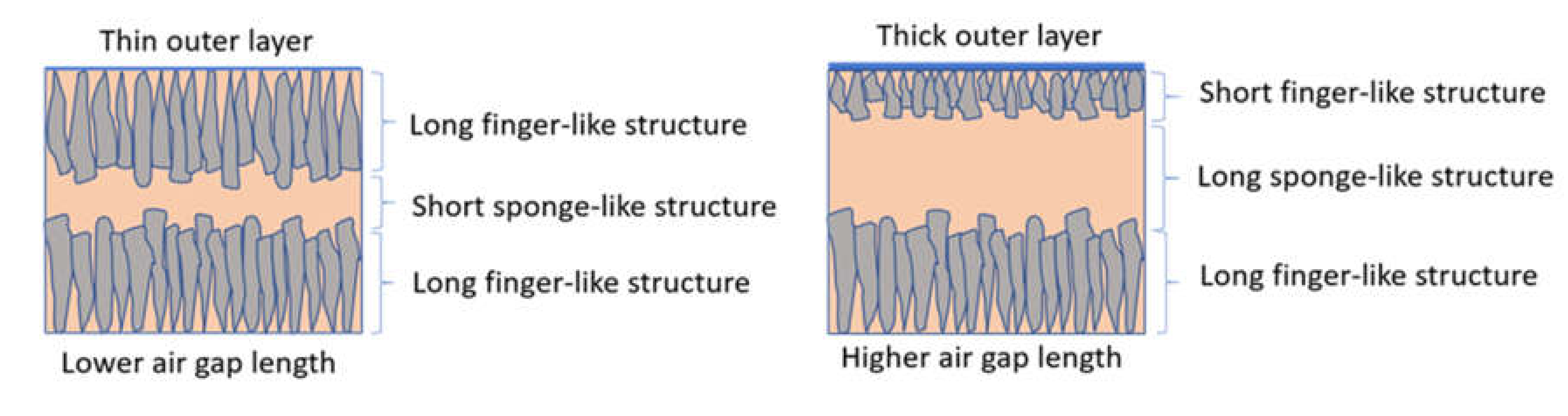
3.3. Effect of Air Gap Length on Membrane Pore Size and Morphology
3.4. Effect of Air Gap on Membrane Hydrophilicity
3.5. Effect of Air Gap on Membrane Porosity
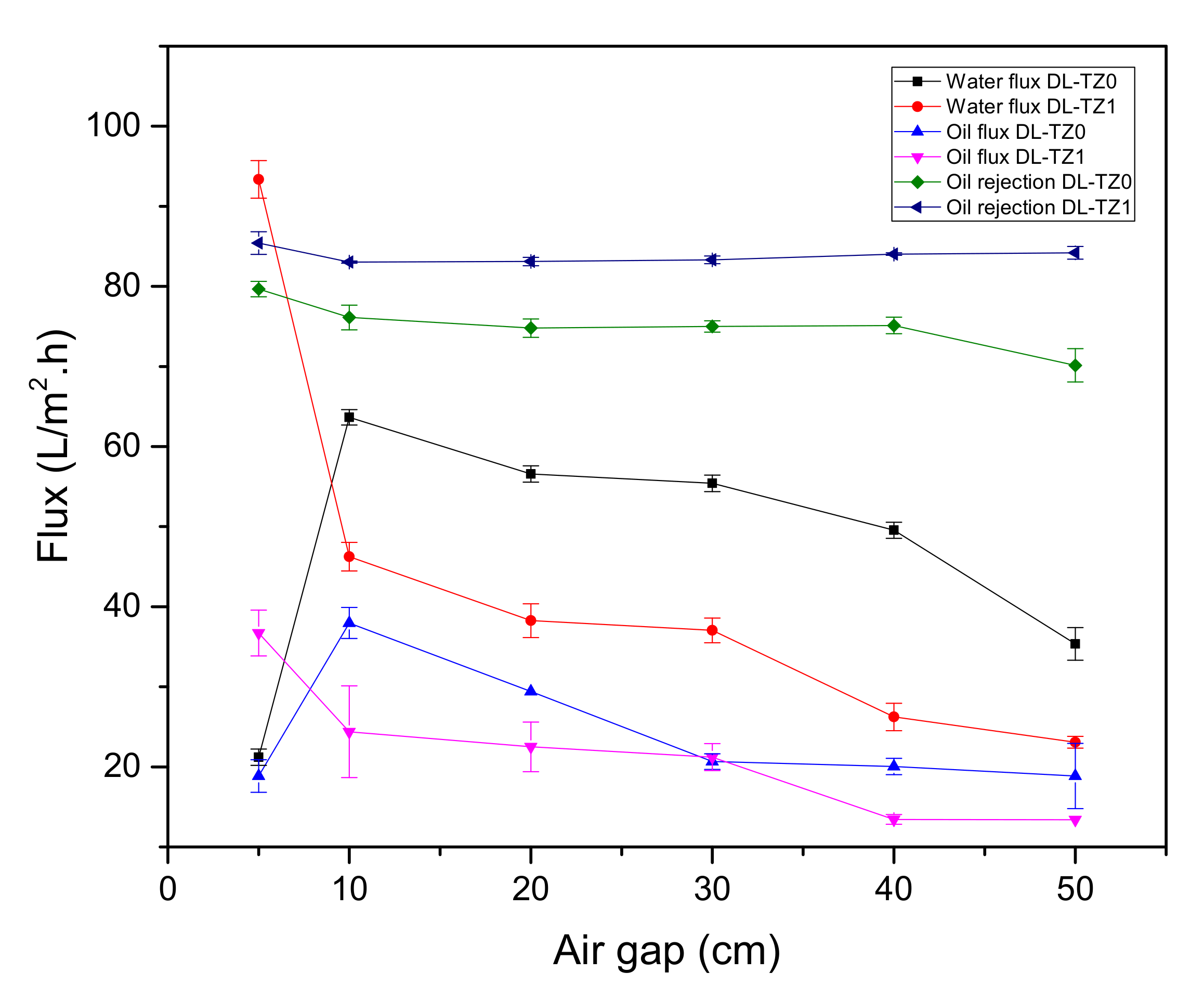
3.6. Effect of Air Gap on Membrane Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alade, A.O.; Jameel, A.T.; Muyubi, S.A.; Abdul Karim, M.I.; Alam, M.Z. Removal of oil and grease as emerging pollutants of concern (EPC) in wastewater stream. IIUM Eng. J. 2018, 12, 161–169. [Google Scholar]
- Elanchezhiyan, S.S.; Meenakshi, S. Encapsulation of metal ions between the biopolymeric layer beads for tunable action on oil particles adsorption from oily wastewater. J. Mol. Liq. 2018, 255, 429–438. [Google Scholar] [CrossRef]
- Saini, B.; Sinha, M.K.; Dash, S.K. Mitigation of HA, BSA and oil/water emulsion fouling of PVDF Ultrafiltration Membranes by SiO2-g-PEGMA nanoparticles. J. Water Process Eng. 2018, 30. [Google Scholar] [CrossRef]
- Sarioglu Cebeci, M.; Gökçek, Ö.B. Investigation of the treatability of molasses and industrial oily wastewater mixture by an anaerobic membrane hybrid system. J. Environ. Manag. 2018, 224, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Guria, C.; Mandal, A. Synthesis, characterization and performance studies of mixed–matrix poly(vinyl chloride)–bentonite ultrafiltration membrane for the treatment of saline oily wastewater. Process Saf. Environ. Prot. 2018, 116, 703–717. [Google Scholar] [CrossRef]
- Ong, C.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.I. Preparation and characterization of PVDF–TiO2 composite membranes blended with different Mw of PVP for oily wastewater treatment using submerged membrane system. J. Tekn. 2014, 69, 53–56. [Google Scholar] [CrossRef]
- Yan, L.; Hong, S.; Li, M.L.; Li, Y.S. Application of the Al2O3–PVDF nanocomposite tubular ultrafiltration (UF) membrane for oily wastewater treatment and its antifouling research. Sep. Purif. Technol. 2009, 66, 347–352. [Google Scholar] [CrossRef]
- Jamshidi Gohari, R.; Halakoo, E.; Lau, W.J.; Kassim, M.A.; Matsuura, T.; Ismail, A.F. Novel polyethersulfone (PES)/hydrous manganese dioxide (HMO) mixed matrix membranes with improved anti–fouling properties for oily wastewater treatment process. RSC Adv. 2014, 4, 17587–17596. [Google Scholar] [CrossRef]
- Ong, C.S.; Lau, W.J.; Goh, P.S.; Ng, B.C.; Ismail, A.F. Preparation and characterization of PVDF–PVP–TiO2 composite hollow fiber membranes for oily wastewater treatment using submerged membrane system. Desalin. Water Treat. 2013, 3994, 1–11. [Google Scholar] [CrossRef]
- Ong, C.S.; Lau, W.J.; Goh, P.S.; Ng, B.C.; Matsuura, T.; Ismail, A.F. Effect of PVP molecular weights on the properties of PVDF–TiO2 composite membrane for oily wastewater treatment process. Sep. Sci. Technol. 2014, 49, 2303–2314. [Google Scholar] [CrossRef]
- Mishra, S.B.; Sachan, S.; Mishra, P.K.; Ramesh, M.R. Preparation and Characterisation of PPEES–TiO2 Composite Micro–porous UF Membrane for Oily Water Treatment. Procedia Mater. Sci. 2014, 5, 123–129. [Google Scholar] [CrossRef]
- Moghadam, M.T.; Lesage, G.; Mohammadi, T.; Mericq, J.P.; Mendret, J.; Heran, M.; Faur, C.; Brosillon, S.; Hemmati, M.; Naeimpoor, F. Improved antifouling properties of TiO2/PVDF nanocomposite membranes in UV–coupled ultrafiltration. J. Appl. Polym. Sci. 2015, 132, 41731. [Google Scholar] [CrossRef]
- Jamshidi Gohari, R.; Korminouri, F.; Lau, W.J.; Ismail, A.F.; Matsuura, T.; Chowdhury, M.N.K.; Halakoo, E.; Jamshidi Gohari, M.S. A novel super–hydrophilic PSf/HAO nanocomposite ultrafiltration membrane for efficient separation of oil/water emulsion. Sep. Pur. Technol. 2015, 150, 13–20. [Google Scholar] [CrossRef]
- Tang, Y.P.; Chan, J.X.; Chung, T.S.; Weber, M.; Staudt, C.; Maletzko, C. Simultaneously covalent and ionic bridging towards antifouling of GO–imbedded nanocomposite hollow fiber membranes. J. Mater. Chem. A 2015, 3, 10573–10584. [Google Scholar] [CrossRef]
- Ali, I.; Suhail, M.; Alothman, Z.A.; Alwarthan, A. Recent advances in syntheses, properties and applications of TiO2 nanostructures. RSC Adv. 2018, 8, 30125–30147. [Google Scholar] [CrossRef]
- Razmjou, A.; Resosudarmo, A.; Holmes, R.L.; Li, H.; Mansouri, J.; Chen, V. The effect of modified TiO2 nanoparticles on the polyethersulfone ultrafiltration hollow fiber membranes. Desalination 2012, 287, 271–280. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Sadeghi, M.T.; Khazaei, M. Improving oleophobicity and hydrophilicity of superhydrophobic surface by TiO2–based coatings. Mater. Res. Exp. 2018, 5, 085010. [Google Scholar] [CrossRef]
- Rezaei, M.; Ismail, A.F.; Hashemifard, S.A.; Matsuura, T. Preparation and characterization of PVDF–montmorillonite mixed matrix hollow fiber membrane for gas–liquid contacting process. Chem. Eng. Res. Des. 2014, 92, 2449–2460. [Google Scholar] [CrossRef]
- Koutahzadeh, N.; Esfahani, M.R.; Arce, P.E. Sequential Use of UV/H2O2—(PSF/TiO2/MWCNT) Mixed matrix membranes for dye removal in water purification: Membrane permeation, fouling, rejection, and decolorization. Environ. Eng. Sci. 2016, 33, 430–440. [Google Scholar] [CrossRef]
- Davey, C.J.; Leak, D.; Patterson, D.A. Hybrid and mixed matrix membranes for separations from fermentations. Membranes 2016, 6, 17. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Shen, H.M.; Xu, Z.-L. PVDF—TiO2 Composite hollow fiber ultrafiltration membranes prepared by TiO2 sol–gel method blending method. J. Appl. Polym. Sci. 2009, 113, 1763–1772. [Google Scholar] [CrossRef]
- Kubiak, A.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Titania-based hybrid materials with ZnO, ZrO2 and MoS2: A review. Materials 2018, 11, 2295. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Nakahara, C.; Ota, K.; Inagaki, M. Direct formation of zirconia–doped titania with stable anatase–type structure by thermal hydrolysis. J. Am. Ceram. Soc. 2002, 85, 1333–1335. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Sotto, A.; Kim, J.; Arsuaga, J.M.; Del Rosario, G.; Martínez, A.; Nam, D.; Luis, P.; Van Der Bruggen, B. Binary metal oxides for composite ultrafiltration membranes. J. Mater. Chem. A 2014, 2, 7054–7064. [Google Scholar] [CrossRef]
- Khan, S.; Kim, J.; Sotto, A.; Van der Bruggen, B. Humic acid fouling in a submerged photocatalytic membrane reactor with binary TiO2–ZrO2 particles. J. Ind. Eng. Chem. 2015, 21, 779–786. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, K.Y.; Chung, T. Dual–Layer Hollow fibers with enhanced flux as novel forward osmosis membranes for water production. Environ. Sci. Technol. 2009, 43, 2800–2805. [Google Scholar] [CrossRef]
- Fu, F.J.; Zhang, S.; Sun, S.P.; Wang, K.Y.; Chung, T.S. POSS–containing delamination–free dual–layer hollow fiber membranes for forward osmosis and osmotic power generation. J. Membr. Sci. 2013, 443, 144–155. [Google Scholar] [CrossRef]
- Shi, H.; Xue, L.; Gao, A.; Zhou, Q. Dual layer hollow fiber PVDF ultra–filtration membranes containing Ag nano–particle loaded zeolite with longer term anti–bacterial capacity in salt water. Water Sci. Technol. 2016, 73, 2159–2167. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Photocatalytic degradation of nonylphenol by immobilized TiO2 in dual layer hollow fibre membranes. Chem. Eng. J. 2015, 269, 255–261. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Photocatalytic degradation of nonylphenol using co–extruded dual–layer hollow fibre membranes incorporated with a different ratio of TiO2/PVDF. React. Funct. Polym. 2016, 99, 80–87. [Google Scholar] [CrossRef]
- Sun, S.P.; Wang, K.Y.; Peng, N.; Hatton, T.A.; Chung, T.S. Novel polyamide–imide/cellulose acetate dual–layer hollow fiber membranes for nanofiltration. J. Membr. Sci. 2010, 363, 232–242. [Google Scholar] [CrossRef]
- Liu, T.Y.; Zhang, R.X.; Li, Q.; Van der Bruggen, B.; Wang, X.L. Fabrication of a novel dual–layer (PES/PVDF) hollow fiber ultrafiltration membrane for wastewater treatment. J. Membr. Sci. 2014, 472, 119–132. [Google Scholar] [CrossRef]
- Zuo, J.; Chung, T.S.; O’Brien, G.S.; Kosar, W. Hydrophobic/hydrophilic PVDF/Ultem® dual–layer hollow fiber membranes with enhanced mechanical properties for vacuum membrane distillation. J. Membr. Sci. 2017, 523, 103–110. [Google Scholar] [CrossRef]
- Khayet, M. The effects of air gap length on the internal and external morphology of hollow fiber membranes. Chem. Eng. Sci. 2003, 58, 3091–3104. [Google Scholar] [CrossRef]
- Korminouri, F.; Rahbari–Sisakht, M.; Matsuura, T.; Ismail, A.F. Surface modification of polysulfone hollow fiber membrane spun under different air–gap lengths for carbon dioxide absorption in membrane contactor system. Chem. Eng. J. 2015, 264, 453–461. [Google Scholar] [CrossRef]
- Fang, C.; Rajabzadeh, S.; Wu, H.-C.; Zhang, X.; Kato, N.; Kunimatsu, M.; Yoshioka, T.; Matsuyama, H. Effect of mass transfer at the interface of the polymer solution and extruded solvent during the air gap on membrane structures and performances in TIPS process using triple–orifice spinneret. J. Membr. Sci 2020, 595, 117513. [Google Scholar]
- Mubashir, M.; Yeong, Y.F.; Chew, T.L.; Lau, K.K. Optimization of spinning parameters on the fabrication of NH2–MIL–53(Al)/cellulose acetate (CA) hollow fiber mixed matrix membrane for CO2 separation. Sep. Purif. Technol. 2019, 215, 32–43. [Google Scholar] [CrossRef]
- Le, N.L.; Tang, Y.P.; Chung, T.S. The development of high–performance 6FDA–NDA/DABA/POSS/Ultem® dual–layer hollow fibers for ethanol dehydration via pervaporation. J. Membr. Sci. 2013, 447, 163–176. [Google Scholar] [CrossRef]
- Neppolian, B.; Wang, Q.; Yamashita, H.; Choi, H. Synthesis and characterization of ZrO2-TiO2 binary oxide semiconductor nanoparticles: Application and interparticle electron transfer process. Appl. Catal. A Gen. 2007, 333, 264–271. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Jiang, G.; He, G. Hierarchically macro–mesoporous ZrO2-TiO2 composites with enhanced photocatalytic activity. Ceram. Int. 2015, 41, 5749–5757. [Google Scholar] [CrossRef]
- Wang, P.; Teoh, M.M.; Chung, T.S. Morphological architecture of dual–layer hollow fiber for membrane distillation with higher desalination performance. Water Res. 2011, 45, 5489–5500. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Modi, A.; Bellare, J. Enhanced flux and antifouling property on municipal wastewater of polyethersulfone hollow fiber membranes by embedding carboxylated multi–walled carbon nanotubes and a vitamin E derivative. Sep. Purif. Technol. 2020, 235, 116199. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Lau, C.-H.; Zhang, N.-Q.; Bai, Y.-P.; Shao, L. Mussel–inspired tailoring of membrane wettability for harsh water treatment. J. Mater. Chem. A 2015, 3, 2650–2657. [Google Scholar] [CrossRef]
- Ong, C.S.; Lau, W.J.; Goh, P.S.; Ng, B.C.; Ismail, A.F. Investigation of submerged membrane photocatalytic reactor (sMPR) operating parameters during oily wastewater treatment process. Desalination 2014, 353, 48–56. [Google Scholar] [CrossRef]
- Mavinakere Ramesh, A.; Shivanna, S. Visible light assisted photocatalytic degradation of chromium (VI) by using nanoporous Fe2O3. J. Mater. 2018, 2018, 1593947. [Google Scholar] [CrossRef]
- Dong, Z.; Iqbal, S.; Zhao, Z. Preparation of ergosterol–loaded nanostructured lipid carriers for enhancing oral bioavailability and antidiabetic nephropathy effects. AAPS PharmSciTech 2020, 21, 64. [Google Scholar] [CrossRef]
- Abo El–Reesh, G.Y.; Farghali, A.A.; Taha, M.; Mahmoud, R.K. Novel synthesis of Ni/Fe layered double hydroxides using urea and glycerol and their enhanced adsorption behavior for Cr(VI) removal. Sci. Rep. 2020, 10, 587. [Google Scholar] [CrossRef]
- Garcia–Ivars, J.; Alcaina–Miranda, M.I.; Iborra–Clar, M.I.; Mendoza–Roca, J.A.; Pastor–Alcañiz, L. Enhancement in hydrophilicity of different polymer phase–inversion ultrafiltration membranes by introducing PEG/Al2O3 nanoparticles. Sep. Purif. Technol. 2014, 128, 45–57. [Google Scholar] [CrossRef]
- Galiano, F.; Song, X.; Marino, T.; Boerrigter, M.; Saoncella, O.; Simone, S.; Faccini, M.; Chaumette, C.; Drioli, E.; Figoli, A. Novel photocatalytic PVDF/Nano–TiO2 hollow fibers for Environmental remediation. Polymers 2018, 10, 1134. [Google Scholar] [CrossRef]
- Karim, Z.; Adnan, R.; Ansari, M.S. Low concentration of silver nanoparticles not only enhances the activity of horseradish peroxidase but alter the structure also. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Morphological study of co–extruded dual–layer hollow fiber membranes incorporated with different TiO2 loadings. J. Membr. Sci. 2015, 479, 123–131. [Google Scholar] [CrossRef]
- Perlich, J.; Kaune, G.; Memesa, M.; Gutmann, J.S.; Müller–Buschbaum, P. Sponge–like structures for application in photovoltaics. Phil. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1783–1798. [Google Scholar] [CrossRef] [PubMed]
- Purkait, M.K.; Sinha, M.K.; Mondal, P.; Singh, R. Introduction to membranes. Interface Sci. Technol. 2018, 25, 1–37. [Google Scholar]
- Villalobos, L.F.; Hilke, R.; Akhtar, F.H.; Peinemann, K.-V. Fabrication of polybenzimidazole/palladium nanoparticles hollow fiber membranes for hydrogen purification. Adv. Energy Mater. 2017, 1701567. [Google Scholar] [CrossRef]
- Kajekar, A.J.; Dodamani, B.M.; Isloor, A.M.; Karim, Z.A.; Cheer, N.B.; Ismail, A.F.; Shilton, S.J. Preparation and characterization of novel PSf/PVP/PANI–nanofiber nanocomposite hollow fiber ultrafiltration membranes and their possible applications for hazardous dye rejection. Desalination 2015, 365, 117–125. [Google Scholar] [CrossRef]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Surface science techniques. In Springer Series in Surface Sciences; Bracco, G., Holst, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51, pp. 3–34. ISBN 9783642342431. [Google Scholar]
- Li, Y.; Cao, C.; Chung, T.S.; Pramoda, K.P. Fabrication of dual-layer polyethersulfone (PES) hollow fiber membranes with an ultrathin dense-selective layer for gas. J. Membr. Sci. 2004, 245, 53–60. [Google Scholar] [CrossRef]






| Parameters | Values |
|---|---|
| Outer dope composition (wt%) | PVDF/ZrO2-TiO2/NMP/PVP (15/1/80/5) |
| Inner dope composition (wt%) | PVDF/NMP/PVP (18/77/5) |
| Outer dope flowrate (cm3/min) | 2.48 |
| Inner dope flowrate (cm3/min) | 1.2 |
| Bore fluid | Distilled water |
| Bore fluid flowrate (cm3/min) | 0.73 |
| Air gap (cm) | 5 |
| Take up speed (cm/s) | Free fall |
| Spinneret dimension | 1.2/0.8/0.4 |
| Samples | Porosity | Samples | Porosity |
|---|---|---|---|
| DL-ZT0–5 | 49.7 | DL-ZT1–5 | 45.5 |
| DL-ZT0–10 | 50.9 | DL-ZT1–10 | 46.3 |
| DL-ZT0–20 | 60.0 | DL-ZT1–20 | 47.2 |
| DL-ZT0–30 | 58.5 | DL-ZT1–30 | 46.9 |
| DL-ZT0–40 | 56.9 | DL-ZT1–40 | 47.0 |
| DL-ZT0–50 | 58.1 | DL-ZT1–50 | 46.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaacob, N.; Goh, P.S.; Ismail, A.F.; Mohd Nazri, N.A.; Ng, B.C.; Zainal Abidin, M.N.; Yogarathinam, L.T. ZrO2-TiO2 Incorporated PVDF Dual-Layer Hollow Fiber Membrane for Oily Wastewater Treatment: Effect of Air Gap. Membranes 2020, 10, 124. https://doi.org/10.3390/membranes10060124
Yaacob N, Goh PS, Ismail AF, Mohd Nazri NA, Ng BC, Zainal Abidin MN, Yogarathinam LT. ZrO2-TiO2 Incorporated PVDF Dual-Layer Hollow Fiber Membrane for Oily Wastewater Treatment: Effect of Air Gap. Membranes. 2020; 10(6):124. https://doi.org/10.3390/membranes10060124
Chicago/Turabian StyleYaacob, Nurshahnawal, Pei Sean Goh, Ahmad Fauzi Ismail, Noor Aina Mohd Nazri, Be Cheer Ng, Muhammad Nizam Zainal Abidin, and Lukka Thuyavan Yogarathinam. 2020. "ZrO2-TiO2 Incorporated PVDF Dual-Layer Hollow Fiber Membrane for Oily Wastewater Treatment: Effect of Air Gap" Membranes 10, no. 6: 124. https://doi.org/10.3390/membranes10060124
APA StyleYaacob, N., Goh, P. S., Ismail, A. F., Mohd Nazri, N. A., Ng, B. C., Zainal Abidin, M. N., & Yogarathinam, L. T. (2020). ZrO2-TiO2 Incorporated PVDF Dual-Layer Hollow Fiber Membrane for Oily Wastewater Treatment: Effect of Air Gap. Membranes, 10(6), 124. https://doi.org/10.3390/membranes10060124







