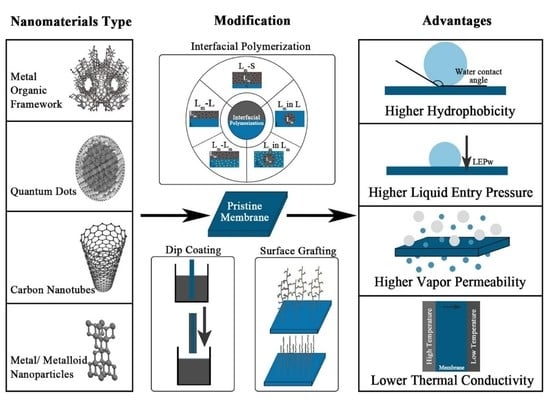
Recent Developments in Nanomaterials-Modified Membranes for Improved Membrane Distillation Performance
Abstract
:1. Introduction
1.1. Timeline of MD Membranes
1.2. New Generation Nanomaterials-based MD Membrane
2. Fundamentals of MD Membrane
2.1. Membrane Materials and Structure
2.2. MD Membrane Perquisites
2.3. MD Membrane Modification
2.3.1. Surface Grafting
2.3.2. Plasma Polymerization
2.3.3. Interfacial Polymerization
2.3.4. Dip Coating
2.4. State of the Art of Nanomaterials Doped MD Membrane
3. Incorporation of Nanomaterials for Enhanced Performance
3.1. Metalloid and Metal Oxides Based Nanoparticles
3.2. Carbon Materials
3.2.1. Carbon Nanotubes (CNTs)
3.2.2. Graphene and Graphene Oxide (GO)
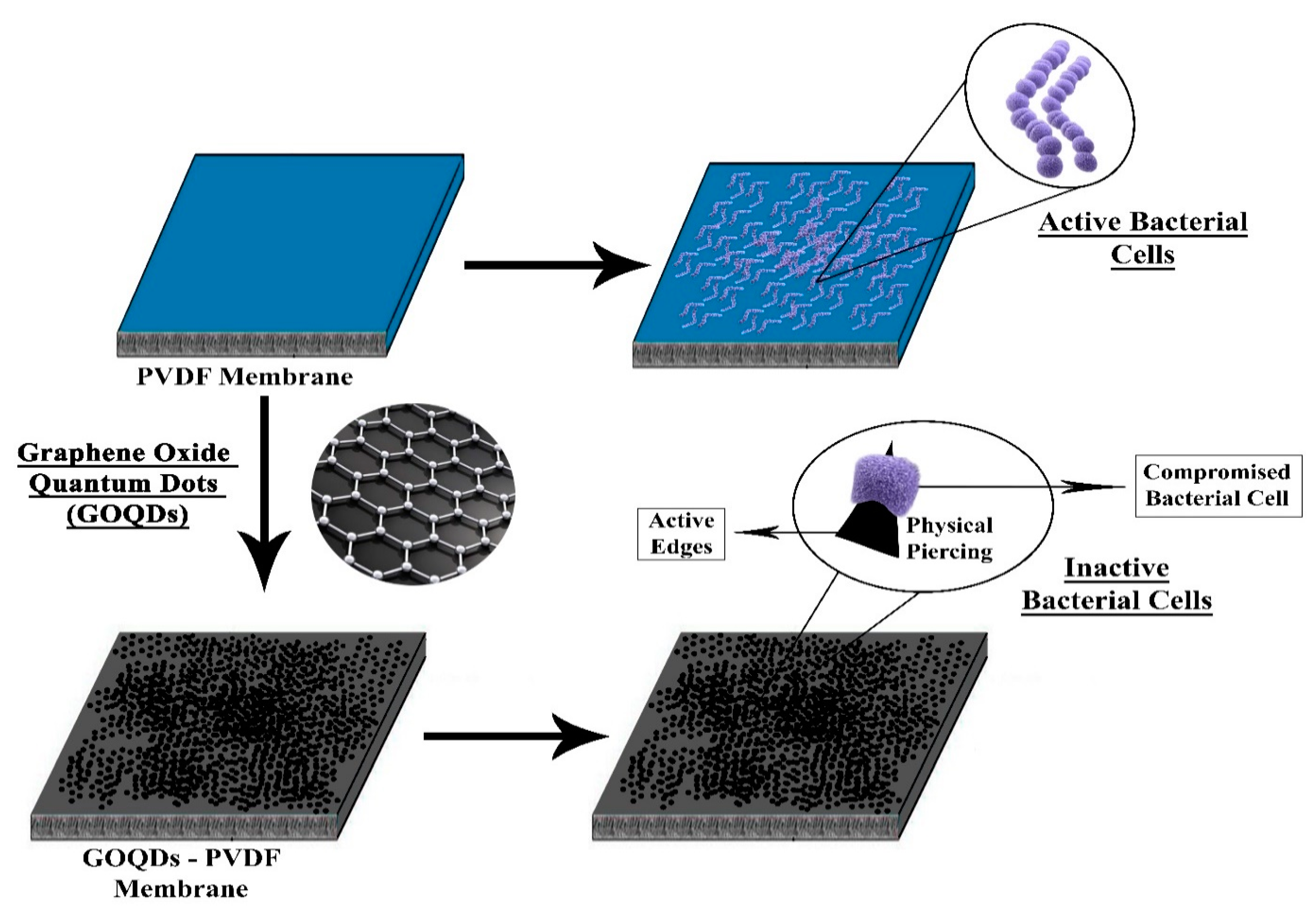
3.2.3. Quantum Dots (QDs)
3.3. Metal Organic Framework (MOFs)
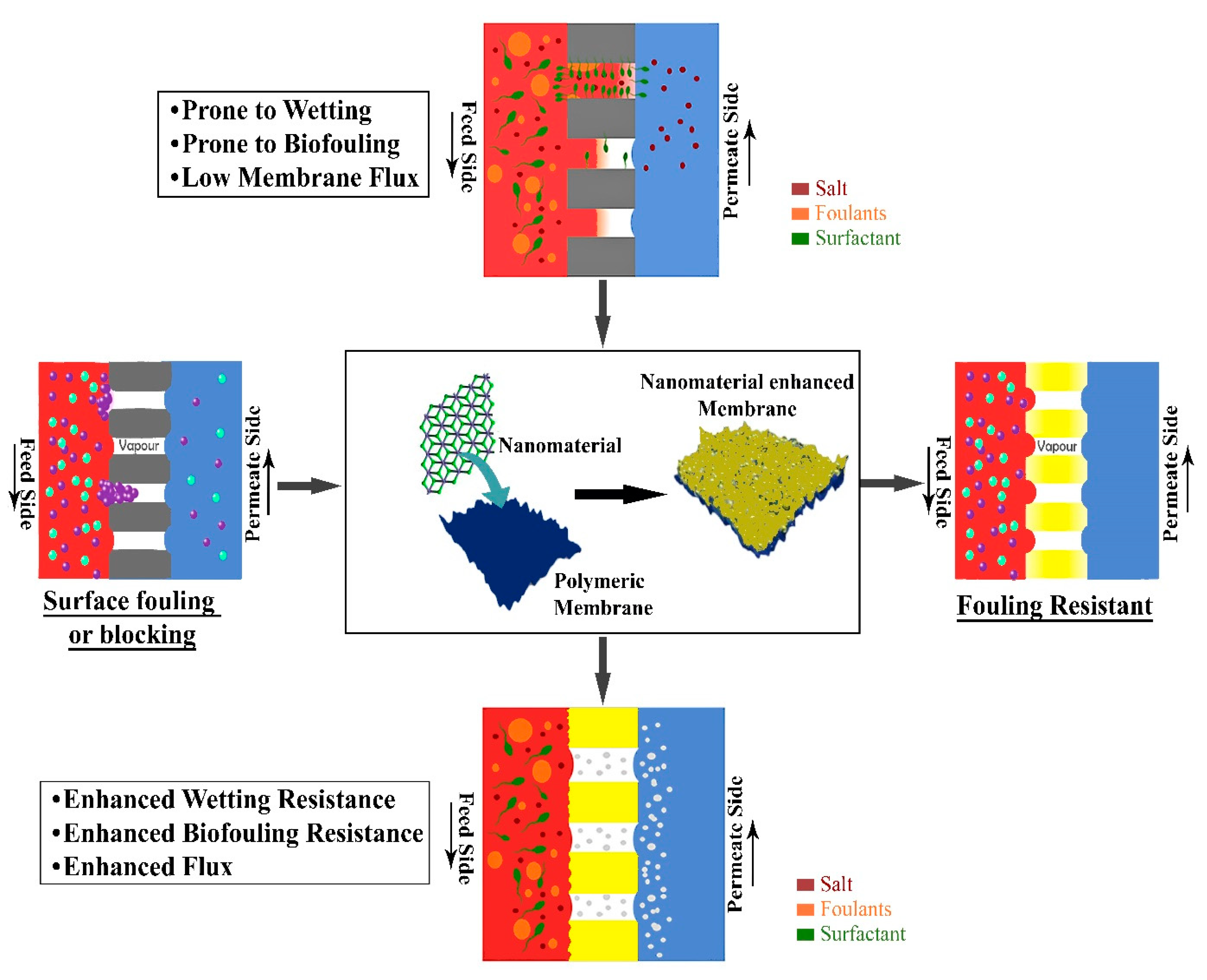
4. Nanomaterials for Fouling Control in MD Process
5. Current Challenges and Future Outlook
- Efficient synthesis methods: The use of nanomaterials poses many barriers to successful membrane modification, such as membrane pore blocking and non-uniform dispersal of membrane nanomaterials, among others [1,136]. Hence, it is important to test and devise an efficient membrane synthesis method according to the requirements for successful membrane modification. Otherwise, the entire process may fail because the membranes will not be efficient and successful in their position;
- Appropriate integration of the materials: The nanomaterials used to modify the membranes must adhere or embed in the membranes appropriately; otherwise, they may leach out over time [136]. The different commonly used nanomaterials, such as CNTs and metal oxide NPs, among others, could be linked to the membranes via different groups, like -OH, present on their surface with the functional groups present in the membrane matrix via hydrogen or covalent bonds. This will not only make the membrane effective and efficient but also enhance its stability and life;
- Stability of the fillers: The stability of the filler material is very important as it defines the overall membrane effectiveness and efficiency [135,136,137]. They must be firmly embedded with the aid of various bonds in the membrane matrix. The firmly embedded filler material in the membrane satisfies that while in use, the filler material will not disintegrate from the membrane. The membrane can, therefore, be utilized for longer periods of time without losing its structural and functional integrity. However, presently much work is needed for developing such filler materials as well as synthesis methods that approve the stability of the fillers to cent percent;
- Conservation of the functional properties: It is very important to conserve the required functional attributes of membranes for which they are sought. Currently, however, there is a definite loss of functional characteristics during membrane synthesis, meaning that the membranes cannot show the theoretical extent of their functional attributes. In addition, there is a loss of functionality over time during membrane operations. Therefore, there is a requirement of a sustainable synthesis method that is capable of withholding the functional capacity of the membranes to the best;
- Membrane strength: The filler materials, sometimes instead of increasing the strength of the membrane, make them weak and brittle. Therefore, this aspect should also be studied carefully before using any filler for the modification of membranes;
- Membrane fouling: Fouling is one of the most important factors in membrane science that inhibits the use of membranes in large-scale applications. However, there are lots of studies carried out specifically to eradicate this single problem, but it still persists [2]. Therefore, there is a need to tackle this problem appropriately for better employment of membranes for large-scale applications.
- (a)
- Risk of particle aggregation while incorporating nanomaterials onto the membrane;
- (b)
- Utilization of cheap nanomaterials in order to reduce the overall cost;
- (c)
- Rougher membrane surface may enhance membrane fouling, which must be optimized;
- (d)
- Risk of peeling off or particle washout after a long-term experiment.
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Purkait, M.K.; Singh, R.; Haldar, D.; Mondal, P. Thermal Induced Membrane Separation Processes; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Purkait, M.K.; Singh, R. Membrane Technology in Separation Science; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Purkait, M.K.; Sinha, M.K.; Mondal, P.; Singh, R. Stimuli Responsive Polymeric Membranes: Smart Polymeric Membranes; Academic Press: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.S.; Sangeetha, D.; Chang, H.M.; Thanh, C.N.D.; Le, Q.H.; Ku, H.M. Developments in forward osmosis and membrane distillation for desalination of waters. Environ. Chem. Lett. 2018, 16, 1247–1265. [Google Scholar] [CrossRef]
- Kochkodan, V.; Hilal, N. A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 2015, 356, 187–207. [Google Scholar] [CrossRef]
- Gilron, J.; Song, L.; Sirkar, K.K. Design for cascade of crossflow direct contact membrane distillation. Ind. Eng. Chem. Res. 2007, 46, 2324–2334. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N.J.D. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Ray, S.S.; Dommati, H.; Wang, J.-C.; Chen, S.-S. Solvent based Slurry Stereolithography 3D printed hydrophilic ceramic membrane for ultrafiltration application. Ceram. Int. 2020, 46, 12480–12488. [Google Scholar] [CrossRef]
- Bodell, B.R. Distillation of Saline Water Using Silicone Rubber Membrane. U.S. Patent US3361645A, 2 January 1968. [Google Scholar]
- Amaya-Vías, D.; López-Ramírez, J.A. Techno-economic assessment of air and water gap membrane distillation for Seawater desalination under different heat source scenarios. Water 2019, 11, 2117. [Google Scholar] [CrossRef] [Green Version]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.D.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.S.; Deb, C.K.; Chang, H.M.; Chen, S.S.; Ganesapillai, M. Crosslinked PVDF-HFP-based hydrophobic membranes incorporated with CNF for enhanced stability and permeability in membrane distillation. J. Appl. Polym. Sci. 2019, 136, 48021. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Cui, Z.; Drioli, E.; Wang, Z.; Zhao, S. Enhancing wetting resistance of poly (vinylidene fluoride) membranes for vacuum membrane distillation. Desalination 2017, 415, 58–66. [Google Scholar] [CrossRef]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; You-Sheng, O.Y.; Chen, Y.B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Nthunya, L.N.; Gutierrez, L.; Derese, S.; Nxumalo, E.N.; Verliefde, A.R.; Mamba, B.B.; Mhlanga, S.D. A review of nanoparticle-enhanced membrane distillation membranes: Membrane synthesis and applications in water treatment. J. Chem. Technol. Biotechnol. 2019, 94, 2757–2771. [Google Scholar] [CrossRef]
- Wang, Y.; He, G.; Shao, Y.; Zhang, D.; Ruan, X.; Xiao, W.; Li, X.; Wu, X.; Jiang, X.J.S.; Technology, P. Enhanced performance of superhydrophobic polypropylene membrane with modified antifouling surface for high salinity water treatment. Sep. Purif. Technol. 2019, 214, 11–20. [Google Scholar] [CrossRef]
- Barati, G. Science and Engineering of Superhydrophobic Surfaces: Review of Corrosion Resistance. Chem. Mech. Stab. Arab. J. Chem. 2018, 13, 1763–1802. [Google Scholar] [CrossRef]
- Deka, B.J.; Guo, J.; Khanzada, N.K.; An, A.K. Omniphobic re-entrant PVDF membrane with ZnO nanoparticles composite for desalination of low surface tension oily seawater. Water Res. 2019, 165, 114982. [Google Scholar] [CrossRef]
- Natarajan, K.J.C. Antibiofilm activity of epoxy/Ag-TiO2 polymer nanocomposite coatings against Staphylococcus aureus and Escherichia coli. Coatings 2015, 5, 95–114. [Google Scholar]
- Furukawa, H.; Cordova, K.E.O.; Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Hernandez, B.V.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Kadhom, M.; Deng, B. Metal-organic frameworks (MOFs) in water filtration membranes for desalination and other applications. Appl. Mater. Today 2018, 11, 219–230. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Abd Hamid, S.B.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Zhao, D.L.; Chung, T.-S. Applications of carbon quantum dots (CQDs) in membrane technologies: A review. Water Res. 2018, 147, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Dumee, L.; Velleman, L.; Sears, K.; Hill, M.; Schutz, J.; Finn, N.; Duke, M.; Gray, S. Control of porosity and pore size of metal reinforced carbon nanotube membranes. Membranes 2011, 1, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Nasreen, S.A.A.N.; Sundarrajan, S.; Nizar, S.A.S.; Balamurugan, R.; Ramakrishna, S. Advancement in electrospun nanofibrous membranes modification and their application in water treatment. Membranes 2013, 3, 266–284. [Google Scholar] [CrossRef]
- Ma, L.; Dong, X.; Chen, M.; Zhu, L.; Wang, C.; Yang, F.; Dong, Y. Fabrication and water treatment application of carbon nanotubes (CNTs)-based composite membranes: A review. Membranes 2017, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Lippmann, G. Endosmose entre deux liquides de même composition chimique et de températures différentes. Compt. Rend 1907, 145, 104–105. [Google Scholar]
- Aubert, M. Thermo-osmose. Verlag Nicht Ermittelbar; Gauthier-Villars: Paris, France, 1912. [Google Scholar]
- Villalobos García, J.; Dow, N.; Milne, N.; Zhang, J.; Naidoo, L.; Gray, S.; Duke, M. Membrane distillation trial on textile wastewater containing surfactants using hydrophobic and hydrophilic-coated polytetrafluoroethylene (PTFE) membranes. Membranes 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Nawi, M.; Izati, N.; Bilad, M.R.; Zolkhiflee, N.; Nordin, N.A.H.; Lau, W.J.; Narkkun, T.; Faungnawakij, K.; Arahman, N.; Mahlia, T.M.I. Development of a novel corrugated polyvinylidene difluoride membrane via improved imprinting technique for membrane distillation. Polymers 2019, 11, 865. [Google Scholar] [CrossRef] [Green Version]
- Gryta, M. Effectiveness of water desalination by membrane distillation process. Membranes 2012, 2, 415–429. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Chung, T.S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. How to optimize the membrane properties for membrane distillation: A review. Ind. Eng. Chem. Res. 2016, 55, 9333–9343. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Pervaporation and vacuum membrane distillation processes: Modeling and experiments. AIChE J. 2004, 50, 1697–1712. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Li, P. Effect of polymer dope concentration on the morphology and performance of PES/PDMS hollow fiber composite membrane for gas separation. PES 2017, 500, 72. [Google Scholar] [CrossRef]
- Kim, J.; Van der Bruggen, B.J.E.P. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Rana, D.; Lan, C.Q.; Matsuura, T.J.S.; Technology, P. Effects of hydrophilic silica nanoparticles and backing material in improving the structure and performance of VMD PVDF membranes. Sep. Purif. Technol. 2016, 157, 60–71. [Google Scholar] [CrossRef]
- Efome, J.E.; Baghbanzadeh, M.; Rana, D.; Matsuura, T.; Lan, C.Q.J.D. Effects of superhydrophobic SiO2 nanoparticles on the performance of PVDF flat sheet membranes for vacuum membrane distillation. Desalination 2015, 373, 47–57. [Google Scholar] [CrossRef]
- Attia, H.; Alexander, S.; Wright, C.J.; Hilal, N.J.D. Superhydrophobic electrospun membrane for heavy metals removal by air gap membrane distillation (AGMD). Desalination 2017, 420, 318–329. [Google Scholar] [CrossRef] [Green Version]
- Bhadra, M.; Roy, S.; Mitra, S.J.D. Desalination across a graphene oxide membrane via direct contact membrane distillation. Desalination 2016, 378, 37–43. [Google Scholar] [CrossRef]
- Roy, S.; Bhadra, M.; Mitra, S.J.S.; Technology, P. Enhanced desalination via functionalized carbon nanotube immobilized membrane in direct contact membrane distillation. Sep. Purif. Technol. 2014, 136, 58–65. [Google Scholar] [CrossRef]
- Huang, F.Y.; Arning, A. Performance comparison between polyvinylidene fluoride and polytetrafluoroethylene hollow fiber membranes for direct contact membrane distillation. Membranes 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B.J.S.; Technology, P. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- El-Bourawi, M.S.; Ding, Z.; Ma, R.; Khayet, M. A framework for better understanding membrane distillation separation process. J. Membr. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M. Superhydrophobic surfaces. Curr. Opin. Colloid Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Johnson, D.J.; Oatley-Radcliffe, D.L.; Hilal, N.J.D. State of the art review on membrane surface characterisation: Visualisation, verification and quantification of membrane properties. Desalination 2018, 434, 12–36. [Google Scholar] [CrossRef] [Green Version]
- Laganà, F.; Barbieri, G.; Drioli, E. Direct contact membrane distillation: Modelling and concentration experiments. J. Membr. Sci. 2000, 166, 1–11. [Google Scholar] [CrossRef]
- Srisurichan, S.; Jiraratananon, R.; Fane, A.G. Mass transfer mechanisms and transport resistances in direct contact membrane distillation process. J. Membr. Sci. 2006, 277, 186–194. [Google Scholar] [CrossRef]
- Razmjou, A.; Mansouri, J.; Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Ray, S.S.; Lee, H.-K.; Kwon, Y.-N. Review on blueprint of designing anti-wetting polymeric membrane surfaces for enhanced membrane distillation performance. Polymers 2020, 12, 23. [Google Scholar]
- Ju, J.; Fejjari, K.; Cheng, Y.; Liu, M.; Li, Z.; Kang, W.; Liao, Y. Engineering hierarchically structured superhydrophobic PTFE/POSS nanofibrous membranes for membrane distillation. Desalination 2020, 486, 114481. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y.M. Application and modification of poly (vinylidene fluoride)(PVDF) membranes–a review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Alaoui, O.T.; Yang, H.; Mbareck, C. Dry-cast process for synthetic microporous membranes: Physico-chemical analyses through morphological studies. J. Membr. Sci. 2010, 358, 13–25. [Google Scholar] [CrossRef]
- Kim, J.-K.; Taki, K.; Ohshima, M. Preparation of a unique microporous structure via two step phase separation in the course of drying a ternary polymer solution. Langmuir 2007, 23, 12397–12405. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, A.; Krupa, A.; Czech, T. Modern electrostatic devices and methods for exhaust gas cleaning: A brief review. J. Electrost. 2007, 65, 133–155. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Li, C.-W.; Nguyen, N.C.; Nguyen, H.T. A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv. 2016, 6, 85495–85514. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Nguyen, N.C.; Hsu, H.-T.; Nguyen, H.T.; Chang, C.-T. Poly (vinyl alcohol) incorporated with surfactant based electrospun nanofibrous layer onto polypropylene mat for improved desalination by using membrane distillation. Desalination 2017, 414, 18–27. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Nguyen, N.C.; Nguyen, H.T. Electrospinning: A Versatile Fabrication Technique for Nanofibrous Membranes for Use in Desalination. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–273. [Google Scholar]
- Al-Furaiji, M.; Arena, J.T.; Ren, J.; Benes, N.; Nijmeijer, A.; McCutcheon, J.R. Triple-layer nanofiber membranes for treating high salinity brines using direct contact membrane distillation. Membranes 2019, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Zare, S.; Kargari, A. Membrane properties in membrane distillation. In Emerging Technologies for Sustainable Desalination Handbook; Elsevier: Amsterdam, The Netherlands, 2018; pp. 107–156. [Google Scholar]
- Low, S.-C.; Ng, Q.-H. Progress of stimuli responsive membranes in water treatment. In Advanced Nanomaterials for Membrane Synthesis and its Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 69–99. [Google Scholar]
- Wei, C.C.; Li, K. Preparation and characterization of a robust and hydrophobic ceramic membrane via an improved surface grafting technique. Ind. Eng. Chem. Res. 2009, 48, 3446–3452. [Google Scholar] [CrossRef]
- El-Arnaouty, M.; Abdel Ghaffar, A.; Eid, M.; Aboulfotouh, M.E.; Taher, N.; Soliman, E.-S. Nano-modification of polyamide thin film composite reverse osmosis membranes by radiation grafting. J. Radiat. Res. Appl. Sci. 2018, 11, 204–216. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.K.; Kim, W.; Kim, Y.M.; Kwon, Y.N. Surface modification of polyvinylidene fluoride membrane for enhanced wetting resistance. Appl. Surf. Sci. 2019, 491, 32–42. [Google Scholar] [CrossRef]
- Akhavan, B.; Jarvis, K.; Majewski, P. Plasma polymer-functionalized silica particles for heavy metals removal. ACS Appl. Mater. Interfaces 2015, 7, 4265–4274. [Google Scholar] [CrossRef]
- Song, Y.; Fan, J.-B.; Wang, S. Recent progress in interfacial polymerization. Mater. Chem. Front. 2017, 1, 1028–1040. [Google Scholar] [CrossRef]
- Baig, M.I.; Ingole, P.G.; Jeon, J.D.; Hong, S.U.; Choi, W.K.; Lee, H.K. Water vapor transport properties of interfacially polymerized thin film nanocomposite membranes modified with graphene oxide and GO-TiO2 nanofillers. Chem. Eng. J. 2019, 373, 1190–1202. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Chang, H.-M.; Thanh, C.N.D.; Le, H.Q.; Nguyen, N.C. Enhanced desalination using a three-layer OTMS based superhydrophobic membrane for a membrane distillation process. RSC Adv. 2018, 8, 9640–9650. [Google Scholar] [CrossRef] [Green Version]
- Han, S.W.; Kim, K.D.; Seo, H.O.; Kim, I.H.; Jeon, C.S.; An, J.E.; Kim, J.H.; Uhm, S.; Kim, Y.D. Oil–Water Separation Using Superhydrophobic PET Membranes Fabricated Via Simple Dip-Coating of PDMS–SiO2 Nanoparticles. Macromol. Mater. Eng. 2017, 302, 1700218. [Google Scholar] [CrossRef]
- Yimsiri, P.; Mackley, M.R.J.C.E.S. Spin and dip coating of light-emitting polymer solutions: Matching experiment with modelling. Chem. Eng. Sci. 2006, 61, 3496–3505. [Google Scholar] [CrossRef]
- Nassrullah, H.; Makanjuola, O.; Janajreh, I.; AlMarzooqi, F.A.; Hashaikeh, R. Incorporation of nanosized LTL zeolites in dual-layered PVDF-HFP/cellulose membrane for enhanced membrane distillation performance. J. Membr. Sci 2020, 118298. [Google Scholar] [CrossRef]
- Hamzah, N.; Leo, C.J.S.; Technology, P. Fouling prevention in the membrane distillation of phenolic-rich solution using superhydrophobic PVDF membrane incorporated with TiO2 nanoparticles. Sep. Purif. Technol. 2016, 167, 79–87. [Google Scholar] [CrossRef]
- Razmjou, A.; Arifin, E.; Dong, G.; Mansouri, J.; Chen, V. Superhydrophobic modification of TiO2 nanocomposite PVDF membranes for applications in membrane distillation. J. Membr. Sci. 2012, 415, 850–863. [Google Scholar] [CrossRef]
- Ye, H.; Li, X.; Deng, L.; Li, P.; Zhang, T.; Wang, X.; Hsiao, B.S. Silver nanoparticle-enabled photothermal nanofibrous membrane for light-driven membrane distillation. Ind. Eng. Chem. Res. 2019, 58, 3269–3281. [Google Scholar] [CrossRef]
- Chen, L.H.; Huang, A.; Chen, Y.R.; Chen, C.H.; Hsu, C.C.; Tsai, F.Y.; Tung, K.L. Omniphobic membranes for direct contact membrane distillation: Effective deposition of zinc oxide nanoparticles. Desalination 2018, 428, 255–263. [Google Scholar] [CrossRef]
- Huang, Y.X.; Wang, Z.; Hou, D.; Lin, S. Coaxially electrospun super-amphiphobic silica-based membrane for anti-surfactant-wetting membrane distillation. J. Membr. Sci. 2017, 531, 122–128. [Google Scholar] [CrossRef]
- Han, M.; Dong, T.; Hou, D.; Yao, J.; Han, L. Carbon nanotube based Janus composite membrane of oil fouling resistance for direct contact membrane distillation. J. Membr. Sci. 2020, 118078. [Google Scholar] [CrossRef]
- Yan, K.K.; Jiao, L.; Lin, S.; Ji, X.; Lu, Y.; Zhang, L. Superhydrophobic electrospun nanofiber membrane coated by carbon nanotubes network for membrane distillation. Desalination 2018, 437, 26–33. [Google Scholar] [CrossRef]
- Grasso, G.; Galiano, F.; Yoo, M.J.; Mancuso, R.; Park, H.B.; Gabriele, B.; Figoli, A.; Drioli, E. Development of graphene-PVDF composite membranes for membrane distillation. J. Membr. Sci. 2020, 604, 118017. [Google Scholar] [CrossRef]
- Jafari, A.; Kebria, M.R.S.; Rahimpour, A.; Bakeri, G. Graphene quantum dots modified polyvinylidenefluride (PVDF) nanofibrous membranes with enhanced performance for air Gap membrane distillation. Chem. Eng. Process. Process Intensif. 2018, 126, 222–231. [Google Scholar] [CrossRef]
- Zhou, R.; Rana, D.; Matsuura, T.; Lan, C.Q. Effects of multi-walled carbon nanotubes (MWCNTs) and integrated MWCNTs/SiO2 nano-additives on PVDF polymeric membranes for vacuum membrane distillation. Sep. Purif. Technol. 2019, 217, 154–163. [Google Scholar] [CrossRef]
- Damodar, R.A.; You, S.-J.; Chou, H.-H. Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes. J. Hazard. Mater. 2009, 172, 1321–1328. [Google Scholar] [CrossRef]
- Monteserín, C.; Blanco, M.; Murillo, N.; Pérez-Márquez, A.; Maudes, J.; Gayoso, J.; Manuel Laza, J.; Hernáez, E.; Aranzabe, E.; Vilas, J.L. Novel Antibacterial and Toughened Carbon-Fibre/Epoxy Composites by the Incorporation of TiO2 Nanoparticles Modified Electrospun Nanofibre Veils. Polymers 2019, 11, 1524. [Google Scholar]
- Dong, Z.-Q.; Ma, X.-H.; Xu, Z.-L.; Gu, Z.-Y. Superhydrophobic modification of PVDF–SiO2 electrospun nanofiber membranes for vacuum membrane distillation. RSC Adv. 2015, 5, 67962–67970. [Google Scholar] [CrossRef]
- Samaha, M.A.; Gad-el-Hak, M. Polymeric Slippery Coatings: Nature and Applications. Polymers 2014, 6, 1266–1311. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zheng, R.; Wang, J.; Liu, Y.; Wang, Y.; Li, X.-M.; He, T. Laminated PTFE membranes to enhance the performance in direct contact membrane distillation for high salinity solution. Desalination 2017, 424, 140–148. [Google Scholar] [CrossRef]
- Ray, S.S.; Gandhi, M.; Chen, S.-S.; Chang, H.-M.; Dan, C.T.N.; Le, H.Q. Anti-wetting behaviour of a superhydrophobic octadecyltrimethoxysilane blended PVDF/recycled carbon black composite membrane for enhanced desalination. Environ. Sci. Water Res. Technol. 2018, 4, 1612–1623. [Google Scholar] [CrossRef]
- Lee, J.K.Y.; Chen, N.; Peng, S.; Li, L.; Tian, L.; Thakor, N.; Ramakrishna, S. Polymer-based composites by electrospinning: Preparation functionalization with nanocarbons. Prog. Polym. Sci. 2018, 86, 40–84. [Google Scholar] [CrossRef]
- Liu, W.-W.; Chai, S.-P.; Mohamed, A.R.; Hashim, U. Synthesis and characterization of graphene and carbon nanotubes: A review on the past and recent developments. J. Ind. Eng. Chem. 2014, 20, 1171–1185. [Google Scholar] [CrossRef]
- Elmarghany, M.R.; El-Shazly, A.H.; Rajabzadeh, S.; Salem, M.S.; Shouman, M.A.; Sabry, M.N.; Matsuyama, H.; Nady, N. Triple-layer nanocomposite membrane prepared by electrospinning based on modified PES with carbon nanotubes for membrane distillation applications. Membranes 2020, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Rashid, M.; Ralph, S.F. Carbon nanotube membranes: Synthesis, properties, and future filtration applications. Nanomaterials 2017, 7, 99. [Google Scholar] [CrossRef] [Green Version]
- Gethard, K.; Mitra, S. Carbon nanotube enhanced membrane distillation for online preconcentration of trace pharmaceuticals in polar solvents. Analyst 2011, 136, 2643–2648. [Google Scholar] [CrossRef]
- Bhadra, M.; Roy, S.; Mitra, S. A bilayered structure comprised of functionalized carbon nanotubes for desalination by membrane distillation. ACS Appl. Mater. Interfaces 2016, 8, 19507–19513. [Google Scholar] [CrossRef]
- Silva, T.L.; Morales-Torres, S.; Figueiredo, J.L.; Silva, A.M. Multi-walled carbon nanotube/PVDF blended membranes with sponge-and finger-like pores for direct contact membrane distillation. Desalination 2015, 357, 233–245. [Google Scholar] [CrossRef]
- An, A.K.; Lee, E.-J.; Guo, J.; Jeong, S.; Lee, J.-G.; Ghaffour, N. Enhanced vapor transport in membrane distillation via functionalized carbon nanotubes anchored into electrospun nanofibres. Sci. Rep. 2017, 7, 41562. [Google Scholar]
- Dumée, L.F.; Sears, K.; Schütz, J.; Finn, N.; Huynh, C.; Hawkins, S.; Duke, M.; Gray, S. Characterization and evaluation of carbon nanotube Bucky-Paper membranes for direct contact membrane distillation. J. Membr. Sci. 2010, 351, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Alsebaeai, M.K.; Otitoju, T.A.; Makhtar, M.B.M.Z.; Ahmad, A.L. Membrane distillation: Progress in the improvement of dedicated membranes for enhanced hydrophobicity and desalination performance. J. Ind. Eng. Chem. 2020, 86, 13–34. [Google Scholar] [CrossRef]
- Seo, D.H.; Pineda, S.; Woo, Y.C.; Xie, M.; Murdock, A.T.; Ang, E.Y.; Jiao, Y.; Park, M.J.; Lim, S.I.; Lawn, M. Anti-fouling graphene-based membranes for effective water desalination. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Woo, Y.C.; Kim, Y.; Shim, W.-G.; Tijing, L.D.; Yao, M.; Nghiem, L.D.; Choi, J.-S.; Kim, S.-H.; Shon, H.K. Graphene/PVDF flat-sheet membrane for the treatment of RO brine from coal seam gas produced water by air gap membrane distillation. J. Membr. Sci. 2016, 513, 74–84. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Faki, B.; Luque-Alled, J.M.; Alberto, M.; Vijayaraghavan, A.; Holmes, S.M.; Szekely, G.; Badawy, M.I.; Shokri, N. Flux-enhanced PVDF mixed matrix membranes incorporating APTS-functionalized graphene oxide for membrane distillation. J. Membr. Sci. 2018, 554, 309–323. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F.; Li, F.; Xia, Y.; Li, Y. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Zahirifar, J.; Karimi-Sabet, J.; Moosavian, S.M.A.; Hadi, A.; Khadiv-Parsi, P. Fabrication of a novel octadecylamine functionalized graphene oxide/PVDF dual-layer flat sheet membrane for desalination via air gap membrane distillation. Desalination 2018, 428, 227–239. [Google Scholar] [CrossRef]
- Ma, J.; Ping, D.; Dong, X. Recent developments of graphene oxide-based membranes: A review. Membranes 2017, 7, 52. [Google Scholar]
- Zeng, Z.; Yu, D.; He, Z.; Liu, J.; Xiao, F.-X.; Zhang, Y.; Wang, R.; Bhattacharyya, D.; Tan, T.T.Y. Graphene oxide quantum dots covalently functionalized PVDF membrane with significantly-enhanced bactericidal and antibiofouling performances. Sci. Rep. 2016, 6, 20142. [Google Scholar] [CrossRef] [Green Version]
- Lei, S.; Zeng, M.; Huang, D.; Wang, L.; Zhang, L.; Xi, B.; Ma, W.; Chen, G.; Cheng, Z. Synergistic High-flux Oil–Saltwater Separation and Membrane Desalination with Carbon Quantum Dots Functionalized Membrane. ACS Sustain. Chem. Eng. 2019, 7, 13708–13716. [Google Scholar] [CrossRef]
- Zhao, G.; Hu, R.; Li, J.; Zhu, H. Graphene oxide quantum dots embedded polysulfone membranes with enhanced hydrophilicity, permeability and antifouling performance. Sci. China Mater. 2019, 62, 1177–1187. [Google Scholar] [CrossRef] [Green Version]
- Lecaros, R.L.G.; Deseo, K.M.; Hung, W.-S.; Tayo, L.L.; Hu, C.-C.; An, Q.-F.; Tsai, H.-A.; Lee, K.-R.; Lai, J.-Y. Influence of integrating graphene oxide quantum dots on the fine structure characterization and alcohol dehydration performance of pervaporation composite membrane. J. Membr. Sci. 2019, 576, 36–47. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Li, Q.; Zhang, G. Metal−organic framework composite membranes: Synthesis and separation applications. Chem. Eng. Sci. 2015, 135, 232–257. [Google Scholar] [CrossRef]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Lalia, B.S.; Guillen, E.; Arafat, H.A.; Hashaikeh, R. Nanocrystalline cellulose reinforced PVDF-HFP membranes for membrane distillation application. Desalination 2014, 332, 134–141. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wang, J.; Gascon, J.; Li, J.; Van der Bruggen, B. Metal–organic frameworks based membranes for liquid separation. Chem. Soc. Rev. 2017, 46, 7124–7144. [Google Scholar] [CrossRef]
- Zuo, J.; Chung, T.-S. Metal–organic framework-functionalized alumina membranes for vacuum membrane distillation. Water 2016, 8, 586. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.; Zhao, L.; Li, N.; Smith, S.J.; Wu, D.; Zhang, J.; Ng, D.; Wu, C.; Martinez, M.R.; Batten, M.P. Aluminum fumarate MOF/PVDF hollow fiber membrane for enhancement of water flux and thermal efficiency in direct contact membrane distillation. J. Membr. Sci. 2019, 588, 117204. [Google Scholar] [CrossRef]
- Yang, F.; Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C. Metal–organic frameworks supported on nanofiber for desalination by direct contact membrane distillation. ACS Appl. Mater. Interfaces 2018, 10, 11251–11260. [Google Scholar] [CrossRef]
- Su, P.; Zhang, X.; Li, Y.; Chen, H.; Meng, Q.; Zhang, G. Distillation of alcohol/water solution in hybrid metal–organic framework hollow fibers. AIChE J. 2019, 65, e16693. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Yang, F.; Cong, Y.; Lan, C.Q. Triple-layered nanofibrous metal-organic frameworks-based membranes for desalination by direct contact membrane distillation. ACS Sustain. Chem. Eng. 2020, 8, 6601–6610. [Google Scholar] [CrossRef]
- Hamzah, N.; Leo, C.; Ooi, B. Superhydrophobic PVDF/TiO2-SiO2 membrane with hierarchical roughness in membrane distillation for water recovery from phenolic rich solution containing surfactant. Chin. J. Polym. Sci. 2019, 37, 609–616. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, R.; Fane, A.G. Engineering superhydrophobic surface on poly (vinylidene fluoride) nanofiber membranes for direct contact membrane distillation. J. Membr. Sci. 2013, 440, 77–87. [Google Scholar] [CrossRef]
- Hu, C.; Yang, Z.; Sun, Q.; Ni, Z.; Yan, G.; Wang, Z. Facile Preparation of a Superhydrophobic iPP Microporous Membrane with Micron-Submicron Hierarchical Structures for Membrane Distillation. Polymers 2020, 12, 962. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Loh, C.-H.; Wang, R.; Fane, A.G. Electrospun superhydrophobic membranes with unique structures for membrane distillation. ACS Appl. Mater. Interfaces 2014, 6, 16035–16048. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; An, A.K.; Hadi, P.; Lee, S.; Woo, Y.C.; Shon, H.K. Advanced multi-nozzle electrospun functionalized titanium dioxide/polyvinylidene fluoride-co-hexafluoropropylene (TiO2/PVDF-HFP) composite membranes for direct contact membrane distillation. J. Membr. Sci. 2017, 524, 712–720. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Gutierrez, L.; Verliefde, A.R.; Mhlanga, S.D. Enhanced flux in direct contact membrane distillation using superhydrophobic PVDF nanofibre membranes embedded with organically modified SiO2 nanoparticles. J. Chem. Technol. Biotechnol. 2019, 94, 2826–2837. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Quan, X.; Zhao, H.; Chen, S.; Yi, G.; Du, L. High desalination permeability, wetting and fouling resistance on superhydrophobic carbon nanotube hollow fiber membrane under self-powered electrochemical assistance. J. Membr. Sci. 2016, 514, 501–509. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Shim, W.-G.; He, T.; Choi, J.-S.; Kim, S.-H.; Shon, H.K. Superhydrophobic nanofiber membrane containing carbon nanotubes for high-performance direct contact membrane distillation. J. Membr. Sci. 2016, 502, 158–170. [Google Scholar] [CrossRef]
- Guo, J.; Deka, B.J.; Kim, K.-J.; An, A.K. Regeneration of superhydrophobic TiO2 electrospun membranes in seawater desalination by water flushing in membrane distillation. Desalination 2019, 468, 114054. [Google Scholar] [CrossRef]
- Hou, D.; Lin, D.; Ding, C.; Wang, D.; Wang, J. Fabrication and characterization of electrospun superhydrophobic PVDF-HFP/SiNPs hybrid membrane for membrane distillation. Sep. Purif. Technol. 2017, 189, 82–89. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Liu, J.; Li, B.; Wang, S. Fabrication of hierarchical poly (vinylidene fluoride) micro/nano-composite membrane with anti-fouling property for membrane distillation. J. Membr. Sci. 2017, 535, 258–267. [Google Scholar] [CrossRef]
- Salehi, S.; Jahanshahi, M.; Peyravi, M. Poly (vinylidene difluoride) Membrane Assisted by Modified ZnO/ZIF Nanoparticles for Membrane Distillation. Chem. Eng. Technol. 2018, 41, 1994–2004. [Google Scholar] [CrossRef]
- Ragunath, S.; Roy, S.; Mitra, S. Carbon nanotube immobilized membrane with controlled nanotube incorporation via phase inversion polymerization for membrane distillation based desalination. Sep. Purif. Technol. 2018, 194, 249–255. [Google Scholar] [CrossRef]
- Intrchom, W.; Roy, S.; Humoud, M.S.; Mitra, S. Immobilization of graphene oxide on the permeate side of a membrane distillation membrane to enhance flux. Membranes 2018, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Purkait, M.K. Role of poly (2-acrylamido-2-methyl-1-propanesulfonic acid) in the modification of polysulfone membranes for ultrafiltration. J. Appl. Polym. Sci. 2017, 134, 45290. [Google Scholar] [CrossRef]
- Singh, R.; Yadav, V.; Purkait, M.K. Cu2O photocatalyst modified antifouling polysulfone mixed matrix membrane for ultrafiltration of protein and visible light driven photocatalytic pharmaceutical removal. Sep. Purif. Technol. 2019, 212, 191–204. [Google Scholar] [CrossRef]
- Singh, R.; Purkait, M.K. Evaluation of mPEG effect on the hydrophilicity and antifouling nature of the PVDF-co-HFP flat sheet polymeric membranes for humic acid removal. J. Water Process Eng. 2016, 14, 9–18. [Google Scholar] [CrossRef]









| Criteria | Description | Desired Value | Ref. |
|---|---|---|---|
| Liquid Entry Pressure (LEPw) | LEPw is the pressure required for the liquid to overcome the forces of hydrophobicity and penetrate the pores of the membrane. It is desired for the external pressure to be less than LEPw to allow the proper functioning of an MD system. It is expressed using LEPw = B is a geometric pore coefficient (equal to 1 for cylindrical pores), γ is liquid surface tension, θ is the contact angle, and rmax is the maximum pore radius. | LEPw > 250 kPa | [47] |
| Mean Pore Size and Pore Size distribution | Permeability depends on the mean pore size. Larger pore size allows a greater area for mass transfer, thereby increasing the overall membrane flux. However, increasing the pore size reduces the LEPw, hence it is necessary to find the optimum pore size to find a balance between LEPw and membrane permeability Pore size distribution (PSD) indicates the variation in pore size and hence the variation in mass transfer and heat transfer mechanism with it, throughout the surface. Overall, PSD has a minimal effect on MD performance. | Mean Pore Size = 100 nm–1 μm | [48] |
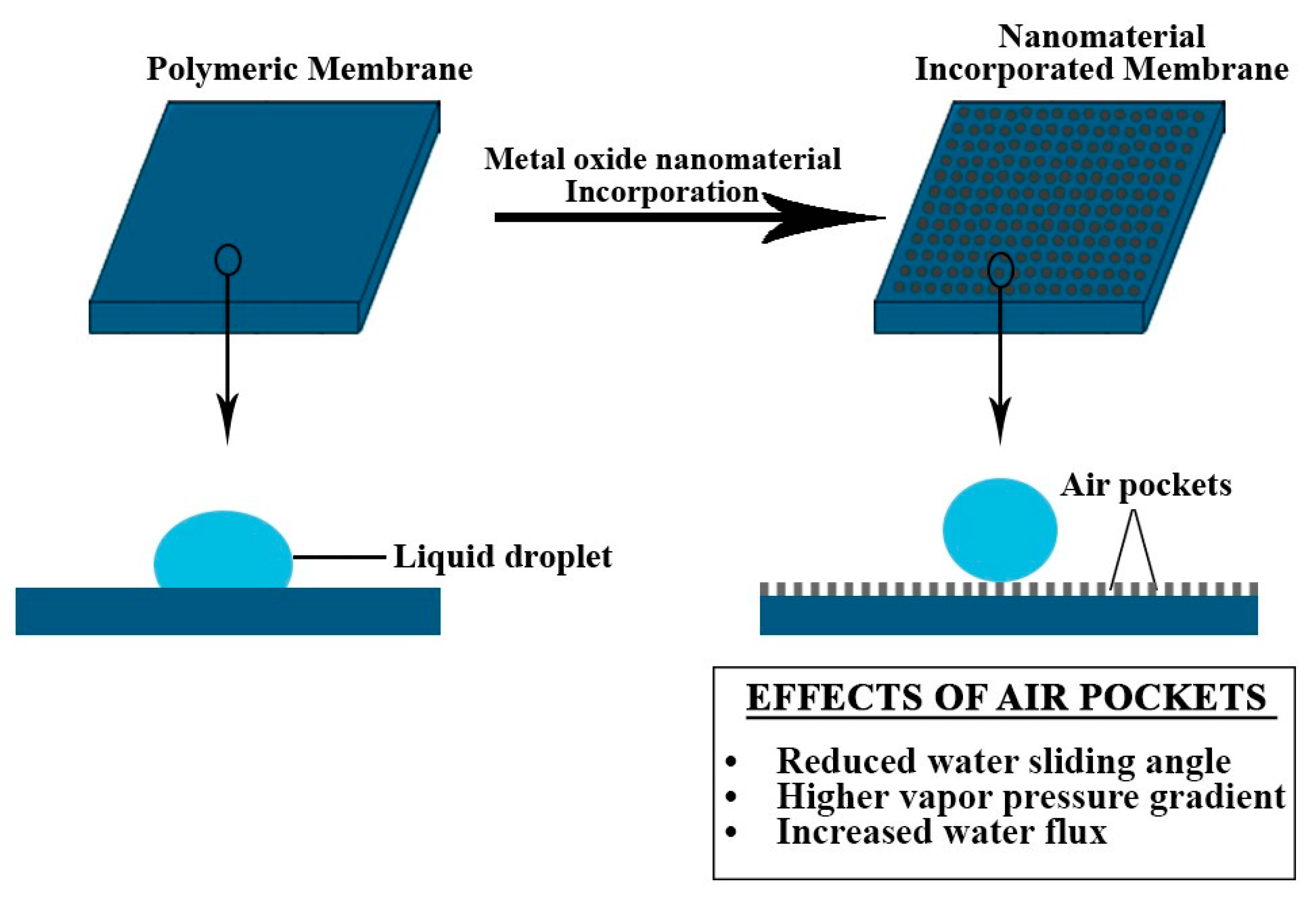
| Hydrophobicity | Hydrophobicity is a crucial aspect when the fabrication material for the membrane is chosen. It is quantified with respect to the contact angle (θCA) of water between the liquid surface and the membrane surface. | θCA > 90° (Hydrophobic) θCA > 150° (Superhydrophobic) | [49] |
| Chemical Resistance | The material used for membrane fabrication must show good resistance to chemicals (acids, bases, surfactants) to prevent membrane fouling and consequent wetting. | - | [50] |
| Thermal Conductivity | Membranes are desired to have a low thermal conductivity in the MD operation as it directly relates to the heat transfer through the membrane. Increased heat transfer would affect the vapor pressure equilibrium, thereby reducing the transmembrane flux. | 0.1–0.5 W m−1 K−1 is the range commonly observed in the literature | [47] |
| Membrane Thickness | Optimum membrane thickness is required as it has major effects on the thermal conductivity and the membrane flux. Even though reducing the membrane thickness increases the membrane flux, it severely reduces the thermal resistance. | 30–60 μm | [51] |
| Membrane Porosity | Membrane porosity refers to the fraction of voids present in the membrane to the total volume of the membrane. Increasing membrane porosity improves the flux transfer as well as the thermal resistance of the membrane; however, it is achieved at the expense of the mechanical strength of the membrane. | ɛ > 80% | [4] |
| Tortuosity | The irregularities of membrane pores from the ideal cylindrical pores are quantified by tortuosity. Highly tortuous structures result in lower flux as the vapor molecules suffer deviation from the direct path of transport. | 1.1–3.9 has been observed for most MD systems | [52] |
| Tensile Strength | The membrane material should possess adequate tensile strength to be assembled and fixed in membrane modules as the operational pressures are much less compared with RO, UF, and MF. | 3.4–54.9 MPa is commonly observed for most MD membranes. | [53] |
| Sliding Angle | Sliding angle is another criterion along with contact angle used to measure surface hydrophobicity. Lower sliding angle indicates higher hydrophobicity as the water droplets do not adhere to the membrane surface. | <10° | [54] |
| Surface Roughness | Microstructure roughness results in the formation of air pockets which results in improving membrane hydrophobicity. | Optimized surface roughness provides air layers which ultimately leads to higher hydrophobicity | [55] |
| Membrane | Nanomaterials | MD Type | Category | Pore Size (µm) | Flux (L m−2 h−1) | Contact Angle (°) | Ref. |
|---|---|---|---|---|---|---|---|
| PVDF-HFP/Si(NPs) | Silica | DCMD | Metalloid | 1.28 | 48.6 | >150° | [75] |
| PVDF- TiO2(NPs) | Titanium dioxide | DCMD | Metal oxide | 0.4 ± 0.05 | 2.5 | 140° | [76] |
| FTCS-TiO2-PVDF | Titanium dioxide | DCMD | Metal oxide | 0.45 | 30 | 163 ± 3° | [77] |
| S-PVDF-20 | Silver | UVMD | Metallic | 0.475 | 2.1 | 148 ± 2.1° | [78] |
| OMNI (ZnO-GF) | Zinc oxide | DCMD | Metal oxide | 0.4 | 11.4 ± 0.9 | 152.8 ± 1° | [79] |
| FAS-SiNPs-SFM | Silica | DCMD | Metalloid | 0.85 | 21.9 ± 1.2 | - | [80] |
| PVDF-SiO2(NPs) | Silicon dioxide | VMD | Metalloid | 0.14 | 2.8 | 94° | [42] |
| PVDF-Al2O3(NPs) | Aluminium oxide | AGMD | Metal oxide | 0.370 | 20 | 153° | [43] |
| PVDF-M-CNT | Carbon nanotubes | DCMD | Carbon | 0.14 | 35.1 ± 0.7 | - | [81] |
| PVDF-CNTs | Carbon nanotubes | VMD | Carbon | 0.20 | 28.5 | 159° | [82] |
| GNP-Polyethene | Graphene | DCMD | Carbon | 0.15 | 16.7 | 123° | [83] |
| GQDs-PVDF | Graphene quantum dots | AGMD | Quantum dots | 0.0049 | 17.6 | >125° | [84] |
| MWCNTs/ SiO2-PVDF | Multi-walled carbon nanotubes and silicon dioxide | VMD | Carbon | 0.09 | 2.5 | 91 ± 2.1° | [85] |
| Membrane | QD Type | Contact Angle (°) | Application | Ref. |
|---|---|---|---|---|
| (GQDs)/PVDF | Graphene quantum dots | >125° |
| [84] |
| C18-CQDs | Carbon quantum dots | 152.2 ± 1.25° |
| [109] |
| GOQDs-PVDF | Graphene oxide quantum dots | 34.3 ± 2.6° |
| [108] |
| GOQDs-PSF | Graphene oxide quantum dots | 65° |
| [110] |
| PVAx-GOQD300 | Graphene oxide quantum dots | 53.8 ± 0.1° |
| [111] |
| Membrane | MOFs Type | Membrane Type | MD Module | Contact Angle (°) | LEPw (KPa) | Ref. |
|---|---|---|---|---|---|---|
| MOF-functionalized alumina tub | NH2-MIL-53(Al) | Tubular | (VMD) Vacuum membrane distillation | - | 300 | [116] |
| ZIF-8/PDMS | ZIF-8 | Hollow fiber | (DCMD) Direct contact membrane distillation | 130° | - | [119] |
| (Iron-BTC)/PVDF | Iron-BTC | Flat sheet membrane | (DCMD) Direct contact membrane distillation | 138.06 ± 2.18° | 82.73 | [118] |
| AlFu MOF/PVDF | AlFu | Hollow fiber | (DCMD) Direct contact membrane distillation | >100° | - | [117] |
| MOFs/SiO2-PVDF | MOF-808 | Flat sheet membrane | (DCMD) Direct contact membrane distillation | 140.8° | 86.2 ± 3.2 | [120] |
| Base Polymer | Nanomaterial | Mode of Fabrication | Configuration | Water Contact Angle (°) | LEPw (kPa) | Mean Pore Diameter (µm) | Performance Characteristics | Ref. |
|---|---|---|---|---|---|---|---|---|
| PVDF | TiO2 | Phase inversion | DCMD | 112 ± 1.4 | 64 ± 3 | 0.44 ± 0.02 | Self-cleaning effects under UV light with a higher flux recovery ratio as compared with unmodified PVDF | [76] |
| PVDF-co-HPF | FTES-functionalized TiO2 | Electrospinning followed by electrospraying to coat TiO2 NPs | DCMD | 157 ± 1.6 | - | 0.52 | Mitigation of membrane fouling with regenerative abilities for long-term performance | [129] |
| PVDF-HPF | Si | Electrospinning | DCMD | > 150 | 76.4 | 1.70 | Total of 99.99% salt rejection over 240 h of desalination experiments showing long-term permeability | [130] |
| PVDF | PFOTS-modified SiO2 | Immersion deposition | DCMD | 161.5 | - | 0.2 ± 0.01 | Steady operation over 156 h with feed consisting of NaCl (100 g/L), CaCl2 (1.26 g/L), and humic Acid (10 mg/L) | [131] |
| PVDF | FAS-modified SiO2 (8% wt) | Electrospinning | VMD | 160.5 ± 2.3 | 195 | 0.26 ± 0.02 | Pore wetting prevented due to high LEPw value showing a permeate flux of around 30 l. m−2h−1 | [88] |
| PVDF | Aluminum fumarate MOF (1%) | Dry-jet wet phase inversion | DCMD | > 100 | >200 | 0.3 | Stable salt rejection of 99.9% for 3.5 wt% NaCl solution over 50 h of operation | [117] |
| PVDF | ZnO NPs modified by silane and coupled with ZIF-8 crystal | Phase inversion | DCMD | 70 | 100 | - | Modified membrane did not have a definite trend for permeate flux due to blocking of pores possibly due to wetting and scaling | [132] |
| PVDF | Triple-layered membrane with SiO2 (hydrophobic) blended in PVDF, PAN-MOFs, SiO2 (hydrophilic) blended in PVDF | Electrospinning | DCMD | 140.8 ± 9.9 | 86.2 | 0.31−1.22 | Hydrophobic SiO2-NPs increase the permeate flux while MOFs increase the pore size of the middle layer that contributed to superior DCMD performance for 5 h with low permeate conductivity | [120] |
| PVDF | CNTs | Electrospinning followed by spray gun to coat CNTs | VMD | 159.3 | 188 | 0.2 | Even though the membrane had a stable performance for 14 h of operation, increasing the CNT loading beyond a point did not improve pure water flux due to the increased thickness of the membrane | [133] |
| PTFE | GO | Dropwise coating of PVDF-GO onto flat sheet PTFE membranes | DCMD | 75 ± 2 | - | 0.2 | Hydrophilic properties of GO improve mass transfer coefficient, thus improving membrane flux with stable performance for 60 days of operation | [134] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinha Ray, S.; Singh Bakshi, H.; Dangayach, R.; Singh, R.; Deb, C.K.; Ganesapillai, M.; Chen, S.-S.; Purkait, M.K. Recent Developments in Nanomaterials-Modified Membranes for Improved Membrane Distillation Performance. Membranes 2020, 10, 140. https://doi.org/10.3390/membranes10070140
Sinha Ray S, Singh Bakshi H, Dangayach R, Singh R, Deb CK, Ganesapillai M, Chen S-S, Purkait MK. Recent Developments in Nanomaterials-Modified Membranes for Improved Membrane Distillation Performance. Membranes. 2020; 10(7):140. https://doi.org/10.3390/membranes10070140
Chicago/Turabian StyleSinha Ray, Saikat, Harshdeep Singh Bakshi, Raghav Dangayach, Randeep Singh, Chinmoy Kanti Deb, Mahesh Ganesapillai, Shiao-Shing Chen, and Mihir Kumar Purkait. 2020. "Recent Developments in Nanomaterials-Modified Membranes for Improved Membrane Distillation Performance" Membranes 10, no. 7: 140. https://doi.org/10.3390/membranes10070140
APA StyleSinha Ray, S., Singh Bakshi, H., Dangayach, R., Singh, R., Deb, C. K., Ganesapillai, M., Chen, S.-S., & Purkait, M. K. (2020). Recent Developments in Nanomaterials-Modified Membranes for Improved Membrane Distillation Performance. Membranes, 10(7), 140. https://doi.org/10.3390/membranes10070140