Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives
Abstract
:1. Introduction
2. Research Method, Rationale and Structure of the Review
3. Electrodialysis Process Fundamentals
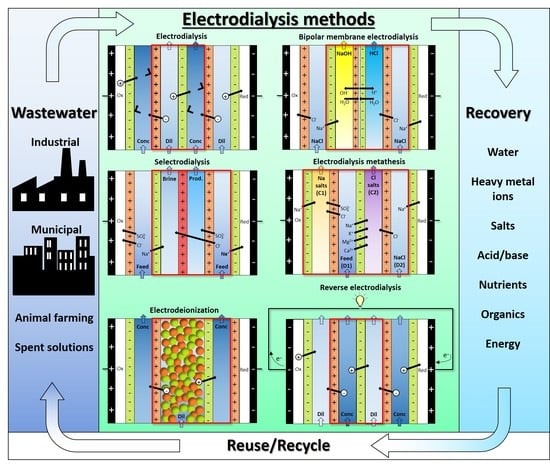
3.1. Working Principle and Design/Operating Features of ED Processes
3.2. Ion Exchange Membranes and Mass Transfer
3.3. Performance Parameters
4. Industrial Wastewater
4.1. Separation of Heavy Metal Ions
4.1.1. Nickel
4.1.2. Copper
4.1.3. Zinc
4.1.4. Chromium
4.1.5. Cadmium
4.1.6. Lead
4.1.7. Mixtures and Other Heavy Metal Ions
4.2. Regeneration of Acid/Base, Salt Conversion
4.2.1. Effluents with Heavy Metal Ions
4.2.2. Effluents without Heavy Metal Ions
4.2.3. Spent Solutions from Chemical Absorption of Flue Gases
4.2.4. Effluents with Organic Matter
4.3. Desalination
4.3.1. Oil and Gas Extraction
4.3.2. Refineries and Petrochemical Industries
4.3.3. Coal Mines
4.3.4. Power Plants
4.4. Treatment of Other Wastewaters
5. Municipal Wastewater and Other Effluents
5.1. Desalination of Municipal WWTP Effluents
5.2. Energy Recovery
5.3. Recovery of Nutrients and VFAs
5.3.1. Municipal WWTP Effluents
5.3.2. Excess Sludge Sidestreams
5.3.3. Human Urine
5.3.4. Animal Farming
5.4. Regeneration of Liquid Desiccant Solutions for Air Conditioning
6. Waste Brine from Desalination or Ion Exchange
6.1. Water and Salt Recovery
6.1.1. BWRO Brine
6.1.2. SWRO Brine
6.1.3. WWRO Brine
6.1.4. IX Spent Brine
6.2. Salt Conversion into Acid and Base
6.2.1. BWRO Brine and SWRO Brine
6.2.2. WWRO Brine and IX Spent Brine
6.3. Energy Recovery
7. Discussion, Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AEL | Anion exchange layer |
| AEM | Anion exchange membrane |
| BM | Bipolar membrane |
| BMED | Bipolar membrane electrodialysis |
| BMSED | Bipolar membrane selectrodialysis |
| BWRO | Brackish water reverse osmosis |
| CEDI | Continuous electrodeionisation |
| CEL | Cation exchange layer |
| CEM | Cation exchange membrane |
| COP | Coefficient of performance |
| ED | Electrodialysis |
| EDI | Electrodeionisation |
| EDL | Electrical double layer |
| EDM | Electrodialysis metathesis |
| EDR | Electrodialysis reversal |
| EDTA | Ethylenediaminetetraacetic acid |
| FO | Forward osmosis |
| HPAM | Partially hydrolysed polyacrylamide |
| IEM | Ion exchange membrane |
| IX | Ion-exchange |
| IXR | Ion-exchange resin |
| MCDI | Membrane capacitive deionisation |
| MD | Membrane distillation |
| MF | Microfiltration |
| MVA | Monovalent selective anion exchange membrane |
| MVC | Monovalent selective cation exchange membrane |
| MVM | Monovalent selective ion exchange membrane |
| NF | Nanofiltration |
| NOM | Natural organic matter |
| RED | Reverse electrodialysis |
| REDI | Reverse electrodeionisation |
| RO | Reverse osmosis |
| SED | Selectrodialysis |
| SWRO | Seawater reverse osmosis |
| TDS | Total Dissolved Solids |
| UF | Ultrafiltration |
| VFA | Volatile fatty acid |
| WWRO | Wastewater reverse osmosis |
| WWTP | Wastewater treatment plant |
| ZLD | Zero liquid discharge |
References
- Tong, T.; Elimelech, M. The Global Rise of Zero Liquid Discharge for Wastewater Management: Drivers, Technologies, and Future Directions. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef]
- Ahirrao, S. Zero Liquid Discharge Solutions. In Industrial Wastewater Treatment, Recycling and Reuse; Butterworth-Heinemann: Oxford, UK, 2014; pp. 489–520. ISBN 9780444634030. [Google Scholar]
- Yaqub, M.; Lee, W. Zero-liquid discharge (ZLD) technology for resource recovery from wastewater: A review. Sci. Total Environ. 2019, 681, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Voulvoulis, N. Water reuse from a circular economy perspective and potential risks from an unregulated approach. Curr. Opin. Environ. Sci. Health 2018, 2, 32–45. [Google Scholar] [CrossRef]
- European Comission. Report from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the Implementation of the Circular Economy Action Plan; European Comission: Brusselles, Belgium, 2019. [Google Scholar]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, H. Ion.-Exchange Membrane Separation Processes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 044450236X. [Google Scholar]
- Tanaka, Y. Ion. Exchange Membranes: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2007; Volume 12, ISBN 0927-5193. [Google Scholar]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K. Recent developments on ion-exchange membranes and electro-membrane processes. Adv. Colloid Interface Sci. 2006, 119, 97–130. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Xu, T.; Huang, C. Electrodialysis-Based separation technologies: A critical review. AiCHE J. 2008, 54, 3147–3159. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhou, M.; Yan, B.; Sun, X.; Liu, Y.; Wang, Y.; Xu, T.; Zhang, Y. Waste conversion and resource recovery from wastewater by ion exchange membranes: State-of-the-art and perspective. Ind. Eng. Chem. Res. 2018, 57, 6025–6039. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Sajjad, A.-A.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis Desalination for Water and Wastewater: A Review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar]
- Fidaleo, M.; Moresi, M. Electrodialysis Applications in The Food Industry. Adv. Food Nutr. Res. 2006, 51, 265–360. [Google Scholar]
- Huang, C.; Xu, T.; Zhang, Y.; Xue, Y.; Chen, G. Application of electrodialysis to the production of organic acids: State-of-the-art and recent developments. J. Membr. Sci. 2007, 288, 1–12. [Google Scholar] [CrossRef]
- Electrodialysis and Water Reuse: Novel Approaches; Moura Bernardes, A.; Zoppas Ferreira, J.; Siqueira Rodrigues, M.A. (Eds.) Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783642402494. [Google Scholar]
- López-Garzón, C.S.; Straathof, A.J.J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Gurreri, L.; Cipollina, A.; Tamburini, A.; Micale, G. Electrodialysis for wastewater treatment—Part I: Fundamentals and municipal effluents. In Current Trends and Future Developments on (Bio-) Membranes-Membrane Technology for Water and Wastewater Treatment-Advances and Emerging Processes; Basile, A., Comite, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 141–192. ISBN 9780128168233. [Google Scholar]
- Gurreri, L.; Cipollina, A.; Tamburini, A.; Micale, G. Electrodialysis for wastewater treatment—Part II: Industrial effluents. In Current Trends and Future Developments on (Bio-) Membranes-Membrane Technology for Water and Wastewater Treatment-Advances and Emerging Processes; Basile, A., Comite, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–241. ISBN 9780128168233. [Google Scholar]
- Gurreri, L.; Battaglia, G.; Tamburini, A.; Cipollina, A.; Micale, G.; Ciofalo, M. Multi-physical modelling of reverse electrodialysis. Desalination 2017, 423, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, G.; Gurreri, L.; Airò Farulla, G.; Cipollina, A.; Pirrotta, A.; Micale, G.; Ciofalo, M. Membrane Deformation and Its Effects on Flow and Mass Transfer in the Electromembrane Processes. Int. J. Mol. Sci. 2019, 20, 1840. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, G.; Gurreri, L.; Airò Farulla, G.; Cipollina, A.; Pirrotta, A.; Micale, G.; Ciofalo, M. Pressure-Induced Deformation of Pillar-Type Profiled Membranes and Its Effects on Flow and Mass Transfer. Computation 2019, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, G.; Gurreri, L.; Cipollina, A.; Pirrotta, A.; Velizarov, S.; Ciofalo, M.; Micale, G. Fluid–Structure Interaction and Flow Redistribution in Membrane-Bounded Channels. Energies 2019, 12, 4259. [Google Scholar] [CrossRef] [Green Version]
- Pawlowski, S.; Crespo, J.; Velizarov, S. Profiled Ion Exchange Membranes: A Comprehensible Review. Int. J. Mol. Sci. 2019, 20, 165. [Google Scholar] [CrossRef] [Green Version]
- Lindstrand, V.; Sundström, G.; Jönsson, A.S. Fouling of electrodialysis membranes by organic substances. Desalination 2000, 128, 91–102. [Google Scholar] [CrossRef]
- Strathmann, H.; Krol, J.J.; Rapp, H.J.; Eigenberger, G. Limiting current density and water dissociation in bipolar membranes. J. Membr. Sci. 1997, 125, 123–142. [Google Scholar] [CrossRef] [Green Version]
- Mareev, S.A.; Evdochenko, E.; Wessling, M.; Kozaderova, O.A.; Niftaliev, S.I.; Pismenskaya, N.D.; Nikonenko, V.V. A comprehensive mathematical model of water splitting in bipolar membranes: Impact of the spatial distribution of fixed charges and catalyst at bipolar junction. J. Membr. Sci. 2020, 603, 118010. [Google Scholar] [CrossRef]
- Pan, J.; Hou, L.; Wang, Q.; He, Y.; Wu, L.; Mondal, A.N.; Xu, T. Preparation of bipolar membranes by electrospinning. Mater. Chem. Phys. 2017, 186, 484–491. [Google Scholar] [CrossRef]
- Shen, C.; Wycisk, R.; Pintauro, P.N. High performance electrospun bipolar membrane with a 3D junction. Energy Environ. Sci. 2017, 10, 1435–1442. [Google Scholar] [CrossRef]
- Pourcelly, G. Electrodialysis with bipolar membranes: Principles, optimization, and applications. Russ. J. Electrochem. 2002, 38, 919–926. [Google Scholar] [CrossRef]
- Jaroszek, H.; Dydo, P. Ion-exchange membranes in chemical synthesis-a review. Open Chem. 2016, 14, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Xu, T. Electrodialysis with bipolar membranes for sustainable development. Environ. Sci. Technol. 2006, 40, 5233–5243. [Google Scholar] [CrossRef]
- Mani, K.N.; Chlanda, F.P.; Byszewski, C.H. Aquatech membrane technology for recovery of acid/base values for salt streams. Desalination 1988, 68, 149–166. [Google Scholar] [CrossRef]
- Mani, K.N. Electrodialysis water splitting technology. J. Membr. Sci. 1991, 58, 117–138. [Google Scholar] [CrossRef]
- Zhang, Y.; Paepen, S.; Pinoy, L.; Meesschaert, B.; Van Der Bruggen, B. Selectrodialysis: Fractionation of divalent ions from monovalent ions in a novel electrodialysis stack. Sep. Purif. Technol. 2012, 88, 191–201. [Google Scholar] [CrossRef]
- Alhéritière, C.; Ernst, W.R.; Davis, T.A. Metathesis of magnesium and sodium salt systems by electrodialysis. Desalination 1998, 115, 189–198. [Google Scholar] [CrossRef]
- Chen, Q.-B.; Ren, H.; Tian, Z.; Sun, L.; Wang, J. Conversion and pre-concentration of SWRO reject brine into high solubility liquid salts (HSLS) by using electrodialysis metathesis. Sep. Purif. Technol. 2019, 213, 587–598. [Google Scholar] [CrossRef]
- Alvarado, L.; Chen, A. Electrodeionization: Principles, strategies and applications. Electrochim. Acta 2014, 132, 583–597. [Google Scholar] [CrossRef]
- Hakim, A.N.; Khoiruddin, K.; Ariono, D.; Wenten, I.G. Ionic Separation in Electrodeionization System: Mass Transfer Mechanism and Factor Affecting Separation Performance. Sep. Purif. Rev. 2019, 1–23. [Google Scholar] [CrossRef]
- Wood, J.; Gifford, J.; Arba, J.; Shaw, M. Production of ultrapure water by continuous electrodeionization. Desalination 2010, 250, 973–976. [Google Scholar] [CrossRef]
- Dzyazko, Y.S.; Belyakov, V.N. Purification of a diluted nickel solution containing nickel by a process combining ion exchange and electrodialysis. Desalination 2004, 162, 179–189. [Google Scholar] [CrossRef]
- Feng, X.; Wu, Z.; Chen, X. Removal of metal ions from electroplating effluent by EDI process and recycle of purified water. Sep. Purif. Technol. 2007, 57, 257–263. [Google Scholar] [CrossRef]
- Mahmoud, A.; Hoadley, A.F.A. An evaluation of a hybrid ion exchange electrodialysis process in the recovery of heavy metals from simulated dilute industrial wastewater. Water Res. 2012, 46, 3364–3376. [Google Scholar] [CrossRef]
- Souilah, O.; Akretche, D.E.; Amara, M. Water reuse of an industrial effluent by means of electrodeionisation. Desalination 2004, 167, 49–54. [Google Scholar] [CrossRef]
- Spoor, P.B.; Koene, L.; ter Veen, W.R.; Janssen, L.J.J. Continuous deionization of a dilute nickel solution. Chem. Eng. J. 2002, 85, 127–135. [Google Scholar] [CrossRef]
- Spoor, P.B.; Grabovska, L.; Koene, L.; Janssen, L.J.J.; Ter Veen, W.R. Pilot scale deionisation of a galvanic nickel solution using a hybrid ion-exchange/electrodialysis system. Chem. Eng. J. 2002, 89, 193–202. [Google Scholar] [CrossRef]
- Park, S.; Kwak, R. Microscale electrodeionization: In situ concentration profiling and flow visualization. Water Res. 2020, 170, 115310. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Snyder, S.W.; Ma, H.W.; Lin, Y.J.; Chiang, P.C. Energy-efficient resin wafer electrodeionization for impaired water reclamation. J. Clean. Prod. 2018, 174, 1464–1474. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Tufa, R.A.; Pawlowski, S.; Veerman, J.; Bouzek, K.; Fontananova, E.; di Profio, G.; Velizarov, S.; Goulão Crespo, J.; Nijmeijer, K.; Curcio, E. Progress and prospects in reverse electrodialysis for salinity gradient energy conversion and storage. Appl. Energy 2018, 225, 290–331. [Google Scholar] [CrossRef]
- Cipollina, A.; Micale, G.; Tamburini, A.; Tedesco, M.; Gurreri, L.; Veerman, J.; Grasman, S. Reverse electrodialysis: Applications. In Sustainable Energy from Salinity Gradients; Cipollina, A., Micale, G., Eds.; Woodhead Publishing: Cambridge, UK; Elsevier: Amsterdam, The Netherlands, 2016; pp. 135–180. ISBN 9780081003237. [Google Scholar]
- Tamburini, A.; Cipollina, A.; Tedesco, M.; Gurreri, L.; Ciofalo, M.; Micale, G. The REAPower Project: Power Production From Saline Waters and Concentrated Brines. In Current Trends and Future Developments on (Bio-) Membranes-Membrane Desalination Systems: The Next Generation; Basile, A., Curcio, E., Inamuddin, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 407–448. [Google Scholar]
- Tian, H.; Wang, Y.; Pei, Y.; Crittenden, J.C. Unique applications and improvements of reverse electrodialysis: A review and outlook. Appl. Energy 2020, 262, 114482. [Google Scholar] [CrossRef]
- Avci, A.H.; Tufa, R.A.; Fontananova, E.; Di Profio, G.; Curcio, E. Reverse Electrodialysis for energy production from natural river water and seawater. Energy 2018, 165, 512–521. [Google Scholar] [CrossRef]
- Sata, T. Ion. Exchange Membranes: Preparation, Characterization, Modification and Application; Royal Society of Chemistry: Cambridge, UK, 2004; ISBN 978-0-85404-590-7. [Google Scholar]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Kontturi, K.; Murtomäki, L.; Manzanares, J.A. Ionic Transport. Processes in Electrochemistry and Membrane Science; Oxford University Press Inc.: New York, NY, USA, 2008; ISBN 978-0-19–953381–7. [Google Scholar]
- Manzanares, J.A.; Vergara, G.; Mafé, S.; Kontturi, K.; Viinikka, P. Potentiometric Determination of Transport Numbers of Ternary Electrolyte Systems in Charged Membranes. J. Phys. Chem. B 1998, 102, 1301–1307. [Google Scholar] [CrossRef]
- Kraaijeveld, G.; Sumberova, V.; Kuindersma, S.; Wesselingh, H. Modelling electrodialysis using the Maxwell-Stefan description. Chem. Eng. J. Biochem. Eng. J. 1995, 57, 163–176. [Google Scholar] [CrossRef]
- Plntauro, P.N.; Bennion, D.N. Mass Transport of Electrolytes in Membranes. 1. Development of Mathematical Transport Model. Ind. Eng. Chem. Fundam. 1984, 23, 230–234. [Google Scholar] [CrossRef]
- Wesselingh, J.A.; Vonk, P.; Kraaijeveld, G. Exploring the Maxwell-Stefan description of ion exchange. Chem. Eng. J. Biochem. Eng. J. 1995, 57, 75–89. [Google Scholar] [CrossRef]
- Sata, T. Studies on ion exchange membranes with permselectivity for specific ions in electrodialysis. J. Membr. Sci. 1994, 93, 117–135. [Google Scholar] [CrossRef]
- Balster, J.; Yildirim, M.H.; Stamatialis, D.F.; Ibanez, R.; Lammertink, R.G.H.; Jordan, V.; Wessling, M. Morphology and microtopology of cation-exchange polymers and the origin of the overlimiting current. J. Phys. Chem. B 2007, 111, 2152–2165. [Google Scholar] [CrossRef]
- Długołecki, P.; Anet, B.; Metz, S.J.; Nijmeijer, K.; Wessling, M. Transport limitations in ion exchange membranes at low salt concentrations. J. Membr. Sci. 2010, 346, 163–171. [Google Scholar] [CrossRef]
- Galama, A.H.; Vermaas, D.A.; Veerman, J.; Saakes, M.; Rijnaarts, H.H.M.; Post, J.W.; Nijmeijer, K. Membrane resistance: The effect of salinity gradients over a cation exchange membrane. J. Membr. Sci. 2014, 467, 279–291. [Google Scholar] [CrossRef]
- Gohil, G.S.; Shahi, V.K.; Rangarajan, R. Comparative studies on electrochemical characterization of homogeneous and heterogeneous type of ion-exchange membranes. J. Membr. Sci. 2004, 240, 211–219. [Google Scholar] [CrossRef]
- Mehdizadeh, S.; Yasukawa, M.; Abo, T.; Kakihana, Y.; Higa, M. Effect of spacer geometry on membrane and solution compartment resistances in reverse electrodialysis. J. Membr. Sci. 2019, 572, 271–280. [Google Scholar] [CrossRef]
- Galama, A.H.; Hoog, N.A.; Yntema, D.R. Method for determining ion exchange membrane resistance for electrodialysis systems. Desalination 2016, 380, 1–11. [Google Scholar] [CrossRef]
- Silva, R.F.; De Francesco, M.; Pozio, A. Tangential and normal conductivities of Nafion® membranes used in polymer electrolyte fuel cells. J. Power Sources 2004, 134, 18–26. [Google Scholar] [CrossRef]
- Kamcev, J.; Sujanani, R.; Jang, E.S.; Yan, N.; Moe, N.; Paul, D.R.; Freeman, B.D. Salt concentration dependence of ionic conductivity in ion exchange membranes. J. Membr. Sci. 2018, 547, 123–133. [Google Scholar] [CrossRef]
- Zhu, S.; Kingsbury, R.S.; Call, D.F.; Coronell, O. Impact of solution composition on the resistance of ion exchange membranes. J. Membr. Sci. 2018, 554, 39–47. [Google Scholar] [CrossRef]
- Larchet, C.; Nouri, S.; Auclair, B.; Dammak, L.; Nikonenko, V. Application of chronopotentiometry to determine the thickness of diffusion layer adjacent to an ion-exchange membrane under natural convection. Adv. Colloid Interface Sci. 2008, 139, 45–61. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Nikonenko, V.V. Effect of structural membrane inhomogeneity on transport properties. J. Membr. Sci. 1993, 79, 181–198. [Google Scholar] [CrossRef]
- Veerman, J. The Effect of the NaCl Bulk Concentration on the Resistance of Ion Exchange Membranes—Measuring and Modeling. Energies 2020, 13, 1946. [Google Scholar] [CrossRef] [Green Version]
- Porada, S.; van Egmond, W.J.; Post, J.W.; Saakes, M.; Hamelers, H.V.M. Tailoring ion exchange membranes to enable low osmotic water transport and energy efficient electrodialysis. J. Membr. Sci. 2018, 552, 22–30. [Google Scholar] [CrossRef]
- Kamcev, J.; Doherty, C.M.; Lopez, K.P.; Hill, A.J.; Paul, D.R.; Freeman, B.D. Effect of fixed charge group concentration on salt permeability and diffusion coefficients in ion exchange membranes. J. Membr. Sci. 2018, 566, 307–316. [Google Scholar] [CrossRef]
- Kamcev, J.; Paul, D.R.; Manning, G.S.; Freeman, B.D. Predicting salt permeability coefficients in highly swollen, highly charged ion exchange membranes. ACS Appl. Mater. Interfaces 2017, 9, 4044–4056. [Google Scholar] [CrossRef]
- Kamcev, J.; Paul, D.R.; Manning, G.S.; Freeman, B.D. Ion Diffusion Coefficients in Ion Exchange Membranes: Significance of Counterion Condensation. Macromolecules 2018, 51, 5519–5529. [Google Scholar] [CrossRef]
- Ciofalo, M.; Di Liberto, M.; Gurreri, L.; La Cerva, M.; Scelsi, L.; Micale, G. Mass transfer in ducts with transpiring walls. Int. J. Heat Mass Transf. 2019, 132, 1074–1086. [Google Scholar] [CrossRef]
- Spiegler, K.S. Polarization at ion exchange membrane-solution interfaces. Desalination 1971, 9, 367–385. [Google Scholar] [CrossRef]
- Helfferich, F. Ion. Exchange; McGraw-Hill: New York, NY, USA, 1962. [Google Scholar]
- Levich, V.G. Physicochemical Hydrodynamics; Prentice-Hall: Englewood Cliffs, NJ, USA, 1962. [Google Scholar]
- Krol, J.J.; Wessling, M.; Strathmann, H. Concentration polarization with monopolar ion exchange membranes: Current-voltage curves and water dissociation. J. Membr. Sci. 1999, 162, 145–154. [Google Scholar] [CrossRef]
- Kwak, R.; Guan, G.; Peng, W.K.; Han, J. Microscale electrodialysis: Concentration profiling and vortex visualization. Desalination 2013, 308, 138–146. [Google Scholar] [CrossRef]
- Rubinstein, I.; Shtilman, L. Voltage against current curves of cation exchange membranes. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1979, 75, 231–246. [Google Scholar] [CrossRef]
- Lee, H.J.; Strathmann, H.; Moon, S.H. Determination of the limiting current density in electrodialysis desalination as an empirical function of linear velocity. Desalination 2006, 190, 43–50. [Google Scholar] [CrossRef]
- Urtenov, M.K.; Uzdenova, A.M.; Kovalenko, A.V.; Nikonenko, V.V.; Pismenskaya, N.D.; Vasil’eva, V.I.; Sistat, P.; Pourcelly, G. Basic mathematical model of overlimiting transfer enhanced by electroconvection in flow-through electrodialysis membrane cells. J. Membr. Sci. 2013, 447, 190–202. [Google Scholar] [CrossRef]
- La Cerva, M.; Gurreri, L.; Tedesco, M.; Cipollina, A.; Ciofalo, M.; Tamburini, A.; Micale, G. Determination of limiting current density and current efficiency in electrodialysis units. Desalination 2018, 445, 138–148. [Google Scholar] [CrossRef]
- Cowan, D.A.; Brown, J.H. Effect of Turbulence on Limiting Current in Electrodialysis Cells. Ind. Eng. Chem. 1959, 51, 1445–1448. [Google Scholar] [CrossRef]
- Ben Sik Ali, M.; Mnif, A.; Hamrouni, B. Modelling of the limiting current density of an electrodialysis process by response surface methodology. Ionics (Kiel) 2018, 24, 617–628. [Google Scholar] [CrossRef]
- Forgacs, C.; Ishibashi, N.; Leibovitz, J.; Sinkovic, J.; Spiegler, K.S. Polarization at ion-exchange membranes in electrodialysis. Desalination 1972, 10, 181–214. [Google Scholar] [CrossRef]
- Kharkats, Y.I. Mechanism of “supralimiting” currents at ion-exchange mebrane/electrolyte interfaces. Sov. Electrochem. 1985, 21, 917–920. [Google Scholar]
- Simons, R. The origin and elimination of water splitting in ion exchange membranes during water demineralisation by electrodialysis. Desalination 1979, 28, 41–42. [Google Scholar] [CrossRef]
- Simons, R. Water splitting in ion exchange membranes. Electrochim. Acta 1985, 30, 275–282. [Google Scholar] [CrossRef]
- Nikonenko, V.; Urtenov, M.; Mareev, S. Pourcelly Mathematical Modeling of the Effect of Water Splitting on Ion Transfer in the Depleted Diffusion Layer Near an Ion-Exchange Membrane. Membranes 2020, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Uzdenova, A. 2D Mathematical Modelling of Overlimiting Transfer Enhanced by Electroconvection in Flow-Through Electrodialysis Membrane Cells in Galvanodynamic Mode. Membranes 2019, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Uzdenova, A.; Urtenov, M. Potentiodynamic and Galvanodynamic Regimes of Mass Transfer in Flow-Through Electrodialysis Membrane Systems: Numerical Simulation of Electroconvection and Current-Voltage Curve. Membranes 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Nikonenko, V.V.; Pismenskaya, N.D.; Belova, E.I.; Sistat, P.; Huguet, P.; Pourcelly, G.; Larchet, C. Intensive current transfer in membrane systems: Modelling, mechanisms and application in electrodialysis. Adv. Colloid Interface Sci. 2010, 160, 101–123. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Kovalenko, A.V.; Urtenov, M.K.; Pismenskaya, N.D.; Han, J.; Sistat, P.; Pourcelly, G. Desalination at overlimiting currents: State-of-the-art and perspectives. Desalination 2014, 342, 85–106. [Google Scholar] [CrossRef]
- Mareev, S.A.; Nebavskiy, A.V.; Nichka, V.S.; Urtenov, M.K.; Nikonenko, V.V. The nature of two transition times on chronopotentiograms of heterogeneous ion exchange membranes: 2D modelling. J. Membr. Sci. 2019, 575, 179–190. [Google Scholar] [CrossRef]
- Nikonenko, V.; Nebavsky, A.; Mareev, S.; Kovalenko, A.; Urtenov, M.; Pourcelly, G. Modelling of Ion Transport in Electromembrane Systems: Impacts of Membrane Bulk and Surface Heterogeneity. Appl. Sci. 2018, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Andersen, M.B.; Wang, K.M.; Schiffbauer, J.; Mani, A. Confinement effects on electroconvective instability. Electrophoresis 2017, 38, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Pham, S.V.; Kwon, H.; Kim, B.; White, J.K.; Lim, G.; Han, J. Helical vortex formation in three-dimensional electrochemical systems with ion-selective membranes. Phys. Rev. E 2016, 93, 033114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karatay, E.; Druzgalski, C.L.; Mani, A. Simulation of chaotic electrokinetic transport: Performance of commercial software versus custom-built direct numerical simulation codes. J. Colloid Interface Sci. 2015, 446, 67–76. [Google Scholar] [CrossRef]
- Zaltzman, B.; Rubinstein, I. Electro-osmotic slip and electroconvective instability. J. Fluid Mech. 2007, 579, 173–226. [Google Scholar] [CrossRef]
- Krol, J.J.; Wessling, M.; Strathmann, H. Chronopotentiometry and overlimiting ion transport through monopolar ion exchange membranes. J. Membr. Sci. 1999, 162, 155–164. [Google Scholar] [CrossRef]
- Rubinstein, I. Electroconvection at an electrically inhomogeneous permselective interface. Phys. Fluids A 1991, 3, 2301–2309. [Google Scholar] [CrossRef]
- Maletzki, F.; Rösler, H.W.; Staude, E. Ion transfer across electrodialysis membranes in the overlimiting current range: Stationary voltage current characteristics and current noise power spectra under different conditions of free convection. J. Membr. Sci. 1992, 71, 105–116. [Google Scholar] [CrossRef]
- Rubinstein, I.; Staude, E.; Kedem, O. Role of the membrane surface in concentration polarization at ion-exchange membrane. Desalination 1988, 69, 101–114. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Nikonenko, V.V.; Pismenskaya, N.D.; Laktionov, E.V.; Urtenov, M.K.; Strathmann, H.; Wessling, M.; Koops, G.H. Coupled transport phenomena in overlimiting current electrodialysis. Sep. Purif. Technol. 1998, 14, 255–267. [Google Scholar] [CrossRef]
- Dukhin, S.S. Electrokinetic phenomena of the second kind and their applications. Adv. Colloid Interface Sci. 1991, 35, 173–196. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B.; Kedem, O. Electric fields in and around ion-exchange membranes. J. Membr. Sci. 1997, 125, 17–21. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, H.-J.; Moon, S.-H. Effects of Electrolytes on the Transport Phenomena in a Cation-Exchange Membrane. J. Colloid Interface Sci. 2001, 238, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, R.; Stamatialis, D.F.; Wessling, M. Role of membrane surface in concentration polarization at cation exchange membranes. J. Membr. Sci. 2004, 239, 119–128. [Google Scholar] [CrossRef]
- Pismenskaia, N.; Sistat, P.; Huguet, P.; Nikonenko, V.; Pourcelly, G. Chronopotentiometry applied to the study of ion transfer through anion exchange membranes. J. Membr. Sci. 2004, 228, 65–76. [Google Scholar] [CrossRef]
- Volodina, E.; Pismenskaya, N.; Nikonenko, V.; Larchet, C.; Pourcelly, G. Ion transfer across ion-exchange membranes with homogeneous and heterogeneous surfaces. J. Colloid Interface Sci. 2005, 285, 247–258. [Google Scholar] [CrossRef]
- Gil, V.V.; Andreeva, M.A.; Jansezian, L.; Han, J.; Pismenskaya, N.D.; Nikonenko, V.V.; Larchet, C.; Dammak, L. Impact of heterogeneous cation-exchange membrane surface modification on chronopotentiometric and current–voltage characteristics in NaCl, CaCl2and MgCl2solutions. Electrochim. Acta 2018, 281, 472–485. [Google Scholar] [CrossRef]
- Nebavskaya, K.A.; Sarapulova, V.V.; Sabbatovskiy, K.G.; Sobolev, V.D.; Pismenskaya, N.D.; Sistat, P.; Cretin, M.; Nikonenko, V.V. Impact of ion exchange membrane surface charge and hydrophobicity on electroconvection at underlimiting and overlimiting currents. J. Membr. Sci. 2017, 523, 36–44. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Mareev, S.A.; Pis’menskaya, N.D.; Uzdenova, A.M.; Kovalenko, A.V.; Urtenov, M.K.; Pourcelly, G. Effect of electroconvection and its use in intensifying the mass transfer in electrodialysis (Review). Russ. J. Electrochem. 2017, 53, 1122–1144. [Google Scholar] [CrossRef]
- Butylskii, D.; Moroz, I.; Tsygurina, K.; Mareev, S. Effect of Surface Inhomogeneity of Ion-Exchange Membranes on the Mass Transfer Efficiency in Pulsed Electric Field Modes. Membranes 2020, 10, 40. [Google Scholar] [CrossRef] [Green Version]
- Titorova, V.; Sabbatovskiy, K.; Sarapulova, V.; Kirichenko, E.; Sobolev, V.; Kirichenko, K. Characterization of MK-40 membrane modified by layers of cation exchange and anion exchange polyelectrolytes. Membranes 2020, 10, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabolotsky, V.I.; Nikonenko, V.V.; Pismenskaya, N.D. On the role of gravitational convection in the transfer enhancement of salt ions in the course of dilute solution electrodialysis. J. Membr. Sci. 1996, 119, 171–181. [Google Scholar] [CrossRef]
- Larchet, C.; Zabolotsky, V.I.; Pismenskaya, N.; Nikonenko, V.V.; Tskhay, A.; Tastanov, K.; Pourcelly, G. Comparison of different ED stack conceptions when applied for drinking water production from brackish waters. Desalination 2008, 222, 489–496. [Google Scholar] [CrossRef]
- Isaacson, M.S.; Sonin, A.A. Sherwood Number and Friction Factor Correlations for Electrodialysis Systems, with Application to Process Optimization. Ind. Eng. Chem. Process. Des. Dev. 1976, 15, 313–321. [Google Scholar] [CrossRef]
- Sonin, A.A.; Probstein, R.F. A hydrodynamic theory of desalination by electrodialysis. Desalination 1968, 5, 293–329. [Google Scholar] [CrossRef]
- Malek, P.; Ortiz, J.M.; Richards, B.S.; Schäfer, A.I. Electrodialytic removal of NaCl from water: Impacts of using pulsed electric potential on ion transport and water dissociation phenomena. J. Membr. Sci. 2013, 435, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Tadimeti, J.G.D.; Chattopadhyay, S. Uninterrupted swirling motion facilitating ion transport in electrodialysis. Desalination 2016, 392, 54–62. [Google Scholar] [CrossRef]
- Geraldes, V.; Afonso, M.D. Limiting current density in the electrodialysis of multi-ionic solutions. J. Membr. Sci. 2010, 360, 499–508. [Google Scholar] [CrossRef]
- Sonin, A.A.; Isaacson, M.S. Optimization of Flow Design in Forced Flow Electrochemical Systems, with Special Application to Electrodialysis. Ind. Eng. Chem. Process. Des. Dev. 1974, 13, 241–248. [Google Scholar] [CrossRef]
- Fidaleo, M.; Moresi, M. Optimal strategy to model the electrodialytic recovery of a strong electrolyte. J. Membr. Sci. 2005, 260, 90–111. [Google Scholar] [CrossRef]
- Belfort, G.; Guter, G.A. An experimental study of electrodialysis hydrodynamics. Desalination 1972, 10, 221–262. [Google Scholar] [CrossRef]
- Lee, H.J.; Sarfert, F.; Strathmann, H.; Moon, S.H. Designing of an electrodialysis desalination plant. Desalination 2002, 142, 267–286. [Google Scholar] [CrossRef]
- Tanaka, Y. Limiting current density of an ion-exchange membrane and of an electrodialyzer. J. Membr. Sci. 2005, 266, 6–17. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G.; Ciofalo, M. Flow and mass transfer in spacer-filled channels for reverse electrodialysis: A CFD parametrical study. J. Membr. Sci. 2016, 497, 300–317. [Google Scholar] [CrossRef] [Green Version]
- La Cerva, M.; Di Liberto, M.; Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G.; Ciofalo, M. Coupling CFD with a one-dimensional model to predict the performance of reverse electrodialysis stacks. J. Membr. Sci. 2017, 541, 595–610. [Google Scholar] [CrossRef] [Green Version]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G.; Ciofalo, M. Pressure drop at low Reynolds numbers in woven-spacer-filled channels for membrane processes: CFD prediction and experimental validation. Desalin. Water Treat. 2017, 61, 170–182. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, O.; Takahashi, S.; Nomura, M. Characteristics of flow and mass transfer rate in an electrodialyzer compartment including spacer. Desalination 1983, 46, 225–232. [Google Scholar] [CrossRef]
- Winograd, Y.; Solan, A.; Toren, M. Mass transfer in narrow channels in the presence of turbulence promoters. Desalination 1973, 13, 171–186. [Google Scholar] [CrossRef]
- Da Costa, A.R.; Fane, A.G.; Fell, C.J.D.; Franken, A.C.M. Optimal channel spacer design for ultrafiltration. J. Membr. Sci. 1991, 62, 275–291. [Google Scholar] [CrossRef]
- Koutsou, C.P.; Yiantsios, S.G.; Karabelas, A.J. A numerical and experimental study of mass transfer in spacer-filled channels: Effects of spacer geometrical characteristics and Schmidt number. J. Membr. Sci. 2009, 326, 234–251. [Google Scholar] [CrossRef]
- Campione, A.; Cipollina, A.; Bogle, I.D.L.; Gurreri, L.; Tamburini, A.; Tedesco, M.; Micale, G. A hierarchical model for novel schemes of electrodialysis desalination. Desalination 2019, 465, 79–93. [Google Scholar] [CrossRef]
- Culcasi, A.; Gurreri, L.; Zaffora, A.; Cosenza, A.; Tamburini, A.; Cipollina, A.; Micale, G. Ionic shortcut currents via manifolds in reverse electrodialysis stacks. Desalination 2020, 485, 114450. [Google Scholar] [CrossRef]
- Wagholikar, V.V.; Zhuang, H.; Jiao, Y.; Moe, N.E.; Ramanan, H.; Goh, L.M.; Barber, J.; Lee, K.S.; Lee, H.P.; Fuh, J.Y.H. Modeling cell pair resistance and spacer shadow factors in electro-separation processes. J. Membr. Sci. 2017, 543, 151–162. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, M.S.; Lee, S.Y.; Choi, Y.W.; Jeong, N.J.; Kim, C.S. High power density of reverse electrodialysis with pore-filling ion exchange membranes and a high-open-area spacer. J. Mater. Chem. A 2015, 3, 16302–16306. [Google Scholar] [CrossRef] [Green Version]
- Ciofalo, M.; La Cerva, M.; Di Liberto, M.; Gurreri, L.; Cipollina, A.; Micale, G. Optimization of net power density in Reverse Electrodialysis. Energy 2019, 181, 576–588. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Scarazzato, T.; Panossian, Z.; Tenório, J.A.S.; Pérez-Herranz, V.; Espinosa, D.C.R. A review of cleaner production in electroplating industries using electrodialysis. J. Clean. Prod. 2017, 168, 1590–1602. [Google Scholar] [CrossRef]
- Arar, Ö.; Yüksel, Ü.; Kabay, N.; Yüksel, M. Various applications of electrodeionization (EDI) method for water treatment-A short review. Desalination 2014, 342, 16–22. [Google Scholar] [CrossRef]
- Benvenuti, T.; García-Gabaldón, M.; Ortega, E.M.; Rodrigues, M.A.S.; Bernardes, A.M.; Pérez-Herranz, V.; Zoppas-Ferreira, J. Influence of the co-ions on the transport of sulfate through anion exchange membranes. J. Membr. Sci. 2017, 542, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Martí-Calatayud, M.; García-Gabaldón, M.; Pérez-Herranz, V. Mass Transfer Phenomena during Electrodialysis of Multivalent Ions: Chemical Equilibria and Overlimiting Currents. Appl. Sci. 2018, 8, 1566. [Google Scholar] [CrossRef] [Green Version]
- Nemati, M.; Hosseini, S.M.; Shabanian, M. Novel electrodialysis cation exchange membrane prepared by 2-acrylamido-2-methylpropane sulfonic acid; heavy metal ions removal. J. Hazard. Mater. 2017, 337, 90–104. [Google Scholar] [CrossRef]
- Martí-Calatayud, M.C.; García-Gabaldón, M.; Pérez-Herranz, V. Effect of the equilibria of multivalent metal sulfates on the transport through cation-exchange membranes at different current regimes. J. Membr. Sci. 2013, 443, 181–192. [Google Scholar] [CrossRef]
- Sharma, P.; Shahi, V.K. Assembly of MIL-101(Cr)-sulphonated poly (ether sulfone) membrane matrix for selective electrodialytic separation of Pb2+ from mono-/bi-valent ions. Chem. Eng. J. 2020, 382, 122688. [Google Scholar] [CrossRef]
- Vallois, C.; Sistat, P.; Roualdès, S.; Pourcelly, G. Separation of H+/Cu2+cations by electrodialysis using modified proton conducting membranes. J. Membr. Sci. 2003, 216, 13–25. [Google Scholar] [CrossRef]
- Chang, J.H.; Huang, C.P.; Cheng, S.F.; Shen, S.Y. Transport characteristics and removal efficiency of copper ions in the electrodialysis process under electroconvection operation. Process. Saf. Environ. Prot. 2017, 112, 235–242. [Google Scholar] [CrossRef]
- Barros, K.S.; Scarazzato, T.; Espinosa, D.C.R. Evaluation of the effect of the solution concentration and membrane morphology on the transport properties of Cu (II) through two monopolar cation–exchange membranes. Sep. Purif. Technol. 2018, 193, 184–192. [Google Scholar] [CrossRef]
- Mahmoud, A.; Muhr, L.; Vasiluk, S.; Aleynikoff, A.; Lapicque, F. Investigation of transport phenomena in a hybrid ion exchange-electrodialysis system for the removal of copper ions. J. Appl. Electrochem. 2003, 33, 875–884. [Google Scholar] [CrossRef]
- Scarazzato, T.; Panossian, Z.; García-Gabaldón, M.; Ortega, E.M.; Tenório, J.A.S.; Pérez-Herranz, V.; Espinosa, D.C.R. Evaluation of the transport properties of copper ions through a heterogeneous ion-exchange membrane in etidronic acid solutions by chronopotentiometry. J. Membr. Sci. 2017, 535, 268–278. [Google Scholar] [CrossRef]
- Aouad, F.; Lindheimer, A.; Gavach, C. Transport properties of electrodialysis membranes in the presence of Zn2+ complexes with Cl−. J. Membr. Sci. 1997, 123, 207–223. [Google Scholar] [CrossRef]
- Rodrigues, M.A.S.; Amado, F.D.R.; Bischoff, M.R.; Ferreira, C.A.; Bernardes, A.M.; Ferreira, J.Z. Transport of zinc complexes through an anion exchange membrane. Desalination 2008, 227, 241–252. [Google Scholar] [CrossRef]
- Dalla Costa, R.F.; Antônio Siqueira Rodrigues, M.; Ferreira, J.Z. Transport of Trivalent and Hexavalent Chromium through Different Ion-Selective Membranes in Acidic Aqueous Media. Sep. Sci. Technol. 1998, 33, 1135–1143. [Google Scholar] [CrossRef]
- Vallejo, M.E.; Persin, F.; Innocent, C.; Sistat, P.; Pourcelly, G. Electrotransport of Cr(VI) through an anion exchange membrane. Sep. Purif. Technol. 2000, 21, 61–69. [Google Scholar] [CrossRef]
- Rodrigues, M.A.S.; Dalla Costa, R.F.; Bernardes, A.M.; Zoppas Ferreira, J. Influence of ligand exchange on the treatment of trivalent chromium solutions by electrodialysis. Electrochim. Acta 2001, 47, 753–758. [Google Scholar] [CrossRef]
- Çengeloǧlu, Y.; Tor, A.; Kir, E.; Ersöz, M. Transport of hexavalent chromium through anion-exchange membranes. Desalination 2003, 154, 239–246. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Sohrabnejad, S.; Nabiyouni, G.; Jashni, E.; Van der Bruggen, B.; Ahmadi, A. Magnetic cation exchange membrane incorporated with cobalt ferrite nanoparticles for chromium ions removal via electrodialysis. J. Membr. Sci. 2019, 583, 292–300. [Google Scholar] [CrossRef]
- Jashni, E.; Hosseini, S.M. Promoting the electrochemical and separation properties of heterogeneous cation exchange membrane by embedding 8-hydroxyquinoline ligand: Chromium ions removal. Sep. Purif. Technol. 2020, 234, 116118. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Alibakhshi, H.; Jashni, E.; Parvizian, F.; Shen, J.N.; Taheri, M.; Ebrahimi, M.; Rafiei, N. A novel layer-by-layer heterogeneous cation exchange membrane for heavy metal ions removal from water. J. Hazard. Mater. 2020, 381, 120884. [Google Scholar] [CrossRef]
- Barros, K.S.; Espinosa, D.C.R. Chronopotentiometry of an anion-exchange membrane for treating a synthesized free-cyanide effluent from brass electrodeposition with EDTA as chelating agent. Sep. Purif. Technol. 2018, 201, 244–255. [Google Scholar] [CrossRef]
- Mohammadi, T.; Moheb, A.; Sadrzadeh, M.; Razmi, A. Modeling of metal ion removal from wastewater by electrodialysis. Sep. Purif. Technol. 2005, 41, 73–82. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Razmi, A.; Mohammadi, T. Separation of different ions from wastewater at various operating conditions using electrodialysis. Sep. Purif. Technol. 2007, 54, 147–156. [Google Scholar] [CrossRef]
- Itoi, S.; Nakamura, I.; Kawahara, T. Electrodialytic recovery process of metal finishing waste water. Desalination 1980, 32, 383–389. [Google Scholar] [CrossRef]
- Benvenuti, T.; Krapf, R.S.; Rodrigues, M.A.S.; Bernardes, A.M.; Zoppas-Ferreira, J. Recovery of nickel and water from nickel electroplating wastewater by electrodialysis. Sep. Purif. Technol. 2014, 129, 106–112. [Google Scholar] [CrossRef]
- Benvenuti, T.; Siqueira Rodrigues, M.A.; Bernardes, A.M.; Zoppas-Ferreira, J. Closing the loop in the electroplating industry by electrodialysis. J. Clean. Prod. 2017, 155, 130–138. [Google Scholar] [CrossRef]
- Tzanetakis, N.; Taama, W.M.; Scott, K.; Jachuck, R.J.J.; Slade, R.S.; Varcoe, J. Comparative performance of ion exchange membranes for electrodialysis of nickel and cobalt. Sep. Purif. Technol. 2003, 30, 113–127. [Google Scholar] [CrossRef]
- Li, C.L.; Zhao, H.X.; Tsuru, T.; Zhou, D.; Matsumura, M. Recovery of spent electroless nickel plating bath by electrodialysis. J. Membr. Sci. 1999, 157, 241–249. [Google Scholar] [CrossRef]
- Peng, C.; Jin, R.; Li, G.; Li, F.; Gu, Q. Recovery of nickel and water from wastewater with electrochemical combination process. Sep. Purif. Technol. 2014, 136, 42–49. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Y.; Wang, J. Recovery of Ni2+ and pure water from electroplating rinse wastewater by an integrated two-stage electrodeionization process. J. Clean. Prod. 2015, 92, 257–266. [Google Scholar] [CrossRef]
- Dzyazko, Y.S.; Ponomaryova, L.N.; Rozhdestvenskaya, L.M.; Vasilyuk, S.L.; Belyakov, V.N. Electrodeionization of low-concentrated multicomponent Ni2+-containing solutions using organic-inorganic ion-exchanger. Desalination 2014, 342, 43–51. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, L.; Ge, R.; Zhang, A.; Zhang, C.; Chen, X. Treatment of low-level Cu(II) wastewater and regeneration through a novel capacitive deionization-electrodeionization (CDI-EDI) technology. Chemosphere 2019, 217, 763–772. [Google Scholar] [CrossRef]
- Dermentzis, K. Removal of nickel from electroplating rinse waters using electrostatic shielding electrodialysis/electrodeionization. J. Hazard. Mater. 2010, 173, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, T.; Moheb, A.; Sadrzadeh, M.; Razmi, A. Separation of copper ions by electrodialysis using Taguchi experimental design. Desalination 2004, 169, 21–31. [Google Scholar] [CrossRef]
- Korngold, E.; Kock, K.; Strathmann, H. Electrodialysis in advanced waste water treatment. Desalination 1978, 24, 129–139. [Google Scholar] [CrossRef]
- Chiapello, J.M.; Gal, J.Y. Recovery by electrodialysis of cyanide electroplating rinse waters. J. Membr. Sci. 1992, 68, 283–291. [Google Scholar] [CrossRef]
- Scarazzato, T.; Buzzi, D.C.; Bernardes, A.M.; Romano Espinosa, D.C. Treatment of wastewaters from cyanide-free plating process by electrodialysis. J. Clean. Prod. 2015, 91, 241–250. [Google Scholar] [CrossRef]
- Scarazzato, T.; Panossian, Z.; Tenório, J.A.S.; Pérez-Herranz, V.; Espinosa, D.C.R. Water reclamation and chemicals recovery from a novel cyanide-free copper plating bath using electrodialysis membrane process. Desalination 2018, 436, 114–124. [Google Scholar] [CrossRef]
- Zhelonkina, E.A.; Shishkina, S.V.; Mikhailova, I.Y.; Ananchenko, B.A. Study of electrodialysis of a copper chloride solution at overlimiting currents. Pet. Chem. 2017, 57, 947–953. [Google Scholar] [CrossRef]
- Peng, C.; Liu, Y.; Bi, J.; Xu, H.; Ahmed, A.S. Recovery of copper and water from copper-electroplating wastewater by the combination process of electrolysis and electrodialysis. J. Hazard. Mater. 2011, 189, 814–820. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, J.; Sui, M.; Qu, Y.; Ambuchi, J.J.; Wang, H.; Feng, Y. A combined microbial desalination cell and electrodialysis system for copper-containing wastewater treatment and high-salinity-water desalination. J. Hazard. Mater. 2017, 321, 307–315. [Google Scholar] [CrossRef]
- Song, Y.; Sun, T.; Cang, L.; Wu, S.; Zhou, D. Migration and transformation of Cu(II)-EDTA during electrodialysis accompanied by an electrochemical process with different compartment designs. Electrochim. Acta 2019, 295, 605–614. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Hosamani, K.M.; Aminabhavi, T.M. Potential application of an electrodialysis pilot plant containing ion-exchange membranes in chromium removal. Desalination 2007, 217, 181–190. [Google Scholar] [CrossRef]
- Chen, S.-S.; Li, C.-W.; Hsu, H.-D.; Lee, P.-C.; Chang, Y.-M.; Yang, C.-H. Concentration and purification of chromate from electroplating wastewater by two-stage electrodialysis processes. J. Hazard. Mater. 2009, 161, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.S.L.; Miranda Reis, M.H.; Cardoso, V.L.; De Resende, M.M. Electrodialysis for removal of chromium (VI) from effluent: Analysis of concentrated solution saturation. J. Environ. Chem. Eng. 2019, 7, 103380. [Google Scholar] [CrossRef]
- Alvarado, L.; Ramírez, A.; Rodríguez-Torres, I. Cr(VI) removal by continuous electrodeionization: Study of its basic technologies. Desalination 2009, 249, 423–428. [Google Scholar] [CrossRef]
- Alvarado, L.; Torres, I.R.; Chen, A. Integration of ion exchange and electrodeionization as a new approach for the continuous treatment of hexavalent chromium wastewater. Sep. Purif. Technol. 2013, 105, 55–62. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, X.; Wang, D. Electrically regenerated ion exchange for removal and recovery of Cr(VI) from wastewater. Environ. Sci. Technol. 2007, 41, 1439–1443. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, X.; Yao, P.; Wang, D. Continuous electrodeionization for removal and recovery of Cr(VI) from wastewater. Sep. Purif. Technol. 2009, 67, 123–126. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, X.; Wang, D. Variable effects on the performance of continuous electrodeionization for the removal of Cr(VI) from wastewater. Sep. Purif. Technol. 2009, 68, 357–362. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, H.; Zhang, Y.; Feng, H.; Shehzad, M.A.; Wang, Y.; Xu, T. Complexation Electrodialysis as a general method to simultaneously treat wastewaters with metal and organic matter. Chem. Eng. J. 2018, 348, 952–959. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, H.; Liu, Y.; Chen, R.; Qian, Q.; Van der Bruggen, B. Cr(III) recovery in form of Na2CrO4 from aqueous solution using improved bipolar membrane electrodialysis. J. Membr. Sci. 2020, 604, 118097. [Google Scholar] [CrossRef]
- Zhang, Z.; Liba, D.; Alvarado, L.; Chen, A. Separation and recovery of Cr(III) and Cr(VI) using electrodeionization as an efficient approach. Sep. Purif. Technol. 2014, 137, 86–93. [Google Scholar] [CrossRef]
- Tor, A.; Büyükerkek, T.; Çengeloǧlu, Y.; Ersöz, M. Simultaneous recovery of Cr(III) and Cr(VI) from the aqueous phase with ion-exchange membranes. Desalination 2005, 171, 233–241. [Google Scholar] [CrossRef]
- Raghava Rao, J.; Prasad, B.G.S.; Narasimhan, V.; Ramasami, T.; Shah, P.R.; Khan, A.A. Electrodialysis in the recovery and reuse of chromium from industrial effluents. J. Membr. Sci. 1989, 46, 215–224. [Google Scholar]
- Lambert, J.; Rakib, M.; Durand, G.; Avila-Rodríguez, M. Treatment of solutions containing trivalent chromium by electrodialysis. Desalination 2006, 191, 100–110. [Google Scholar] [CrossRef]
- Lambert, J.; Avila-Rodriguez, M.; Durand, G.; Rakib, M. Separation of sodium ions from trivalent chromium by electrodialysis using monovalent cation selective membranes. J. Membr. Sci. 2006, 280, 219–225. [Google Scholar] [CrossRef]
- Rodrigues, M.A.S.; Amado, F.D.R.; Xavier, J.L.N.; Streit, K.F.; Bernardes, A.M.; Ferreira, J.Z. Application of photoelectrochemical-electrodialysis treatment for the recovery and reuse of water from tannery effluents. J. Clean. Prod. 2008, 16, 605–611. [Google Scholar] [CrossRef]
- Deghles, A.; Kurt, U. Treatment of tannery wastewater by a hybrid electrocoagulation/electrodialysis process. Chem. Eng. Process. Process. Intensif. 2016, 104, 43–50. [Google Scholar] [CrossRef]
- Marder, L.; Sulzbach, G.O.; Bernardes, A.M.; Zoppas Ferreira, J. Removal of cadmium and cyanide from aqueous solutions through electrodialysis. J. Braz. Chem. Soc. 2003, 14, 610–615. [Google Scholar] [CrossRef] [Green Version]
- Marder, L.; Bernardes, A.M.; Zoppas Ferreira, J. Cadmium electroplating wastewater treatment using a laboratory-scale electrodialysis system. Sep. Purif. Technol. 2004, 37, 247–255. [Google Scholar] [CrossRef]
- Mehellou, A.; Delimi, R.; Benredjem, Z.; Saaidia, S.; Allat, L.; Innocent, C. Improving the efficiency and selectivity of Cd 2+ removal using a modified resin in the continuous electropermutation process. Sep. Sci. Technol. 2019, 55, 2049–2060. [Google Scholar]
- Mohammadi, T.; Razmi, A.; Sadrzadeh, M. Effect of operating parameters on Pb2+ separation from wastewater using electrodialysis. Desalination 2004, 167, 379–385. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Mohammadi, T.; Ivakpour, J.; Kasiri, N. Separation of lead ions from wastewater using electrodialysis: Comparing mathematical and neural network modeling. Chem. Eng. J. 2008, 144, 431–441. [Google Scholar] [CrossRef]
- Abou-Shady, A.; Peng, C.; Almeria, O.J.; Xu, H. Effect of pH on separation of Pb (II) and NO3− from aqueous solutions using electrodialysis. Desalination 2012, 285, 46–53. [Google Scholar] [CrossRef]
- Abou-Shady, A.; Peng, C.; Bi, J.; Xu, H.; Almeria, O.J. Recovery of Pb (II) and removal of NO3- from aqueous solutions using integrated electrodialysis, electrolysis, and adsorption process. Desalination 2012, 286, 304–315. [Google Scholar] [CrossRef]
- Gherasim, C.V.; Křivčík, J.; Mikulášek, P. Investigation of batch electrodialysis process for removal of lead ions from aqueous solutions. Chem. Eng. J. 2014, 256, 324–334. [Google Scholar] [CrossRef]
- Barros, K.S.; Ortega, E.M.; Pérez-Herranz, V.; Espinosa, D.C.R. Evaluation of brass electrodeposition at RDE from cyanide-free bath using EDTA as a complexing agent. J. Electroanal. Chem. 2020, 865, 114129. [Google Scholar] [CrossRef]
- Barros, K.S.; Scarazzato, T.; Pérez-Herranz, V.; Espinosa, D.C.R. Treatment of cyanide-free wastewater from brass electrodeposition with edta by electrodialysis: Evaluation of underlimiting and overlimiting operations. Membranes 2020, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Min, K.J.; Choi, S.Y.; Jang, D.; Lee, J.; Park, K.Y. Separation of metals from electroplating wastewater using electrodialysis. Energy Sourcespart. A Recover. Util. Environ. Eff. 2019, 41, 2471–2480. [Google Scholar] [CrossRef]
- Zuo, W.; Zhang, G.; Meng, Q.; Zhang, H. Characteristics and application of multiple membrane process in plating wastewater reutilization. Desalination 2008, 222, 187–196. [Google Scholar] [CrossRef]
- Peng, C.; Meng, H.; Song, S.; Lu, S.; Lopez-Vaidivieso, A. Elimination of Cr(VI) from electroplating wastewater by electrodialysis following chemical precipitation. Sep. Sci. Technol. 2004, 39, 1501–1517. [Google Scholar] [CrossRef]
- Babilas, D.; Dydo, P. Selective zinc recovery from electroplating wastewaters by electrodialysis enhanced with complex formation. Sep. Purif. Technol. 2018, 192, 419–428. [Google Scholar] [CrossRef]
- Babilas, D.; Dydo, P. Zinc salt recovery from electroplating industry wastes by electrodialysis enhanced with complex formation. Sep. Sci. Technol. 2019, 1–9. [Google Scholar] [CrossRef]
- Frioui, S.; Oumeddour, R.; Lacour, S. Highly selective extraction of metal ions from dilute solutions by hybrid electrodialysis technology. Sep. Purif. Technol. 2017, 174, 264–274. [Google Scholar] [CrossRef]
- Cherif, A.T.; Elmidaoui, A.; Gavach, C. Separation of Ag+, Zn2+ and Cu2+ ions by electrodialysis with monovalent cation specific membrane and EDTA. J. Membr. Sci. 1993, 76, 39–49. [Google Scholar] [CrossRef]
- Cifuentes, L.; Crisóstomo, G.; Ibáñez, J.P.; Casas, J.M.; Alvarez, F.; Cifuentes, G. On the electrodialysis of aqueous H2SO4–CuSO4 electrolytes with metallic impurities. J. Membr. Sci. 2002, 207, 1–16. [Google Scholar] [CrossRef]
- Cifuentes, L.; García, I.; Arriagada, P.; Casas, J.M. The use of electrodialysis for metal separation and water recovery from CuSO4-H2SO4-Fe solutions. Sep. Purif. Technol. 2009, 68, 105–108. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, H.; Zhang, M.; Chen, R.; Chen, X.; Zheng, X.; Jin, Y. Cr(VI) recovery from chromite ore processing residual using an enhanced electrokinetic process by bipolar membranes. J. Membr. Sci. 2018, 566, 190–196. [Google Scholar] [CrossRef]
- Reig, M.; Vecino, X.; Valderrama, C.; Gibert, O.; Cortina, J.L. Application of selectrodialysis for the removal of as from metallurgical process waters: Recovery of Cu and Zn. Sep. Purif. Technol. 2018, 195, 404–412. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, X.; Wang, X.; Li, Z.; Wang, Y.; Gao, C. Application of electrodialysis to remove copper and cyanide from simulated and real gold mine effluents. RSC Adv. 2015, 5, 19807–19817. [Google Scholar] [CrossRef]
- Yeon, K.H.; Song, J.H.; Moon, S.H. A study on stack configuration of continuous electrodeionization for removal of heavy metal ions from the primary coolant of a nuclear power plant. Water Res. 2004, 38, 1911–1921. [Google Scholar] [CrossRef]
- Yeon, K.H.; Seong, J.H.; Rengaraj, S.; Moon, S.H. Electrochemical characterization of ion-exchange resin beds and removal of cobalt by electrodeionization for high purity water production. Sep. Sci. Technol. 2003, 38, 443–462. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Xuan, S.; Lin, X.; Luo, X. Variable effects on electrodeionization for removal of Cs+ ions from simulated wastewater. Desalination 2014, 344, 212–218. [Google Scholar] [CrossRef]
- Jiang, B.; Li, F.; Zhao, X. Removal of trace Cs(I), Sr(II), and Co(II) in aqueous solutions using continuous electrodeionization (CEDI). Desalin. Water Treat. 2019, 155, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Zahakifar, F.; Keshtkar, A.R.; Souderjani, E.Z.; Moosavian, M.A. Use of response surface methodology for optimization of thorium(IV) removal from aqueous solutions by electrodeionization (EDI). Prog. Nucl. Energy 2020, 124, 103335. [Google Scholar] [CrossRef]
- Regel-Rosocka, M. A review on methods of regeneration of spent pickling solutions from steel processing. J. Hazard. Mater. 2010, 177, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Sahu, K.K. An overview of the recovery of acid from spent acidic solutions from steel and electroplating industries. J. Hazard. Mater. 2009, 171, 61–75. [Google Scholar] [CrossRef]
- Urano, K.; Ase, T.; Naito, Y. Recovery of acid from wastewater by electrodialysis. Desalination 1984, 51, 213–226. [Google Scholar] [CrossRef]
- Pourcelly, G.; Tugas, I.; Gavach, C. Electrotransport of sulphuric acid in special anion exchange membranes for the recovery of acids. J. Membr. Sci. 1994, 97, 99–107. [Google Scholar] [CrossRef]
- Jia, Y.X.; Li, F.J.; Chen, X.; Wang, M. Model analysis on electrodialysis for inorganic acid recovery and its experimental validation. Sep. Purif. Technol. 2018, 190, 261–267. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Xu, Z.; Zhang, F.; Efome, J.E.; Li, N. Proton blockage membrane with tertiary amine groups for concentration of sulfonic acid in electrodialysis. J. Membr. Sci. 2018, 555, 78–87. [Google Scholar] [CrossRef]
- Guo, R.Q.; Wang, B.B.; Jia, Y.X.; Wang, M. Development of acid block anion exchange membrane by structure design and its possible application in waste acid recovery. Sep. Purif. Technol. 2017, 186, 188–196. [Google Scholar] [CrossRef]
- Bai, T.; Wang, M.; Zhang, B.; Jia, Y.; Chen, Y. Anion-exchange membrane with ion-nanochannels to beat trade-off between membrane conductivity and acid blocking performance for waste acid reclamation. J. Membr. Sci. 2019, 573, 657–667. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Y.; Liu, R.; She, Z.; Tan, M.; Mao, D.; Fu, R.; Zhang, Y. Polymer inclusion membrane (PIM) containing ionic liquid as a proton blocker to improve waste acid recovery efficiency in electrodialysis process. J. Membr. Sci. 2019, 581, 18–27. [Google Scholar] [CrossRef]
- Cong, M.Y.; Jia, Y.X.; Wang, H.; Wang, M. Preparation of acid block anion exchange membrane with quaternary ammonium groups by homogeneous amination for electrodialysis-based acid enrichment. Sep. Purif. Technol. 2020, 238, 116396. [Google Scholar] [CrossRef]
- Bai, T.T.; Cong, M.Y.; Jia, Y.X.; Ma, K.K.; Wang, M. Preparation of self-crosslinking anion exchange membrane with acid block performance from side-chain type polysulfone. J. Membr. Sci. 2020, 599, 117831. [Google Scholar] [CrossRef]
- Paquay, E.; Clarinval, A.M.; Delvaux, A.; Degrez, M.; Hurwitz, H.D. Applications of electrodialysis for acid pickling wastewater treatment. Chem. Eng. J. 2000, 79, 197–201. [Google Scholar] [CrossRef]
- Chapotot, A.; Lopez, V.; Lindheimer, A.; Aouad, N.; Gavach, C. Electrodialysis of acid solutions with metallic divalent salts: Cation-exchange membranes with improved permeability to protons. Desalination 1995, 101, 141–153. [Google Scholar] [CrossRef]
- Xu, T. Electrodialysis processes with bipolar membranes (EDBM) in environmental protection—A review. Resour. Conserv. Recycl. 2002, 37, 1–22. [Google Scholar]
- Baltazar, V.; Harris, G.B.; White, C.W. The selective recovery and concentration of sulphuric acid by electrodialysis. Hydrometallurgy 1992, 30, 463–481. [Google Scholar] [CrossRef]
- Tran, A.T.K.; Mondal, P.; Lin, J.; Meesschaert, B.; Pinoy, L.; Van der Bruggen, B. Simultaneous regeneration of inorganic acid and base from a metal washing step wastewater by bipolar membrane electrodialysis after pretreatment by crystallization in a fluidized pellet reactor. J. Membr. Sci. 2015, 473, 118–127. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, X.; Wang, M.; Wang, B. A win-win strategy for the reclamation of waste acid and conversion of organic acid by a modified electrodialysis. Sep. Purif. Technol. 2016, 171, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Wang, M.; Zhang, B.; Jia, Y.; Chen, Y. Fabrication of proton permselective composite membrane for electrodialysis-based waste acid reclamation. J. Membr. Sci. 2019, 592, 117366. [Google Scholar] [CrossRef]
- Boucher, M.; Turcotte, N.; Guillemette, V.; Lantagne, G.; Chapotot, A.; Pourcelly, G.; Sandeaux, R.; Gavach, C. Recovery of spent acid by electrodialysis in the zinc hydrometallurgy industry: Performance study of different cation-exchange membranes. Hydrometallurgy 1997, 45, 137–160. [Google Scholar] [CrossRef]
- Sistat, P.; Pourcelly, G.; Gavach, C.; Turcotte, N.; Boucher, M. Electrodialysis of acid effluents containing metallic divalent salts: Recovery of acid with a cation-exchange membrane modified in situ. J. Appl. Electrochem. 1997, 27, 65–70. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Jia, Y.X.; Wang, X.L. The improvement of comprehensive transport properties to heterogeneous cation exchange membrane by the covalent immobilization of polyethyleneimine. Sep. Purif. Technol. 2015, 140, 69–76. [Google Scholar] [CrossRef]
- He, Y.; Ge, L.; Ge, Z.J.; Zhao, Z.; Sheng, F.; Liu, X.; Ge, X.; Yang, Z.; Fu, R.; Liu, Z.; et al. Monovalent cations permselective membranes with zwitterionic side chains. J. Membr. Sci. 2018, 563, 320–325. [Google Scholar] [CrossRef]
- Sheng, F.; Afsar, N.U.; Zhu, Y.; Ge, L.; Xu, T. PVA-based mixed matrix membranes comprising ZSM-5 for cations separation. Membranes 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wu, B.; Li, Q.; Wang, Y.; Yu, D.; Wu, L.; Pan, J.; Miao, J.; Xu, T. Electrodialysis with nanofiltration membrane (EDNF) for high-efficiency cations fractionation. J. Membr. Sci. 2016, 498, 192–200. [Google Scholar] [CrossRef]
- Liu, Y.; Ke, X.; Zhu, H.; Chen, R.; Chen, X.; Zheng, X.; Jin, Y.; Van der Bruggen, B. Treatment of raffinate generated via copper ore hydrometallurgical processing using a bipolar membrane electrodialysis system. Chem. Eng. J. 2020, 382, 122956. [Google Scholar] [CrossRef]
- Yuzer, B.; Aydin, M.I.; Hasançebi, B.; Selcuk, H. Application of an electrodialysis process to recover nitric acid from aluminum finishing industry waste. Desalin. Water Treat. 2019, 172, 199–205. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Wang, X.; Wang, Y.; Xu, T. Recovery of hydrochloric acid from simulated chemosynthesis aluminum foils wastewater: An integration of diffusion dialysis and conventional electrodialysis. J. Membr. Sci. 2012, 409–410, 257–263. [Google Scholar] [CrossRef]
- Zhuang, J.X.; Chen, Q.; Wang, S.; Zhang, W.M.; Song, W.G.; Wan, L.J.; Ma, K.S.; Zhang, C.N. Zero discharge process for foil industry waste acid reclamation: Coupling of diffusion dialysis and electrodialysis with bipolar membranes. J. Membr. Sci. 2013, 432, 90–96. [Google Scholar] [CrossRef]
- Aydin, M.I.; Yuzer, B.; Hasancebi, B.; Selcuk, H. Application of electrodialysis membrane process to recovery sulfuric acid and wastewater in the chalcopyrite mining industry. Desalin. Water Treat. 2019, 172, 206–211. [Google Scholar] [CrossRef]
- Heinonen, J.; Zhao, Y.; Van der Bruggen, B. A process combination of ion exchange and electrodialysis for the recovery and purification of hydroxy acids from secondary sources. Sep. Purif. Technol. 2020, 240, 116642. [Google Scholar] [CrossRef]
- Li, M.; Sun, M.; Liu, W.; Zhang, X.; Wu, C.; Wu, Y. Quaternized graphene oxide modified PVA-QPEI membranes with excellent selectivity for alkali recovery through electrodialysis. Chem. Eng. Res. Des. 2020, 153, 875–886. [Google Scholar] [CrossRef]
- Davis, J.R.; Chen, Y.; Baygents, J.C.; Farrell, J. Production of Acids and Bases for Ion Exchange Regeneration from Dilute Salt Solutions Using Bipolar Membrane Electrodialysis. ACS Sustain. Chem. Eng. 2015, 3, 2337–2342. [Google Scholar] [CrossRef]
- Graillon, S.; Persin, F.; Pourcelly, G.; Gavach, C. Development of electrodialysis with bipolar membrane for the treatment of concentrated nitrate effluents. Desalination 1996, 107, 159–169. [Google Scholar] [CrossRef]
- Ben Ali, M.A.; Rakib, M.; Laborie, S.; Viers, P.; Durand, G. Coupling of bipolar membrane electrodialysis and ammonia stripping for direct treatment of wastewaters containing ammonium nitrate. J. Membr. Sci. 2004, 244, 89–96. [Google Scholar]
- Cherif, A.T.; Molenat, J.; Elmidaoui, A. Nitric acid and sodium hydroxide generation by electrodialysis using bipolar membranes. J. Appl. Electrochem. 1997, 27, 1069–1074. [Google Scholar] [CrossRef]
- Monat, L.; Chaudhury, S.; Nir, O. Enhancing the Sustainability of Phosphogypsum Recycling by Integrating Electrodialysis with Bipolar Membranes. ACS Sustain. Chem. Eng. 2020, 8, 2490–2497. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Cao, H.; Wu, X.; Zhao, Z.; Wang, L. Bipolar membrane electrodialysis for generation of hydrochloric acid and ammonia from simulated ammonium chloride wastewater. Water Res. 2016, 89, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yan, H.; Yang, B.; Wu, C.; Zhang, X.; Wang, X. Bipolar membrane electrodialysis for the recycling of ammonium chloride wastewater: Membrane selection and process optimization. Chem. Eng. Res. Des. 2018, 138, 105–115. [Google Scholar] [CrossRef]
- Van Linden, N.; Bandinu, G.L.; Vermaas, D.A.; Spanjers, H.; van Lier, J.B. Bipolar membrane electrodialysis for energetically competitive ammonium removal and dissolved ammonia production. J. Clean. Prod. 2020, 259, 120788. [Google Scholar] [CrossRef]
- Trivedi, G.; Shah, B.; Adhikary, S.; Rangarajan, R. Studies on bipolar membranes: Part III: Conversion of sodium phosphate to phosphoric acid and sodium hydroxide. React. Funct. Polym. 1999, 39, 91–97. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Zhang, X.; Xu, T. Treatment of simulated brominated butyl rubber wastewater by bipolar membrane electrodialysis. Sep. Purif. Technol. 2011, 80, 196–201. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Zhang, X.; Xu, T. Comparative study on the treatment of simulated brominated butyl rubber wastewater by using bipolar membrane electrodialysis (BMED) and conventional electrodialysis (ED). Sep. Purif. Technol. 2013, 110, 164–169. [Google Scholar] [CrossRef]
- Wang, D.; Meng, W.; Lei, Y.; Li, C.; Cheng, J.; Qu, W.; Wang, G.; Zhang, M.; Li, S. The novel strategy for increasing the efficiency and yield of the bipolar membrane electrodialysis by the double conjugate salts stress. Polymers 2020, 12, 343. [Google Scholar] [CrossRef] [Green Version]
- Ghyselbrecht, K.; Huygebaert, M.; Van der Bruggen, B.; Ballet, R.; Meesschaert, B.; Pinoy, L. Desalination of an industrial saline water with conventional and bipolar membrane electrodialysis. Desalination 2013, 318, 9–18. [Google Scholar] [CrossRef]
- Noguchi, M.; Nakamura, Y.; Shoji, T.; Iizuka, A.; Yamasaki, A. Simultaneous removal and recovery of boron from waste water by multi-step bipolar membrane electrodialysis. J. Water Process. Eng. 2018, 23, 299–305. [Google Scholar] [CrossRef]
- Nagasawa, H.; Iizuka, A.; Yamasaki, A.; Yanagisawa, Y. Utilization of bipolar membrane electrodialysis for the removal of boron from aqueous solution. Ind. Eng. Chem. Res. 2011, 50, 6325–6330. [Google Scholar] [CrossRef]
- Sun, M.; Li, M.; Zhang, X.; Wu, C.; Wu, Y. Graphene oxide modified porous P84 co-polyimide membranes for boron recovery by bipolar membrane electrodialysis process. Sep. Purif. Technol. 2020, 232, 115963. [Google Scholar] [CrossRef]
- Reig, M.; Valderrama, C.; Gibert, O.; Cortina, J.L. Selectrodialysis and bipolar membrane electrodialysis combination for industrial process brines treatment: Monovalent-divalent ions separation and acid and base production. Desalination 2016, 399, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.J.; Nagasubramanian, K.; Chlanda, F.P. Membrane electrodialysis process for recovery of sulfur dioxide from power plant stack gases. J. Membr. Sci. 1978, 3, 71–83. [Google Scholar] [CrossRef]
- Liu, K.J.; Chlanda, F.P.; Nagasubramanian, K. Application of bipolar membrane technology: A novel process for control of sulfur dioxide from flue gases. J. Membr. Sci. 1978, 3, 57–70. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, C.; Pi, K.; Huang, J.; Xia, M.; Gerson, A.R. Sustainable treatment of desulfurization wastewater by ion exchange and bipolar membrane electrodialysis hybrid technology. Sep. Purif. Technol. 2019, 211, 330–339. [Google Scholar] [CrossRef]
- Tian, W.; Wang, X.; Fan, C.; Cui, Z. Optimal treatment of hypersaline industrial wastewater via bipolar membrane electrodialysis. ACS Sustain. Chem. Eng. 2019, 7, 12358–12368. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, D.; Zhu, D.; Xu, J.; Jiang, H.; Geng, W.; Wei, W.; Lian, Z. Separation of fluoride and chloride ions from ammonia-based flue gas desulfurization slurry using a two-stage electrodialysis. Chem. Eng. Res. Des. 2019, 147, 73–82. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Yan, H.; Xu, T. Removal of heat stable salts (HSS) from spent alkanolamine wastewater using electrodialysis. J. Ind. Eng. Chem. 2018, 57, 356–362. [Google Scholar] [CrossRef]
- Meng, H.; Zhang, S.; Li, C.; Li, L. Removal of heat stable salts from aqueous solutions of N-methyldiethanolamine using a specially designed three-compartment configuration electrodialyzer. J. Membr. Sci. 2008, 322, 436–440. [Google Scholar] [CrossRef]
- Chen, F.; Chi, Y.; Zhang, M.; Liu, Z.; Fei, X.; Yang, K.; Fu, C. Removal of heat stable salts from N-methyldiethanolamine wastewater by anion exchange resin coupled three-compartment electrodialysis. Sep. Purif. Technol. 2020, 242, 116777. [Google Scholar] [CrossRef]
- Bazhenov, S.; Rieder, A.; Schallert, B.; Vasilevsky, V.; Unterberger, S.; Grushevenko, E.; Volkov, V.; Volkov, A. Reclaiming of degraded MEA solutions by electrodialysis: Results of ED pilot campaign at post-combustion CO2 capture pilot plant. Int. J. Greenh. Gas. Control. 2015, 42, 593–601. [Google Scholar] [CrossRef]
- Grushevenko, E.; Bazhenov, S.; Vasilevsky, V.; Novitsky, E.; Shalygin, M.; Volkov, A. Effect of Carbon Dioxide Loading on Removal of Heat Stable Salts from Amine Solvent by Electrodialysis. Membranes 2019, 9, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iizuka, A.; Hashimoto, K.; Nagasawa, H.; Kumagai, K.; Yanagisawa, Y.; Yamasaki, A. Carbon dioxide recovery from carbonate solutions using bipolar membrane electrodialysis. Sep. Purif. Technol. 2012, 101, 49–59. [Google Scholar] [CrossRef]
- Jiang, C.; Li, S.; Zhang, D.; Yang, Z.; Yu, D.; Chen, X.; Wang, Y.; Xu, T. Mathematical modelling and experimental investigation of CO2 absorber recovery using an electro-acidification method. Chem. Eng. J. 2019, 360, 654–664. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, P.; Cong, W. Cation-exchange membrane fouling and cleaning in bipolar membrane electrodialysis of industrial glutamate production wastewater. Sep. Purif. Technol. 2011, 79, 103–113. [Google Scholar] [CrossRef]
- Shen, J.; Huang, J.; Liu, L.; Ye, W.; Lin, J.; Van der Bruggen, B. The use of BMED for glyphosate recovery from glyphosate neutralization liquor in view of zero discharge. J. Hazard. Mater. 2013, 260, 660–667. [Google Scholar] [CrossRef]
- Ye, W.; Huang, J.; Lin, J.; Zhang, X.; Shen, J.; Luis, P.; Van Der Bruggen, B. Environmental evaluation of bipolar membrane electrodialysis for NaOH production from wastewater: Conditioning NaOH as a CO2 absorbent. Sep. Purif. Technol. 2015, 144, 206–214. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, C.; Wang, Y.; Yang, Z.; Xu, T. Reclamation of Aniline Wastewater and CO2 Capture Using Bipolar Membrane Electrodialysis. ACS Sustain. Chem. Eng. 2016, 4, 5743–5751. [Google Scholar] [CrossRef]
- Loza, N.V.; Loza, S.A.; Romanyuk, N.A.; Kononenko, N.A. Experimental and Theoretical Studies of Electrodialysis of Model Solutions Containing Aniline and Sulfuric Acid. Russ. J. Electrochem. 2019, 55, 871–877. [Google Scholar] [CrossRef]
- Peng, Z.; Sun, Y. Leakage circuit characteristics of a bipolar membrane electrodialyzer with 5 BP-A-C units. J. Membr. Sci. 2020, 597, 117762. [Google Scholar] [CrossRef]
- Schlichter, B.; Mavrov, V.; Erwe, T.; Chmiel, H. Regeneration of bonding agents loaded with heavy metals by electrodialysis with bipolar membranes. J. Membr. Sci. 2004, 232, 99–105. [Google Scholar] [CrossRef]
- Wei, Y.; Li, C.; Wang, Y.; Zhang, X.; Li, Q.; Xu, T. Regenerating sodium hydroxide from the spent caustic by bipolar membrane electrodialysis (BMED). Sep. Purif. Technol. 2012, 86, 49–54. [Google Scholar] [CrossRef]
- Rohman, F.S.; Othman, M.R.; Aziz, N. Modeling of batch electrodialysis for hydrochloric acid recovery. Chem. Eng. J. 2010, 162, 466–479. [Google Scholar] [CrossRef]
- Merkel, A.; Ashrafi, A.M.; Ondrušek, M. The use of electrodialysis for recovery of sodium hydroxide from the high alkaline solution as a model of mercerization wastewater. J. Water Process. Eng. 2017, 20, 123–129. [Google Scholar] [CrossRef]
- Bailly, M. Production of organic acids by bipolar electrodialysis: Realizations and perspectives. Desalination 2002, 144, 157–162. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.; Lu, Y.; Liu, Q.; Jiao, Q.; Wang, X.; Zhang, H. An integrated electrodialysis-biocatalysis-spray-drying process for efficient recycling of keratin acid hydrolysis industrial wastewater. Chem. Eng. J. 2016, 302, 146–154. [Google Scholar] [CrossRef]
- Vertova, A.; Aricci, G.; Rondinini, S.; Miglio, R.; Carnelli, L.; D’Olimpio, P. Electrodialytic recovery of light carboxylic acids from industrial aqueous wastes. J. Appl. Electrochem. 2009, 39, 2051–2059. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, G.; Sun, X.; Jin, B. Recovery of lactic acid from kitchen garbage fermentation broth by four-compartment configuration electrodialyzer. Process. Biochem. 2006, 41, 152–158. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Lang, Q.; Tan, M.; Zhang, Y. Composite anion exchange membrane made by layer-by-layer method for selective ion separation and water migration control. Sep. Purif. Technol. 2018, 192, 278–286. [Google Scholar] [CrossRef]
- Scoma, A.; Varela-Corredor, F.; Bertin, L.; Gostoli, C.; Bandini, S. Recovery of VFAs from anaerobic digestion of dephenolized Olive Mill Wastewaters by Electrodialysis. Sep. Purif. Technol. 2016, 159, 81–91. [Google Scholar] [CrossRef]
- Pan, X.R.; Li, W.W.; Huang, L.; Liu, H.Q.; Wang, Y.K.; Geng, Y.K.; Kwan-Sing Lam, P.; Yu, H.Q. Recovery of high-concentration volatile fatty acids from wastewater using an acidogenesis-electrodialysis integrated system. Bioresour. Technol. 2018, 260, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Wen, J.-L.; Wang, Y.-L.; Wu, Z.-G.; Zhao, P.-J.; Zhang, H.-H.; Wang, J.-J.; Zeng, R.J.; Zhang, F. Impacts of medium composition and applied current on recovery of volatile fatty acids during coupling of electrodialysis with an anaerobic digester. J. Clean. Prod. 2019, 207, 483–489. [Google Scholar] [CrossRef]
- Yu, L.; Guo, Q.; Hao, J.; Jiang, W. Recovery of acetic acid from dilute wastewater by means of bipolar membrane electrodialysis. Desalination 2000, 129, 283–288. [Google Scholar] [CrossRef]
- Yu, L.; Lin, T.; Guo, Q.; Hao, J. Relation between mass transfer and operation parameters in the electrodialysis recovery of acetic acid. Desalination 2003, 154, 147–152. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Wang, Y.; Luo, J.; Xu, T. Recovery of acetic acid from simulated acetaldehyde wastewaters: Bipolar membrane electrodialysis processes and membrane selection. J. Membr. Sci. 2011, 379, 184–190. [Google Scholar] [CrossRef]
- Ferrer, J.S.J.; Laborie, S.; Durand, G.; Rakib, M. Formic acid regeneration by electromembrane processes. J. Membr. Sci. 2006, 280, 509–516. [Google Scholar] [CrossRef]
- Lameloise, M.L.; Lewandowski, R. Recovering l-malic acid from a beverage industry waste water: Experimental study of the conversion stage using bipolar membrane electrodialysis. J. Membr. Sci. 2012, 403–404, 196–202. [Google Scholar] [CrossRef]
- Achoh, A.; Zabolotsky, V.; Melnikov, S. Conversion of water-organic solution of sodium naphtenates into naphtenic acids and alkali by electrodialysis with bipolar membranes. Sep. Purif. Technol. 2019, 212, 929–940. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Millar, G.J.; Couperthwaite, S.J.; Moodliar, C.D. Strategies for the management and treatment of coal seam gas associated water. Renew. Sustain. Energy Rev. 2016, 57, 669–691. [Google Scholar] [CrossRef] [Green Version]
- Onishi, V.C.; Reyes-Labarta, J.A.; Caballero, J.A. Membrane Desalination in Shale Gas Industry: Applications and Perspectives. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Curcio, E., Inamuddin, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–267. ISBN 9780128135518. [Google Scholar]
- Chang, H.; Li, T.; Liu, B.; Vidic, R.D.; Elimelech, M.; Crittenden, J.C. Potential and implemented membrane-based technologies for the treatment and reuse of flowback and produced water from shale gas and oil plays: A review. Desalination 2019, 455, 34–57. [Google Scholar] [CrossRef]
- Hamawand, I.; Yusaf, T.; Hamawand, S.G. Coal seam gas and associated water: A review paper. Renew. Sustain. Energy Rev. 2013, 22, 550–560. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Khajeh, A.; Mesbah, M. Membrane filtration of wastewater from gas and oil production. Environ. Chem. Lett. 2018, 16, 367–388. [Google Scholar] [CrossRef]
- Arthur, J.D.; Langhus, B.G.; Patel, C. Technical Summary of Oil & Gas: Produced Water Treatment Technologies; Tulsa World: Tulsa, OK, USA, 2005. [Google Scholar]
- Sirivedhin, T.; McCue, J.; Dallbauman, L. Reclaiming produced water for beneficial use: Salt removal by electrodialysis. J. Membr. Sci. 2004, 243, 335–343. [Google Scholar] [CrossRef]
- Hao, H.; Huang, X.; Gao, C.; Gao, X. Application of an integrated system of coagulation and electrodialysis for treatment of wastewater produced by fracturing. Desalin. Water Treat. 2014, 55, 2034–2043. [Google Scholar] [CrossRef]
- McGovern, R.K.; Weiner, A.M.; Sun, L.; Chambers, C.G.; Zubair, S.M.; Lienhard, V.J.H. On the cost of electrodialysis for the desalination of high salinity feeds. Appl. Energy 2014, 136, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Peraki, M.; Ghazanfari, E.; Pinder, G.F.; Harrington, T.L. Electrodialysis: An application for the environmental protection in shale-gas extraction. Sep. Purif. Technol. 2016, 161, 96–103. [Google Scholar] [CrossRef]
- McGovern, R.K.; Zubair, S.M.; Lienhard, V.J.H. The cost effectiveness of electrodialysis for diverse salinity applications. Desalination 2014, 348, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Hayes, T.D.; Severin, B.F. Electrodialysis of highly concentrated brines: Effects of calcium. Sep. Purif. Technol. 2017, 175, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Severin, B.F.; Hayes, T.D. Electrodialysis of concentrated brines: Effects of multivalent cations. Sep. Purif. Technol. 2019, 218, 227–241. [Google Scholar] [CrossRef]
- Jing, G.L.; Xing, L.J.; Liu, Y.; Du, W.T.; Han, C.J. Development of a four-grade and four-segment electrodialysis setup for desalination of polymer-flooding produced water. Desalination 2010, 264, 214–219. [Google Scholar] [CrossRef]
- Guo, H.; You, F.; Yu, S.; Li, L.; Zhao, D. Mechanisms of chemical cleaning of ion exchange membranes: A case study of plant-scale electrodialysis for oily wastewater treatment. J. Membr. Sci. 2015, 496, 310–317. [Google Scholar] [CrossRef]
- Jing, G.L.; Wang, X.Y.; Han, C.J. The effect of oilfield polymer-flooding wastewater on anion-exchange membrane performance. Desalination 2008, 220, 386–393. [Google Scholar] [CrossRef]
- Guo, H.; Xiao, L.; Yu, S.; Yang, H.; Hu, J.; Liu, G.; Tang, Y. Analysis of anion exchange membrane fouling mechanism caused by anion polyacrylamide in electrodialysis. Desalination 2014, 346, 46–53. [Google Scholar] [CrossRef]
- Zuo, X.; Wang, L.; He, J.; Li, Z.; Yu, S. SEM-EDX studies of SiO2/PVDF membranes fouling in electrodialysis of polymer-flooding produced wastewater: Diatomite, APAM and crude oil. Desalination 2014, 347, 43–51. [Google Scholar] [CrossRef]
- Wang, T.; Yu, S.; Hou, L. Impacts of HPAM molecular weights on desalination performance of ion exchange membranes and fouling mechanism. Desalination 2017, 404, 50–58. [Google Scholar] [CrossRef]
- Xia, Q.; Guo, H.; Ye, Y.; Yu, S.; Li, L.; Li, Q.; Zhang, R. Study on the fouling mechanism and cleaning method in the treatment of polymer flooding produced water with ion exchange membranes. RSC Adv. 2018, 8, 29947–29957. [Google Scholar] [CrossRef] [Green Version]
- Sosa-Fernandez, P.A.; Post, J.W.; Bruning, H.; Leermakers, F.A.M.; Rijnaarts, H.H.M. Electrodialysis-based desalination and reuse of sea and brackish polymer-flooding produced water. Desalination 2018, 447, 120–132. [Google Scholar] [CrossRef]
- Sosa-Fernandez, P.A.; Post, J.W.; Leermakers, F.A.M.; Rijnaarts, H.H.M.; Bruning, H. Removal of divalent ions from viscous polymer-flooding produced water and seawater via electrodialysis. J. Membr. Sci. 2019, 589, 117251. [Google Scholar] [CrossRef]
- Sosa-Fernandez, P.A.; Miedema, S.J.; Bruning, H.; Leermakers, F.A.M.; Rijnaarts, H.H.M.; Post, J.W. Influence of solution composition on fouling of anion exchange membranes desalinating polymer-flooding produced water. J. Colloid Interface Sci. 2019, 557, 381–394. [Google Scholar] [CrossRef]
- Sosa-Fernandez, P.A.; Post, J.W.; Ramdlan, M.S.; Leermakers, F.A.M.; Bruning, H.; Rijnaarts, H.H.M. Improving the performance of polymer-flooding produced water electrodialysis through the application of pulsed electric field. Desalination 2020, 484, 114424. [Google Scholar] [CrossRef]
- Lopez, A.M.; Dunsworth, H.; Hestekin, J.A. Reduction of the shadow spacer effect using reverse electrodeionization and its applications in water recycling for hydraulic fracturing operations. Sep. Purif. Technol. 2016, 162, 84–90. [Google Scholar] [CrossRef] [Green Version]
- AECOM Inc. Petroleum Refining Water/Wastewater Use and Management; IPIECA: London, UK, 2010. [Google Scholar]
- Parkash, S. Refinery Water Systems. Refin. Process. Handb. 2007, 242–269. [Google Scholar]
- Mikhak, Y.; Torabi, M.M.A.; Fouladitajar, A. Refinery and petrochemical wastewater treatment. In Sustainable Water and Wastewater Processing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 55–91. ISBN 9780128161708. [Google Scholar]
- Gioli, P.; Silingardi, G.E.; Ghiglio, G. High quality water from refinery waste. Desalination 1987, 67, 271–282. [Google Scholar] [CrossRef]
- Venzke, C.D.; Giacobbo, A.; Klauck, C.R.; Viegas, C.; Hansen, E.; Monteiro De Aquim, P.; Antônio, M.; Rodrigues, S.; Moura Bernardes, A. Integrated Membrane Processes (EDR-RO) for Water Reuse in the Petrochemical Industry. J. Membr. Sci. Res. 2018, 4, 218–226. [Google Scholar]
- Hughes, M.; Raubenheimer, A.E.; Viljoen, A.J. Electrodialysis reversal at Tutuka power station, RSA—Seven years’ design and operating experience. Water Sci. Technol. 1992, 25, 277–289. [Google Scholar] [CrossRef]
- Turek, M.; Dydo, P. Electrodialysis reversal of calcium sulphate and calcium carbonate supersaturated solution. Desalination 2003, 158, 91–94. [Google Scholar] [CrossRef]
- Turek, M. Electrodialytic desalination and concentration of coal-mine brine. Desalination 2004, 162, 355–359. [Google Scholar] [CrossRef]
- Turek, M.; Laskowska, E.; Mitko, K.; Chorążewska, M.; Dydo, P.; Piotrowski, K.; Jakóbik-Kolon, A. Application of nanofiltration and electrodialysis for improved performance of a salt production plant. Desalin. Water Treat. 2017, 64, 244–250. [Google Scholar] [CrossRef]
- Mitko, K.; Podleśny, B.; Jakóbik-Kolon, A.; Turek, M. Electrodialytic utilization of coal mine brines. Desalin. Water Treat. 2017, 75, 363–367. [Google Scholar] [CrossRef]
- Turek, M.; Bandura, B.; Dydo, P. Power production from coal-mine brine utilizing reversed electrodialysis. Desalination 2008, 221, 462–466. [Google Scholar] [CrossRef]
- Buzzi, D.C.; Viegas, L.S.; Rodrigues, M.A.S.; Bernardes, A.M.; Tenório, J.A.S. Water recovery from acid mine drainage by electrodialysis. Min. Eng. 2013, 40, 82–89. [Google Scholar] [CrossRef]
- Martí-Calatayud, M.C.; Buzzi, D.C.; García-Gabaldón, M.; Bernardes, A.M.; Tenório, J.A.S.; Pérez-Herranz, V. Ion transport through homogeneous and heterogeneous ion-exchange membranes in single salt and multicomponent electrolyte solutions. J. Membr. Sci. 2014, 466, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Bisselink, R.; de Schepper, W.; Trampé, J.; van den Broek, W.; Pinel, I.; Krutko, A.; Groot, N. Mild desalination demo pilot: New normalization approach to effectively evaluate electrodialysis reversal technology. Water Resour. Ind. 2015, 14, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Irfan, M.; Ge, L.; Wang, Y.; Yang, Z.; Xu, T. Hydrophobic Side Chains Impart Anion Exchange Membranes with High Monovalent-Divalent Anion Selectivity in Electrodialysis. ACS Sustain. Chem. Eng. 2019, 7, 4429–4442. [Google Scholar] [CrossRef]
- Irfan, M.; Xu, T.; Ge, L.; Wang, Y.; Xu, T. Zwitterion structure membrane provides high monovalent/divalent cation electrodialysis selectivity: Investigating the effect of functional groups and operating parameters. J. Membr. Sci. 2019, 588, 117211. [Google Scholar] [CrossRef]
- Irfan, M.; Wang, Y.; Xu, T. Novel electrodialysis membranes with hydrophobic alkyl spacers and zwitterion structure enable high monovalent/divalent cation selectivity. Chem. Eng. J. 2020, 383, 123171. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Koninckx, A.; Vandecasteele, C. Separation of monovalent and divalent ions from aqueous solution by electrodialysis and nanofiltration. Water Res. 2004, 38, 1347–1353. [Google Scholar] [CrossRef]
- Tufa, R.A.; Hnát, J.; Němeček, M.; Kodým, R.; Curcio, E.; Bouzek, K. Hydrogen production from industrial wastewaters: An integrated reverse electrodialysis—Water electrolysis energy system. J. Clean. Prod. 2018, 203, 418–426. [Google Scholar] [CrossRef]
- Luo, K.; Jia, Y.X.; Liu, X.X.; Wang, M. On-line construction of electrodialyzer with mono-cation permselectivity and its application in reclamation of high salinity wastewater. Desalination 2019, 454, 38–47. [Google Scholar] [CrossRef]
- Djouadi Belkada, F.; Kitous, O.; Drouiche, N.; Aoudj, S.; Bouchelaghem, O.; Abdi, N.; Grib, H.; Mameri, N. Electrodialysis for fluoride and nitrate removal from synthesized photovoltaic industry wastewater. Sep. Purif. Technol. 2018, 204, 108–115. [Google Scholar] [CrossRef]
- Kabay, N.; Arar, Ö.; Samatya, S.; Yüksel, Ü.; Yüksel, M. Separation of fluoride from aqueous solution by electrodialysis: Effect of process parameters and other ionic species. J. Hazard. Mater. 2008, 153, 107–113. [Google Scholar] [CrossRef]
- Wang, X.; Li, N.; Li, J.; Feng, J.; Ma, Z.; Xu, Y.; Sun, Y.; Xu, D.; Wang, J.; Gao, X.; et al. Fluoride removal from secondary effluent of the graphite industry using electrodialysis: Optimization with response surface methodology. Front. Env. Sci. Eng. 2019, 13. [Google Scholar] [CrossRef]
- Kabay, N.; Arar, O.; Acar, F.; Ghazal, A.; Yuksel, U.; Yuksel, M. Removal of boron from water by electrodialysis: Effect of feed characteristics and interfering ions. Desalination 2008, 223, 63–72. [Google Scholar] [CrossRef]
- Melnyk, L.; Goncharuk, V.; Butnyk, I.; Tsapiuk, E. Boron removal from natural and wastewaters using combined sorption/ membrane process. Desalination 2005, 185, 147–157. [Google Scholar] [CrossRef]
- Turek, M.; Dydo, P.; Ciba, J.; Trojanowska, J.; Kluczka, J.; Palka-Kupczak, B. Electrodialytic treatment of boron-containing wastewater with univalent permselective membranes. Desalination 2005, 185, 139–145. [Google Scholar] [CrossRef]
- Turek, M.; Dydo, P.; Trojanowska, J.; Bandura, B. Electrodialytic treatment of boron-containing wastewater. Desalination 2007, 205, 185–191. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, X.; Zhao, X.; Li, F. Removal of high level boron in aqueous solutions using continuous electrodeionization (CEDI). Sep. Purif. Technol. 2018, 192, 297–301. [Google Scholar] [CrossRef]
- Kozaderova, O.A.; Kim, K.B.; Gadzhiyeva, C.S.; Niftaliev, S.I. Electrochemical characteristics of thin heterogeneous ion exchange membranes. J. Membr. Sci. 2020, 604, 118081. [Google Scholar] [CrossRef]
- Melnikov, S.S.; Mugtamov, O.A.; Zabolotsky, V.I. Study of electrodialysis concentration process of inorganic acids and salts for the two-stage conversion of salts into acids utilizing bipolar electrodialysis. Sep. Purif. Technol. 2020, 235, 116198. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Q.; Luo, M.; Wang, H.; Qi, X.; Hou, C.H.; Li, F.; Ai, Z.; Junior, J.T.A. Improved bauxite residue dealkalization by combination of aerated washing and electrodialysis. J. Hazard. Mater. 2019, 364, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, E.; Zevenhoven, R. Energy use of flux salt recovery using bipolar membrane electrodialysis for a CO2 mineralisation process. Entropy 2019, 21, 395. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, A.; Yamashita, Y.; Nagasawa, H.; Yamasaki, A.; Yanagisawa, Y. Separation of lithium and cobalt from waste lithium-ion batteries via bipolar membrane electrodialysis coupled with chelation. Sep. Purif. Technol. 2013, 113, 33–41. [Google Scholar] [CrossRef]
- Lindstrand, V.; Jönsson, A.S.; Sundström, G. Organic fouling of electrodialysis membranes with and without applied voltage. Desalination 2000, 130, 73–84. [Google Scholar] [CrossRef]
- Vanoppen, M.; Bakelants, A.F.A.M.; Gaublomme, D.; Schoutteten, K.V.K.M.; Van Den Bussche, J.; Vanhaecke, L.; Verliefde, A.R.D. Properties governing the transport of trace organic contaminants through ion-exchange membranes. Environ. Sci. Technol. 2015, 49, 489–497. [Google Scholar] [CrossRef]
- Han, L.; Galier, S.; Roux-de Balmann, H. Transfer of neutral organic solutes during desalination by electrodialysis: Influence of the salt composition. J. Membr. Sci. 2016, 511, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Galier, S.; Roux-de Balmann, H. A phenomenological model to evaluate the performances of electrodialysis for the desalination of saline water containing organic solutes. Desalination 2017, 422, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Gao, X.; Zhang, Y.; He, Z.; Ji, Z.; Wang, X.; Gao, C. Hybrid RED/ED system: Simultaneous osmotic energy recovery and desalination of high-salinity wastewater. Desalination 2017, 405, 59–67. [Google Scholar] [CrossRef]
- De Schepper, W.; Moraru, M.D.; Jacobs, B.; Oudshoorn, M.; Helsen, J. Electrodialysis of aqueous NaCl-glycerol solutions: A phenomenological comparison of various ion exchange membranes. Sep. Purif. Technol. 2019, 217, 274–283. [Google Scholar] [CrossRef]
- Paltrinieri, L.; Huerta, E.; Puts, T.; Van Baak, W.; Verver, A.B.; Sudhölter, E.J.R.; De Smet, L.C.P.M. Functionalized Anion-Exchange Membranes Facilitate Electrodialysis of Citrate and Phosphate from Model Dairy Wastewater. Environ. Sci. Technol. 2019, 53, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, H.; Aravind, P.; Sundaram, M. Four compartment mono selective electrodialysis for separation of sodium formate from industry wastewater. Chem. Eng. J. 2018, 333, 162–169. [Google Scholar] [CrossRef]
- Chao, Y.M.; Liang, T.M. A feasibility study of industrial wastewater recovery using electrodialysis reversal. Desalination 2008, 221, 433–439. [Google Scholar] [CrossRef]
- Yen, F.C.; You, S.J.; Chang, T.C. Performance of electrodialysis reversal and reverse osmosis for reclaiming wastewater from high-tech industrial parks in Taiwan: A pilot-scale study. J. Environ. Manag. 2017, 187, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Rapp, H.J.; Pfromm, P.H. Electrodialysis for chloride removal from the chemical recovery cycle of a Kraft pulp mill. J. Membr. Sci. 1998, 146, 249–261. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Sridhar, S.; Shaikha, I.N.; Reddy, D.S.; Aminabhavi, T.M. Membrane-based microfiltration/electrodialysis hybrid process for the treatment of paper industry wastewater. Sep. Purif. Technol. 2007, 57, 185–192. [Google Scholar] [CrossRef]
- Lafi, R.; Gzara, L.; Lajimi, R.H.; Hafiane, A. Treatment of textile wastewater by a hybrid ultrafiltration/electrodialysis process. Chem. Eng. Process. Process Intensif. 2018, 132, 105–113. [Google Scholar] [CrossRef]
- Lin, J.; Lin, F.; Chen, X.; Ye, W.; Li, X.; Zeng, H.; Van Der Bruggen, B. Sustainable Management of Textile Wastewater: A Hybrid Tight Ultrafiltration/Bipolar-Membrane Electrodialysis Process for Resource Recovery and Zero Liquid Discharge. Ind. Eng. Chem. Res. 2019, 58, 11003–11012. [Google Scholar] [CrossRef]
- Berkessa, Y.W.; Lang, Q.; Yan, B.; Kuang, S.; Mao, D.; Shu, L.; Zhang, Y. Anion exchange membrane organic fouling and mitigation in salt valorization process from high salinity textile wastewater by bipolar membrane electrodialysis. Desalination 2019, 465, 94–103. [Google Scholar] [CrossRef]
- Tamersit, S.; Bouhidel, K.E.; Zidani, Z. Investigation of electrodialysis anti-fouling configuration for desalting and treating tannery unhairing wastewater: Feasibility of by-products recovery and water recycling. J. Environ. Manag. 2018, 207, 334–340. [Google Scholar] [CrossRef]
- Valero, D.; García-García, V.; Expósito, E.; Aldaz, A.; Montiel, V. Application of electrodialysis for the treatment of almond industry wastewater. J. Membr. Sci. 2015, 476, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Kingsbury, R.S.; Liu, F.; Zhu, S.; Boggs, C.; Armstrong, M.D.; Call, D.F.; Coronell, O. Impact of natural organic matter and inorganic solutes on energy recovery from five real salinity gradients using reverse electrodialysis. J. Membr. Sci. 2017, 541, 621–632. [Google Scholar] [CrossRef]
- Lee, H.J.; Oh, S.J.; Moon, S.H. Recovery of ammonium sulfate from fermentation waste by electrodialysis. Water Res. 2003, 37, 1091–1099. [Google Scholar] [CrossRef]
- Luiz, A.; McClure, D.D.; Lim, K.; Leslie, G.; Coster, H.G.L.; Barton, G.W.; Kavanagh, J.M. Potential upgrading of bio-refinery streams by electrodialysis. Desalination 2017, 415, 20–28. [Google Scholar] [CrossRef]
- Luiz, A.; Mcclure, D.D.; Lim, K.; Coster, H.G.L.; Barton, G.W.; Kavanagh, J.M. Towards a model for the electrodialysis of bio-refinery streams. J. Membr. Sci. 2019, 573, 320–332. [Google Scholar] [CrossRef]
- Barros, L.B.M.; Andrade, L.H.; Drewes, J.E.; Amaral, M.C.S. Investigation of electrodialysis configurations for vinasse desalting and potassium recovery. Sep. Purif. Technol. 2019, 229, 115797. [Google Scholar] [CrossRef]
- Milewski, A.; Czechowicz, D.; Jakóbik-Kolon, A.; Dydo, P. Preparation of Glycidol via Dehydrohalogenation of 3-Chloro-1,2-popanediol Using Bipolar Membrane Electrodialysis. ACS Sustain. Chem. Eng. 2019, 7, 18640–18646. [Google Scholar] [CrossRef]
- Lu, H.; Zou, W.; Chai, P.; Wang, J.; Bazinet, L. Feasibility of antibiotic and sulfate ions separation from wastewater using electrodialysis with ultrafiltration membrane. J. Clean. Prod. 2016, 112, 3097–3105. [Google Scholar] [CrossRef]
- Silva, V.; Poiesz, E.; Van Der Heijden, P. Industrial wastewater desalination using electrodialysis: Evaluation and plant design. J. Appl. Electrochem. 2013, 43, 1057–1067. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhang, X.; Feng, H.; Li, C.; Xu, T. Phosphate recovery from excess sludge by conventional electrodialysis (CED) and electrodialysis with bipolar membranes (EDBM). Ind. Eng. Chem. Res. 2013, 52, 15896–15904. [Google Scholar] [CrossRef]
- Zhang, Y.; Desmidt, E.; Van Looveren, A.; Pinoy, L.; Meesschaert, B.; Van Der Bruggen, B. Phosphate separation and recovery from wastewater by novel electrodialysis. Environ. Sci. Technol. 2013, 47, 5888–5895. [Google Scholar] [CrossRef]
- Abou-Shady, A. Recycling of polluted wastewater for agriculture purpose using electrodialysis: Perspective for large scale application. Chem. Eng. J. 2017, 323, 1–18. [Google Scholar] [CrossRef]
- Besseris, G.J. Concurrent multiresponse multifactorial screening of an electrodialysis process of polluted wastewater using robust non-linear Taguchi profiling. Chemom. Intell. Lab. Syst. 2020, 200, 103997. [Google Scholar] [CrossRef]
- Allison, R.P. Electrodialysis reversal in water reuse applications. Desalination 1995, 103, 11–18. [Google Scholar] [CrossRef]
- Del Pino, M.P.; Durham, B. Wastewater reuse through dual-membrane processes: Opportunities for sustainable water resources. Desalination 1999, 124, 271–277. [Google Scholar] [CrossRef]
- Gotor, A.G.; Pérez Baez, S.O.; Espinoza, C.A.; Bachir, S.I. Membrane processes for the recovery and reuse of wastewater in agriculture. Desalination 2001, 137, 187–192. [Google Scholar] [CrossRef]
- Kim, J.O.; Jung, J.T.; Chung, J. Treatment performance of metal membrane microfiltration and electrodialysis integrated system for wastewater reclamation. Desalination 2007, 202, 343–350. [Google Scholar] [CrossRef]
- Goodman, N.B.; Taylor, R.J.; Xie, Z.; Gozukara, Y.; Clements, A. A feasibility study of municipal wastewater desalination using electrodialysis reversal to provide recycled water for horticultural irrigation. Desalination 2013, 317, 77–83. [Google Scholar] [CrossRef]
- Llanos, J.; Cotillas, S.; Cañizares, P.; Rodrigo, M.A. Novel electrodialysis-electrochlorination integrated process for the reclamation of treated wastewaters. Sep. Purif. Technol. 2014, 132, 362–369. [Google Scholar] [CrossRef]
- Snyder, S.A.; Redding, A.M.; Yoon, Y.; Adham, S.; Wert, E.C.; Cannon, F.S.; Oppenheimer, J.; DeCarolis, J. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 2006, 202, 156–181. [Google Scholar] [CrossRef]
- Guedes-Alonso, R.; Montesdeoca-Esponda, S.; Pacheco-Juárez, J.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. A survey of the presence of pharmaceutical residues in wastewaters. Evaluation of their removal using conventional and natural treatment procedures. Molecules 2020, 25, 1639. [Google Scholar] [CrossRef] [Green Version]
- Gally, C.R.; Benvenuti, T.; Da Trindade, C.D.M.; Rodrigues, M.A.S.; Zoppas-Ferreira, J.; Pérez-Herranz, V.; Bernardes, A.M. Electrodialysis for the tertiary treatment of municipal wastewater: Efficiency of ion removal and ageing of ion exchange membranes. J. Environ. Chem. Eng. 2018, 6, 5855–5869. [Google Scholar] [CrossRef]
- Albornoz, L.L.; Marder, L.; Benvenuti, T.; Bernardes, A.M. Electrodialysis applied to the treatment of an university sewage for water recovery. J. Environ. Chem. Eng. 2019, 7, 102982. [Google Scholar] [CrossRef]
- Zhang, Y.; Pinoy, L.; Meesschaert, B.; Van Der Bruggen, B. A natural driven membrane process for brackish and wastewater treatment: Photovoltaic powered ED and FO hybrid system. Environ. Sci. Technol. 2013, 47, 10548–10555. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; He, Z. Mitigation of Salinity Buildup and Recovery of Wasted Salts in a Hybrid Osmotic Membrane Bioreactor-Electrodialysis System. Environ. Sci. Technol. 2015, 49, 10529–10535. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; He, Z. Electrodialysis recovery of reverse-fluxed fertilizer draw solute during forward osmosis water treatment. Chem. Eng. J. 2017, 330, 550–558. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Co-locating reverse electrodialysis with reverse osmosis desalination: Synergies and implications. J. Membr. Sci. 2017, 539, 305–312. [Google Scholar] [CrossRef]
- Li, W.; Krantz, W.B.; Cornelissen, E.R.; Post, J.W.; Verliefde, A.R.D.; Tang, C.Y. A novel hybrid process of reverse electrodialysis and reverse osmosis for low energy seawater desalination and brine management. Appl. Energy 2013, 104, 592–602. [Google Scholar] [CrossRef]
- Vanoppen, M.; Criel, E.; Walpot, G.; Vermaas, D.A.; Verliefde, A. Assisted reverse electrodialysis—principles, mechanisms, and potential. npj Clean Water 2018, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- La Cerva, M.; Gurreri, L.; Cipollina, A.; Tamburini, A.; Ciofalo, M.; Micale, G. Modelling and cost analysis of hybrid systems for seawater desalination: Electromembrane pre-treatments for Reverse Osmosis. Desalination 2019, 467, 175–195. [Google Scholar] [CrossRef]
- Roman, M.; Van Dijk, L.H.; Gutierrez, L.; Vanoppen, M.; Post, J.W.; Wols, B.A.; Cornelissen, E.R.; Verliefde, A.R.D. Key physicochemical characteristics governing organic micropollutant adsorption and transport in ion-exchange membranes during reverse electrodialysis. Desalination 2019, 468, 114084. [Google Scholar] [CrossRef]
- Nam, J.Y.; Hwang, K.S.; Kim, H.C.; Jeong, H.; Kim, H.; Jwa, E.; Yang, S.C.; Choi, J.; Kim, C.S.; Han, J.H.; et al. Assessing the behavior of the feed-water constituents of a pilot-scale 1000-cell-pair reverse electrodialysis with seawater and municipal wastewater effluent. Water Res. 2019, 148, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Higa, M.; Watanabe, T.; Yasukawa, M.; Endo, N.; Kakihana, Y.; Futamura, H.; Inoue, K.; Miyake, H.; Usui, J.; Hayashi, A.; et al. Sustainable hydrogen production from seawater and sewage treated water using reverse electrodialysis technology. Water Pract. Technol. 2019, 14, 645–651. [Google Scholar] [CrossRef]
- Vanoppen, M.; van Vooren, T.; Gutierrez, L.; Roman, M.; Croué, L.-P.; Verbeken, K.; Philips, J.; Verliefde, A.R.D. Secondary treated domestic wastewater in reverse electrodialysis: What is the best pre-treatment? Sep. Purif. Technol. 2019, 218, 25–42. [Google Scholar] [CrossRef] [Green Version]
- Mehdizadeh, S.; Yasukawa, M.; Suzuki, T.; Higa, M. Reverse electrodialysis for power generation using seawater/municipal wastewater: Effect of coagulation pretreatment. Desalination 2020, 481, 114356. [Google Scholar] [CrossRef]
- Gómez-Coma, L.; Ortiz-Martínez, V.M.; Fallanza, M.; Ortiz, A.; Ibañez, R.; Ortiz, I. Blue energy for sustainable water reclamation in WWTPs. J. Water Process. Eng. 2020, 33, 101020. [Google Scholar] [CrossRef]
- Luque Di Salvo, J.; Cosenza, A.; Tamburini, A.; Micale, G.; Cipollina, A. Long-run operation of a reverse electrodialysis system fed with wastewaters. J. Environ. Manag. 2018, 217, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Mercer, E.; Davey, C.J.; Azzini, D.; Eusebi, A.L.; Tierney, R.; Williams, L.; Jiang, Y.; Parker, A.; Tyrrel, S.; Cartmell, E.; et al. Hybrid membrane distillation reverse electrodialysis configuration for water and energy recovery from human urine: An opportunity for off-grid decentralised sanitation. J. Membr. Sci. 2019, 584, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, L.; Van der Bruggen, B.; Verstraete, W.; Rabaey, K.; Meesschaert, B. Global Phosphorus Scarcity and Full-Scale P-Recovery Techniques: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Tran, A.T.K.; Zhang, Y.; De Corte, D.; Hannes, J.-B.; Ye, W.; Mondal, P.; Jullok, N.; Meesschaert, B.; Pinoy, L.; Van der Bruggen, B. P-recovery as calcium phosphate from wastewater using an integrated selectrodialysis/crystallization process. J. Clean. Prod. 2014, 77, 140–151. [Google Scholar] [CrossRef]
- Tran, A.T.K.; Zhang, Y.; Lin, J.; Mondal, P.; Ye, W.; Meesschaert, B.; Pinoy, L.; Van Der Bruggen, B. Phosphate pre-concentration from municipal wastewater by selectrodialysis: Effect of competing components. Sep. Purif. Technol. 2015, 141, 38–47. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Wu, G.; Luo, J.; Wang, S. Development of a selective electrodialysis for nutrient recovery and desalination during secondary effluent treatment. Chem. Eng. J. 2017, 322, 224–233. [Google Scholar] [CrossRef]
- Nur, H.M.; Yüzer, B.; Aydin, M.İ.; Aydin, S.; Öngen, A.; Selçuk, H. Desalination and fate of nutrient transport in domestic wastewater using electrodialysis membrane process. Desalin. Water Treat. 2019, 172, 323–329. [Google Scholar] [CrossRef]
- Rotta, E.H.; Bitencourt, C.S.; Marder, L.; Bernardes, A.M. Phosphorus recovery from low phosphate-containing solution by electrodialysis. J. Membr. Sci. 2019, 573, 293–300. [Google Scholar] [CrossRef]
- Rybalkina, O.; Tsygurina, K.; Melnikova, E.; Mareev, S.; Moroz, I.; Nikonenko, V.; Pismenskaya, N. Partial Fluxes of Phosphoric Acid Anions through Anion-Exchange Membranes in the Course of NaH2PO4 Solution Electrodialysis. Int. J. Mol. Sci. 2019, 20, 3593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pismenskaya, N.; Sarapulova, V.; Nevakshenova, E.; Kononenko, N.; Fomenko, M.; Nikonenko, V. Concentration dependencies of diffusion permeability of anion-exchange membranes in sodium hydrogen carbonate, monosodium phosphate, and potassium hydrogen tartrate solutions. Membranes 2019, 9, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Han, Z.; Lin, X.; Duan, Y.; Du, J.; Ye, Z.; Zhu, J. Study on removal of phosphorus as struvite from synthetic wastewater using a pilot-scale electrodialysis system with magnesium anode. Sci. Total Environ. 2020, 726, 138221. [Google Scholar] [CrossRef]
- Vineyard, D.; Hicks, A.; Karthikeyan, K.G.; Barak, P. Economic analysis of electrodialysis, denitrification, and anammox for nitrogen removal in municipal wastewater treatment. J. Clean. Prod. 2020, 262, 121145. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Wang, Y.; Du, Y.; Feng, H.; Xu, T. Simultaneous recovery of ammonium and phosphorus via the integration of electrodialysis with struvite reactor. J. Membr. Sci. 2015, 490, 65–71. [Google Scholar] [CrossRef]
- Thompson Brewster, E.; Ward, A.J.; Mehta, C.M.; Radjenovic, J.; Batstone, D.J. Predicting scale formation during electrodialytic nutrient recovery. Water Res. 2017, 110, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Ward, A.J.; Arola, K.; Thompson Brewster, E.; Mehta, C.M.; Batstone, D.J. Nutrient recovery from wastewater through pilot scale electrodialysis. Water Res. 2018, 135, 57–65. [Google Scholar] [CrossRef]
- Arola, K.; Ward, A.; Mänttäri, M.; Kallioinen, M.; Batstone, D. Transport of pharmaceuticals during electrodialysis treatment of wastewater. Water Res. 2019, 161, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Van Linden, N.; Spanjers, H.; van Lier, J.B. Application of dynamic current density for increased concentration factors and reduced energy consumption for concentrating ammonium by electrodialysis. Water Res. 2019, 163, 114856. [Google Scholar] [CrossRef]
- Vecino, X.; Reig, M.; Gibert, O.; Valderrama, C.; Cortina, J.L. Integration of liquid-liquid membrane contactors and electrodialysis for ammonium recovery and concentration as a liquid fertilizer. Chemosphere 2020, 245, 125606. [Google Scholar] [CrossRef]
- Gao, F.; Wang, L.; Wang, J.; Zhang, H.; Lin, S. Nutrient recovery from treated wastewater by a hybrid electrochemical sequence integrating bipolar membrane electrodialysis and membrane capacitive deionization. Environ. Sci. Water Res. Technol. 2020, 6, 383–391. [Google Scholar] [CrossRef]
- Tao, B.; Passanha, P.; Kumi, P.; Wilson, V.; Jones, D.; Esteves, S. Recovery and concentration of thermally hydrolysed waste activated sludge derived volatile fatty acids and nutrients by microfiltration, electrodialysis and struvite precipitation for polyhydroxyalkanoates production. Chem. Eng. J. 2016, 295, 11–19. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Latorre-Sánchez, M.; Coll Lozano, C.; Venus, J. Organic fraction of municipal solid waste for the production of L-lactic acid with high optical purity. J. Clean. Prod. 2020, 247, 119165. [Google Scholar] [CrossRef]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The characterization of feces and urine: A review of the literature to inform advanced treatment technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef] [Green Version]
- Escher, B.I.; Pronk, W.; Suter, M.J.F.; Maurer, M. Monitoring the removal efficiency of pharmaceuticals and hormones in different treatment processes of source-separated urine with bioassays. Environ. Sci. Technol. 2006, 40, 5095–5101. [Google Scholar] [CrossRef] [PubMed]
- Pronk, W.; Biebow, M.; Boller, M. Electrodialysis for recovering salts from a urine solution containing micropollutants. Environ. Sci. Technol. 2006, 40, 2414–2420. [Google Scholar] [CrossRef]
- Pronk, W.; Zuleeg, S.; Lienert, J.; Escher, B.; Koller, M.; Berner, A.; Koch, G.; Boller, M. Pilot experiments with electrodialysis and ozonation for the production of a fertiliser from urine. Water Sci. Technol. 2007, 56, 219–227. [Google Scholar] [CrossRef]
- Pronk, W.; Koné, D. Options for urine treatment in developing countries. Desalination 2009, 248, 360–368. [Google Scholar] [CrossRef]
- De Paepe, J.; Lindeboom, R.E.F.; Vanoppen, M.; De Paepe, K.; Demey, D.; Coessens, W.; Lamaze, B.; Verliefde, A.R.D.; Clauwaert, P.; Vlaeminck, S.E. Refinery and concentration of nutrients from urine with electrodialysis enabled by upstream precipitation and nitrification. Water Res. 2018, 144, 76–86. [Google Scholar] [CrossRef]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid-liquid separation of animal slurry in theory and practice. Sustain. Agric. 2009, 2, 953–986. [Google Scholar]
- Nasir, I.M.; Mohd Ghazi, T.I.; Omar, R. Anaerobic digestion technology in livestock manure treatment for biogas production: A review. Eng. Life Sci. 2012, 12, 258–269. [Google Scholar] [CrossRef]
- FAO. Food Outlook, Biannual Report on Global Food Markets; FAO: Rome, Italy, 2017. [Google Scholar]
- Mondor, M.; Masse, L.; Ippersiel, D.; Lamarche, F.; Massé, D.I. Use of electrodialysis and reverse osmosis for the recovery and concentration of ammonia from swine manure. Bioresour. Technol. 2008, 99, 7363–7368. [Google Scholar] [CrossRef]
- Mondor, M.; Ippersiel, D.; Lamarche, F.; Masse, L. Fouling characterization of electrodialysis membranes used for the recovery and concentration of ammonia from swine manure. Bioresour. Technol. 2009, 100, 566–571. [Google Scholar] [CrossRef]
- Ippersiel, D.; Mondor, M.; Lamarche, F.; Tremblay, F.; Dubreuil, J.; Masse, L. Nitrogen potential recovery and concentration of ammonia from swine manure using electrodialysis coupled with air stripping. J. Environ. Manag. 2012, 95, S165–S169. [Google Scholar] [CrossRef]
- Shi, L.; Xie, S.; Hu, Z.; Wu, G.; Morrison, L.; Croot, P.; Hu, H.; Zhan, X. Nutrient recovery from pig manure digestate using electrodialysis reversal: Membrane fouling and feasibility of long-term operation. J. Membr. Sci. 2019, 573, 560–569. [Google Scholar] [CrossRef]
- Shi, L.; Hu, Z.; Simplicio, W.S.; Qiu, S.; Xiao, L.; Harhen, B.; Zhan, X. Antibiotics in nutrient recovery from pig manure via electrodialysis reversal: Sorption and migration associated with membrane fouling. J. Membr. Sci. 2020, 597, 117633. [Google Scholar] [CrossRef]
- Ye, Z.L.; Ghyselbrecht, K.; Monballiu, A.; Pinoy, L.; Meesschaert, B. Fractionating various nutrient ions for resource recovery from swine wastewater using simultaneous anionic and cationic selective-electrodialysis. Water Res. 2019, 160, 424–434. [Google Scholar] [CrossRef]
- Shi, L.; Hu, Y.; Xie, S.; Wu, G.; Hu, Z.; Zhan, X. Recovery of nutrients and volatile fatty acids from pig manure hydrolysate using two-stage bipolar membrane electrodialysis. Chem. Eng. J. 2018, 334, 134–142. [Google Scholar] [CrossRef]
- Duong, H.C.; Ansari, A.J.; Nghiem, L.D.; Cao, H.T.; Vu, T.D.; Nguyen, T.P. Membrane Processes for the Regeneration of Liquid Desiccant Solution for Air Conditioning. Curr. Pollut. Rep. 2019, 5, 308–318. [Google Scholar] [CrossRef]
- Li, X.W.; Zhang, X.S. Photovoltaic-electrodialysis regeneration method for liquid desiccant cooling system. Sol. Energy 2009, 83, 2195–2204. [Google Scholar] [CrossRef]
- Li, X.W.; Zhang, X.S.; Quan, S. Single-stage and double-stage photovoltaic driven regeneration for liquid desiccant cooling system. Appl. Energy 2011, 88, 4908–4917. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, X.S.; Li, X.W. Double-stage photovoltaic/thermal ED regeneration for liquid desiccant cooling system. Energy Build. 2012, 51, 64–72. [Google Scholar] [CrossRef]
- Cheng, Q.; Xu, Y.; Zhang, X.S. Experimental investigation of an electrodialysis regenerator for liquid desiccant. Energy Build. 2013, 67, 419–425. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, X.; Jiao, S. Influence of concentration difference between dilute cells and regenerate cells on the performance of electrodialysis regenerator. Energy 2017, 140, 646–655. [Google Scholar] [CrossRef]
- Cheng, Q.; Jiao, S. Experimental and theoretical research on the current efficiency of the electrodialysis regenerator for liquid desiccant air-conditioning system using LiCl solution. Int. J. Refrig. 2018, 96, 1–9. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, Z.; Al-Jubainawi, A.; Cooper, P.; Nghiem, L.D. Using electrodialysis for regeneration of aqueous lithium chloride solution in liquid desiccant air conditioning systems. Energy Build. 2016, 116, 285–295. [Google Scholar] [CrossRef]
- Al-Jubainawi, A.; Ma, Z.; Guo, Y.; Nghiem, L.D.; Cooper, P.; Li, W. Factors governing mass transfer during membrane electrodialysis regeneration of LiCl solution for liquid desiccant dehumidification systems. Sustain. Cities Soc. 2017, 28, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Al-Jubainawi, A.; Ma, Z. Performance investigation and optimisation of electrodialysis regeneration for LiCl liquid desiccant cooling systems. Appl. Therm. Eng. 2019, 149, 1023–1034. [Google Scholar] [CrossRef]
- Guo, Y.; Al-Jubainawi, A.; Peng, X. Modelling and the feasibility study of a hybrid electrodialysis and thermal regeneration method for LiCl liquid desiccant dehumidification. Appl. Energy 2019, 239, 1014–1036. [Google Scholar] [CrossRef]
- Al-Jubainawi, A.; Ma, Z.; Guo, Y.; Nghiem, L.D. Effect of regulating main governing factors on the selectivity membranes of electrodialysis used for LiCl liquid desiccant regeneration. J. Build. Eng. 2020, 28, 101022. [Google Scholar] [CrossRef]
- Pei, W.; Cheng, Q.; Jiao, S.; Liu, L. Performance evaluation of the electrodialysis regenerator for the lithium bromide solution with high concentration in the liquid desiccant air-conditioning system. Energy 2019, 187, 115928. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, M.; Huang, S.; Su, W.; Zhou, J.; Zhang, X. Performance evaluation on regeneration of high-salt solutions used in air conditioning systems by electrodialysis. J. Membr. Sci. 2019, 582, 224–235. [Google Scholar] [CrossRef]
- International Desalination Association (IDA). Desalination Yearbook 2016–2017; International Desalination Association (IDA): Topsfield, MA, USA, 2017. [Google Scholar]
- Jones, E.; Qadir, M.; van Vliet, M.T.H.; Smakhtin, V.; Kang, S. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Gude, V.G. Desalination and sustainability–An appraisal and current perspective. Water Res. 2016, 89, 87–106. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2012; ISBN 9781118359686. [Google Scholar]
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; Chabib, C.M.; Mustafa, I.; Al Ghaferi, A.; AlMarzooqi, F. Brine management in desalination industry: From waste to resources generation. Desalination 2019, 472, 114187. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.J.; Loizidou, M. Desalination brine disposal methods and treatment technologies—A review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef]
- Korngold, E.; Aronov, L.; Daltrophe, N. Electrodialysis of brine solutions discharged from an RO plant. Desalination 2009, 242, 215–227. [Google Scholar] [CrossRef]
- Oren, Y.; Korngold, E.; Daltrophe, N.; Messalem, R.; Volkman, Y.; Aronov, L.; Weismann, M.; Bouriakov, N.; Glueckstern, P.; Gilron, J. Pilot studies on high recovery BWRO-EDR for near zero liquid discharge approach. Desalination 2010, 261, 321–330. [Google Scholar] [CrossRef]
- Xu, X.; Lin, L.; Ma, G.; Wang, H.; Jiang, W.; He, Q.; Nirmalakhandan, N.; Xu, P. Study of polyethyleneimine coating on membrane permselectivity and desalination performance during pilot-scale electrodialysis of reverse osmosis concentrate. Sep. Purif. Technol. 2018, 207, 396–405. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.; Wu, L.; Shehzad, M.A.; Jiang, C.; Fu, R.; Liu, Z.; Xu, T. Multistage-batch electrodialysis to concentrate high-salinity solutions: Process optimisation, water transport, and energy consumption. J. Membr. Sci. 2019, 570–571, 245–257. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Zhang, Z.; Xu, T. Electrodialysis of concentrated brine from RO plant to produce coarse salt and freshwater. J. Membr. Sci. 2014, 450, 323–330. [Google Scholar] [CrossRef]
- Reig, M.; Casas, S.; Aladjem, C.; Valderrama, C.; Gibert, O.; Valero, F.; Centeno, C.M.; Larrotcha, E.; Cortina, J.L. Concentration of NaCl from seawater reverse osmosis brines for the chlor-alkali industry by electrodialysis. Desalination 2014, 342, 107–117. [Google Scholar] [CrossRef]
- Tanaka, Y.; Reig, M.; Casas, S.; Aladjem, C.; Cortina, J.L. Computer simulation of ion-exchange membrane electrodialysis for salt concentration and reduction of RO discharged brine for salt production and marine environment conservation. Desalination 2015, 367, 76–89. [Google Scholar] [CrossRef]
- Reig, M.; Farrokhzad, H.; Van der Bruggen, B.; Gibert, O.; Cortina, J.L. Synthesis of a monovalent selective cation exchange membrane to concentrate reverse osmosis brines by electrodialysis. Desalination 2015, 375, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Miao, M.; Pan, J.; Sotto, A.; Shen, J.; Gao, C.; Van der Bruggen, B. Separation of divalent ions from seawater concentrate to enhance the purity of coarse salt by electrodialysis with monovalent-selective membranes. Desalination 2017, 411, 28–37. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, J.; Ji, Z.; Wang, B.; Hao, Y.; Guo, X. Concentrating brine from seawater desalination process by nanofiltration-electrodialysis integrated membrane technology. Desalination 2016, 390, 53–61. [Google Scholar] [CrossRef]
- McGovern, R.K.; Zubair, S.M.; Lienhard, V.J.H. The benefits of hybridising electrodialysis with reverse osmosis. J. Membr. Sci. 2014, 469, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.W.; Nayar, K.G.; Swaminathan, J.; Chehayeb, K.M.; Lienhard, V.J.H. Thermodynamic analysis of brine management methods: Zero-discharge desalination and salinity-gradient power production. Desalination 2017, 404, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Chehayeb, K.M.; Farhat, D.M.; Nayar, K.G.; Lienhard, J.H. Optimal design and operation of electrodialysis for brackish-water desalination and for high-salinity brine concentration. Desalination 2017, 420, 167–182. [Google Scholar] [CrossRef]
- Chehayeb, K.M.; Nayar, K.G.; Lienhard, J.H. On the merits of using multi-stage and counterflow electrodialysis for reduced energy consumption. Desalination 2018, 439, 1–16. [Google Scholar] [CrossRef]
- Nayar, K.G.; Fernandes, J.; McGovern, R.K.; Dominguez, K.P.; McCance, A.; Al-Anzi, B.S.; Lienhard, J.H. Cost and energy requirements of hybrid RO and ED brine concentration systems for salt production. Desalination 2019, 456, 97–120. [Google Scholar] [CrossRef]
- Nayar, K.G.; Fernandes, J.; McGovern, R.K.; Al-Anzi, B.S.; Lienhard, J.H. Cost and energy needs of RO-ED-crystallizer systems for zero brine discharge seawater desalination. Desalination 2019, 457, 115–132. [Google Scholar] [CrossRef]
- Cappelle, M.; Walker, W.S.; Davis, T.A. Improving Desalination Recovery Using Zero Discharge Desalination (ZDD): A Process Model for Evaluating Technical Feasibility. Ind. Eng. Chem. Res. 2017, 56, 10448–10460. [Google Scholar] [CrossRef]
- Reahl, E.R. Reclaiming reverse osmosis blowdown with electrodialysis reversal. Desalination 1990, 78, 77–89. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghyselbrecht, K.; Meesschaert, B.; Pinoy, L.; Van der Bruggen, B. Electrodialysis on RO concentrate to improve water recovery in wastewater reclamation. J. Membr. Sci. 2011, 378, 101–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghyselbrecht, K.; Vanherpe, R.; Meesschaert, B.; Pinoy, L.; Van der Bruggen, B. RO concentrate minimization by electrodialysis: Techno-economic analysis and environmental concerns. J. Environ. Manag. 2012, 107, 28–36. [Google Scholar] [CrossRef]
- Tran, A.T.K.; Zhang, Y.; Jullok, N.; Meesschaert, B.; Pinoy, L.; Van der Bruggen, B. RO concentrate treatment by a hybrid system consisting of a pellet reactor and electrodialysis. Chem. Eng. Sci. 2012, 79, 228–238. [Google Scholar] [CrossRef]
- Ghyselbrecht, K.; Van Houtte, E.; Pinoy, L.; Verbauwhede, J.; Van Der Bruggen, B.; Meesschaert, B. Treatment of RO concentrate by means of a combination of a willow field and electrodialysis. Resour. Conserv. Recycl. 2012, 65, 116–123. [Google Scholar] [CrossRef]
- Ribera-Pi, J.; Badia-Fabregat, M.; Espí, J.; Clarens, F.; Jubany, I.; Martínez-Lladó, X. Decreasing environmental impact of landfill leachate treatment by MBR, RO and EDR hybrid treatment. Environ. Technol. (UK) 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Van der Bruggen, B.; Pinoy, L.; Meesschaert, B. Separation of nutrient ions and organic compounds from salts in RO concentrates by standard and monovalent selective ion-exchange membranes used in electrodialysis. J. Membr. Sci. 2009, 332, 104–112. [Google Scholar] [CrossRef]
- Banasiak, L.J.; Van der Bruggen, B.; Schäfer, A.I. Sorption of pesticide endosulfan by electrodialysis membranes. Chem. Eng. J. 2011, 166, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Venzke, C.D.; Giacobbo, A.; Ferreira, J.Z.; Bernardes, A.M.; Rodrigues, M.A.S. Increasing water recovery rate of membrane hybrid process on the petrochemical wastewater treatment. Process. Saf. Environ. Prot. 2018, 117, 152–158. [Google Scholar] [CrossRef]
- Zhao, D.; Lee, L.Y.; Ong, S.L.; Chowdhury, P.; Siah, K.B.; Ng, H.Y. Electrodialysis reversal for industrial reverse osmosis brine treatment. Sep. Purif. Technol. 2019, 213, 339–347. [Google Scholar] [CrossRef]
- Van Linden, N.; Shang, R.; Stockinger, G.; Heijman, B.; Spanjers, H. Separation of natural organic matter and sodium chloride for salt recovery purposes in zero liquid discharge. Water Resour. Ind. 2020, 23, 100117. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Y.; Ma, Z.; Wang, R.; Wang, X.; Wang, J.; Wang, L.; Gao, X.; Gao, J. Response surface modeling and optimization of electrodialysis for reclamation of RO concentrates in coal-fired power plants. Sep. Sci. Technol. 2019, 1–11. [Google Scholar] [CrossRef]
- Qiu, Y.; Ruan, H.; Tang, C.; Yao, L.; Shen, J.; Sotto, A. Study on Recovering High-Concentration Lithium Salt from Lithium-Containing Wastewater Using a Hybrid Reverse Osmosis (RO)–Electrodialysis (ED) Process. ACS Sustain. Chem. Eng. 2019, 7, 13481–13490. [Google Scholar] [CrossRef]
- Vaudevire, E.; Radmanesh, F.; Kolkman, A.; Vughs, D.; Cornelissen, E.; Post, J.; van der Meer, W. Fate and removal of trace pollutants from an anion exchange spent brine during the recovery process of natural organic matter and salts. Water Res. 2019, 154, 34–44. [Google Scholar] [CrossRef]
- Haddad, M.; Bazinet, L.; Barbeau, B. Eco-efficient treatment of ion exchange spent brine via electrodialysis to recover NaCl and minimize waste disposal. Sci. Total Environ. 2019, 690, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, R.; Pérez-González, A.; Gómez, P.; Urtiaga, A.M.; Ortiz, I. Acid and base recovery from softened reverse osmosis (RO) brines. Experimental assessment using model concentrates. Desalination 2013, 309, 165–170. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, C.; Dominguez-Ramos, A.; Ibañez, R.; Chen, Y.; Irabien, A. Valorization of desalination brines by electrodialysis with bipolar membranes using nanocomposite anion exchange membranes. Desalination 2017, 406, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Gonzalez, M.; Diaz-Guridi, P.; Dominguez-Ramos, A.; Ibañez, R.; Irabien, A. Photovoltaic solar electrodialysis with bipolar membranes. Desalination 2018, 433, 155–163. [Google Scholar] [CrossRef]
- Herrero-Gonzalez, M.; Diaz-Guridi, P.; Dominguez-Ramos, A.; Irabien, A.; Ibañez, R. Highly concentrated HCl and NaOH from brines using electrodialysis with bipolar membranes. Sep. Purif. Technol. 2020, 242, 116785. [Google Scholar] [CrossRef]
- Herrero-Gonzalez, M.; Admon, N.; Dominguez-Ramos, A.; Ibañez, R.; Wolfson, A.; Irabien, A. Environmental sustainability assessment of seawater reverse osmosis brine valorization by means of electrodialysis with bipolar membranes. Environ. Sci. Pollut. Res. 2020, 27, 1256–1266. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, X.; Fan, A.; Fu, L.; Gao, C. An innovative beneficial reuse of seawater concentrate using bipolar membrane electrodialysis. J. Membr. Sci. 2014, 449, 119–126. [Google Scholar] [CrossRef]
- Reig, M.; Casas, S.; Gibert, O.; Valderrama, C.; Cortina, J.L. Integration of nanofiltration and bipolar electrodialysis for valorization of seawater desalination brines: Production of drinking and waste water treatment chemicals. Desalination 2016, 382, 13–20. [Google Scholar] [CrossRef]
- Reig, M.; Casas, S.; Valderrama, C.; Gibert, O.; Cortina, J.L. Integration of monopolar and bipolar electrodialysis for valorization of seawater reverse osmosis desalination brines: Production of strong acid and base. Desalination 2016, 398, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Jiang, C.; Wang, Y.; Fu, R.; Liu, Z.; Xu, T. Selectrodialysis with bipolar membrane for the reclamation of concentrated brine from RO plant. Desalination 2018, 442, 8–15. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Oppenheimer, J.; Adham, S.; Kumar, M. Innovative beneficial reuse of reverse osmosis concentrate using bipolar membrane electrodialysis and electrochlorination processes. J. Membr. Sci. 2009, 326, 392–399. [Google Scholar] [CrossRef]
- Wang, M.; Wang, K.; Jia, Y.; Ren, Q. The reclamation of brine generated from desalination process by bipolar membrane electrodialysis. J. Membr. Sci. 2014, 452, 54–61. [Google Scholar] [CrossRef]
- Yip, N.Y.; Brogioli, D.; Hamelers, H.V.M.; Nijmeijer, K. Salinity gradients for sustainable energy: Primer, progress, and prospects. Environ. Sci. Technol. 2016, 50, 12072–12094. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.; Park, Y.G.; Shon, H.; Ahn, C.H.; Kim, S.H. Hybrid desalination processes for beneficial use of reverse osmosis brine: Current status and future prospects. Desalination 2019, 454, 104–111. [Google Scholar] [CrossRef]
- Tufa, R.A.; Noviello, Y.; Di Profio, G.; Macedonio, F.; Ali, A.; Drioli, E.; Fontananova, E.; Bouzek, K.; Curcio, E. Integrated membrane distillation-reverse electrodialysis system for energy-efficient seawater desalination. Appl. Energy 2019, 253, 113551. [Google Scholar] [CrossRef]
- Choi, J.; Oh, Y.; Chae, S.; Hong, S. Membrane capacitive deionization-reverse electrodialysis hybrid system for improving energy efficiency of reverse osmosis seawater desalination. Desalination 2019, 462, 19–28. [Google Scholar] [CrossRef]
- Luo, F.; Wang, Y.; Jiang, C.; Wu, B.; Feng, H.; Xu, T. A power free electrodialysis (PFED) for desalination. Desalination 2017, 404, 138–146. [Google Scholar] [CrossRef]
- Mei, Y.; Li, X.; Yao, Z.; Qing, W.; Fane, A.G.; Tang, C.Y. Simulation of an energy self-sufficient electrodialysis desalination stack for salt removal efficiency and fresh water recovery. J. Membr. Sci. 2020, 598, 117771. [Google Scholar] [CrossRef]
- Kwon, K.; Han, J.; Park, B.H.; Shin, Y.; Kim, D. Brine recovery using reverse electrodialysis in membrane-based desalination processes. Desalination 2015, 362, 1–10. [Google Scholar] [CrossRef]
- Tufa, R.A.; Curcio, E.; Brauns, E.; van Baak, W.; Fontananova, E.; Di Profio, G. Membrane Distillation and Reverse Electrodialysis for Near-Zero Liquid Discharge and low energy seawater desalination. J. Membr. Sci. 2015, 496, 325–333. [Google Scholar] [CrossRef]
- Tufa, R.A.; Rugiero, E.; Chanda, D.; Hnàt, J.; van Baak, W.; Veerman, J.; Fontananova, E.; Di Profio, G.; Drioli, E.; Bouzek, K.; et al. Salinity gradient power-reverse electrodialysis and alkaline polymer electrolyte water electrolysis for hydrogen production. J. Membr. Sci. 2016, 514, 155–164. [Google Scholar] [CrossRef]
- Mehdizadeh, S.; Yasukawa, M.; Kuno, M.; Kawabata, Y.; Higa, M. Evaluation of energy harvesting from discharge solutions in a salt production plant by reverse electrodialysis (RED). Desalination 2019, 467, 95–102. [Google Scholar] [CrossRef]
- Tian, H.; Wang, Y.; Pei, Y. Energy capture from thermolytic solutions and simulated sunlight coupled with hydrogen peroxide production and wastewater remediation. Water Res. 2020, 170, 115318. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, D.; Xu, H. The feasibility and mechanism of reverse electrodialysis enhanced photocatalytic fuel cell-Fenton system on advanced treatment of coal gasification wastewater. Sep. Purif. Technol. 2019, 220, 183–188. [Google Scholar] [CrossRef]
- D’Angelo, A.; Tedesco, M.; Cipollina, A.; Galia, A.; Micale, G.; Scialdone, O. Reverse electrodialysis performed at pilot plant scale: Evaluation of redox processes and simultaneous generation of electric energy and treatment of wastewater. Water Res. 2017, 125, 123–131. [Google Scholar] [CrossRef]
- Ma, P.; Hao, X.; Galia, A.; Scialdone, O. Development of a process for the treatment of synthetic wastewater without energy inputs using the salinity gradient of wastewaters and a reverse electrodialysis stack. Chemosphere 2020, 248, 125994. [Google Scholar] [CrossRef]
- Van Egmond, W.J.; Starke, U.K.; Saakes, M.; Buisman, C.J.N.; Hamelers, H.V.M. Energy efficiency of a concentration gradient flow battery at elevated temperatures. J. Power Sources 2017, 340, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Krakhella, K.W.; Morales, M.; Bock, R.; Seland, F.; Burheim, O.S.; Einarsrud, K.E. Electrodialytic energy storage system: Permselectivity, stack measurements and life-cycle analysis. Energies 2020, 13, 1247. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Eigenberger, G.; Strathmann, H.; Nieken, U. Acid-base flow battery, based on reverse electrodialysis with bi-polar membranes: Stack experiments. Processes 2020, 8, 99. [Google Scholar] [CrossRef] [Green Version]
- Martí-Calatayud, M.C.; Buzzi, D.C.; García-Gabaldón, M.; Ortega, E.; Bernardes, A.M.; Tenório, J.A.S.; Pérez-Herranz, V. Sulfuric acid recovery from acid mine drainage by means of electrodialysis. Desalination 2014, 343, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Dara, S.; Bonakdarpour, A.; Ho, M.; Govindarajan, R.; Wilkinson, D.P. Conversion of saline waste-water and gaseous carbon dioxide to (bi)carbonate salts, hydrochloric acid and desalinated water for on-site industrial utilization. React. Chem. Eng. 2019, 4, 141–150. [Google Scholar] [CrossRef]
- Wu, D.; Chen, G.Q.; Hu, B.; Deng, H. Feasibility and energy consumption analysis of phenol removal from salty wastewater by electro-electrodialysis. Sep. Purif. Technol. 2019, 215, 44–50. [Google Scholar] [CrossRef]
- Magro, C.; Paz-Garcia, J.M.; Ottosen, L.M.; Mateus, E.P.; Ribeiro, A.B. Sustainability of construction materials: Electrodialytic technology as a tool for mortars production. J. Hazard. Mater. 2019, 363, 421–427. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Li, L.; Zhang, S. Separation of olefin/paraffin by electrodialysis. Sep. Purif. Technol. 2019, 218, 20–24. [Google Scholar] [CrossRef]
- Xia, A.; Wei, P.; Sun, C.; Show, P.L.; Huang, Y.; Fu, Q. Hydrogen fermentation of organic wastewater with high ammonium concentration via electrodialysis system. Bioresour. Technol. 2019, 288, 121560. [Google Scholar] [CrossRef]
- Raschitor, A.; Llanos, J.; Rodrigo, M.A.; Cañizares, P. Is it worth using the coupled electrodialysis/electro-oxidation system for the removal of pesticides? Process modelling and role of the pollutant. Chemosphere 2020, 246, 125781. [Google Scholar] [CrossRef]
- Liu, M.J.; Neo, B.S.; Tarpeh, W.A. Building an operational framework for selective nitrogen recovery via electrochemical stripping. Water Res. 2020, 169, 115226. [Google Scholar] [CrossRef]
- Hara, K.; Kishimoto, N.; Kato, M.; Otsu, H. Efficacy of a two-compartment electrochemical flow cell introduced into a reagent-free UV/chlorine advanced oxidation process. Chem. Eng. J. 2020, 388, 124385. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.M.; Villen-Guzman, M.; Vereda-Alonso, C.; Gomez-Lahoz, C.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Recovery of Li and Co from LiCoO2 via hydrometallurgical-electrodialytic treatment. Appl. Sci. 2020, 10, 2367. [Google Scholar] [CrossRef] [Green Version]
- Sadyrbaeva, T.Z. Removal of chromium(VI) from aqueous solutions using a novel hybrid liquid membrane—electrodialysis process. Chem. Eng. Process. Process. Intensif. 2016, 99, 183–191. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.; Lang, Q.; Tan, M.; Ratanatamskul, C.; Lee, M.; Liu, Y.; Zhang, Y. A comprehensive investigation on the components in ionic liquid-based polymer inclusion membrane for Cr(VI) transport during electrodialysis. J. Membr. Sci. 2020, 604, 118016. [Google Scholar] [CrossRef]
- Meng, X.; Li, J.; Lv, Y.; Feng, Y.; Zhong, Y. Electro-membrane extraction of cadmium(II) by bis(2-ethylhexyl) phosphate/kerosene/polyvinyl chloride polymer inclusion membrane. J. Hazard. Mater. 2020, 386, 121990. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhu, T.; He, Z. Minimizing effects of chloride and calcium towards enhanced nutrient recovery from sidestream centrate in a decoupled electrodialysis driven by solar energy. J. Clean. Prod. 2020, 263, 121419. [Google Scholar] [CrossRef]
- Alkhadra, M.A.; Conforti, K.M.; Gao, T.; Tian, H.; Bazant, M.Z. Continuous Separation of Radionuclides from Contaminated Water by Shock Electrodialysis. Environ. Sci. Technol. 2020, 45, 527–536. [Google Scholar] [CrossRef]
- Lee, J.B.; Park, K.K.; Eum, H.M.; Lee, C.W. Desalination of a thermal power plant wastewater by membrane capacitive deionization. Desalination 2006, 196, 125–134. [Google Scholar] [CrossRef]
- Xu, P.; Drewes, J.E.; Heil, D.; Wang, G. Treatment of brackish produced water using carbon aerogel-based capacitive deionization technology. Water Res. 2008, 42, 2605–2617. [Google Scholar] [CrossRef]
- Liang, P.; Yuan, L.; Yang, X.; Zhou, S.; Huang, X. Coupling ion-exchangers with inexpensive activated carbon fiber electrodes to enhance the performance of capacitive deionization cells for domestic wastewater desalination. Water Res. 2013, 47, 2523–2530. [Google Scholar] [CrossRef]
- Oren, Y. Capacitive deionization (CDI) for desalination and water treatment—Past, present and future (a review). Desalination 2008, 228, 10–29. [Google Scholar] [CrossRef]
- Anderson, M.A.; Cudero, A.L.; Palma, J. Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: Will it compete? Electrochim. Acta 2010, 55, 3845–3856. [Google Scholar] [CrossRef]
- Liu, P.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Separation and recovery of heavy metal ions and salt ions from wastewater by 3D graphene-based asymmetric electrodes: Via capacitive deionization. J. Mater. Chem. A 2017, 5, 14748–14757. [Google Scholar] [CrossRef]
- Choi, J.; Dorji, P.; Shon, H.K.; Hong, S. Applications of capacitive deionization: Desalination, softening, selective removal, and energy efficiency. Desalination 2019, 449, 118–130. [Google Scholar] [CrossRef]
- Pawlowski, S.; Huertas, R.M.; Galinha, C.F.; Crespo, J.G.; Velizarov, S. On operation of reverse electrodialysis (RED) and membrane capacitive deionisation (MCDI) with natural saline streams: A critical review. Desalination 2020, 476, 114183. [Google Scholar] [CrossRef]
- Folaranmi, G.; Bechelany, M.; Sistat, P.; Cretin, M.; Zaviska, F. Towards Electrochemical Water Desalination Techniques: A Review on Capacitive Deionization, Membrane Capacitive Deionization and Flow Capacitive Deionization. Membranes 2020, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, C.; Liu, H.; Qu, J. Potassium-Ion Recovery with a Polypyrrole Membrane Electrode in Novel Redox Transistor Electrodialysis. Environ. Sci. Technol. 2020, 54, 4592–4600. [Google Scholar] [CrossRef]
- Yang, E.; Chae, K.-J.; Choi, M.-J.; He, Z.; Kim, I.S. Critical review of bioelectrochemical systems integrated with membrane-based technologies for desalination, energy self-sufficiency, and high-efficiency water and wastewater treatment. Desalination 2019, 452, 40–67. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [Green Version]
- Srikanth, S.; Kumar, M.; Puri, S.K. Bio-electrochemical system (BES) as an innovative approach for sustainable waste management in petroleum industry. Bioresour. Technol. 2018, 265, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Huang, X.; Liang, P.; Xiao, K.; Zhou, Y.; Zhang, X.; Logan, B.E. A new method for water desalination using microbial desalination cells. Environ. Sci. Technol. 2009, 43, 7148–7152. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H.M.; Husseini, G.A.; Yousef, S.; Saif, J.; Al-Asheh, S.; Abu Fara, A.; Azzam, S.; Khawaga, R.; Aidan, A. Microbial desalination cell technology: A review and a case study. Desalination 2015, 359, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, A.; Cui, T.; Zhang, J.; Yu, H.; Han, W.; Shen, J.; Li, J.; Sun, X.; Wang, L. Construction and application of a 1-liter upflow-stacked microbial desalination cell. Chemosphere 2020, 248, 126028. [Google Scholar] [CrossRef] [PubMed]
- Jafary, T.; Al-Mamun, A.; Alhimali, H.; Baawain, M.S.; Rahman, M.S.; Rahman, S.; Dhar, B.R.; Aghbashlo, M.; Tabatabaei, M. Enhanced power generation and desalination rate in a novel quadruple microbial desalination cell with a single desalination chamber. Renew. Sustain. Energy Rev. 2020, 127, 109855. [Google Scholar] [CrossRef]
- Lan, J.; Ren, Y.; Lu, Y.; Liu, G.; Luo, H.; Zhang, R. Combined microbial desalination and chemical-production cell with Fenton process for treatment of electroplating wastewater nanofiltration concentrate. Chem. Eng. J. 2019, 359, 1139–1149. [Google Scholar] [CrossRef]
- Li, X.; Jin, X.; Zhao, N.; Angelidaki, I.; Zhang, Y. Novel bio-electro-Fenton technology for azo dye wastewater treatment using microbial reverse-electrodialysis electrolysis cell. Bioresour. Technol. 2017, 228, 322–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Logan, B.E. Microbial reverse electrodialysis cells for synergistically enhanced power production. Environ. Sci. Technol. 2011, 45, 5834–5839. [Google Scholar] [CrossRef]
- Cusick, R.D.; Kim, Y.; Logan, B.E. Energy capture from thermolytic solutions in microbial reverse- electrodialysis cells. Science 2012, 335, 1474–1477. [Google Scholar] [CrossRef] [Green Version]
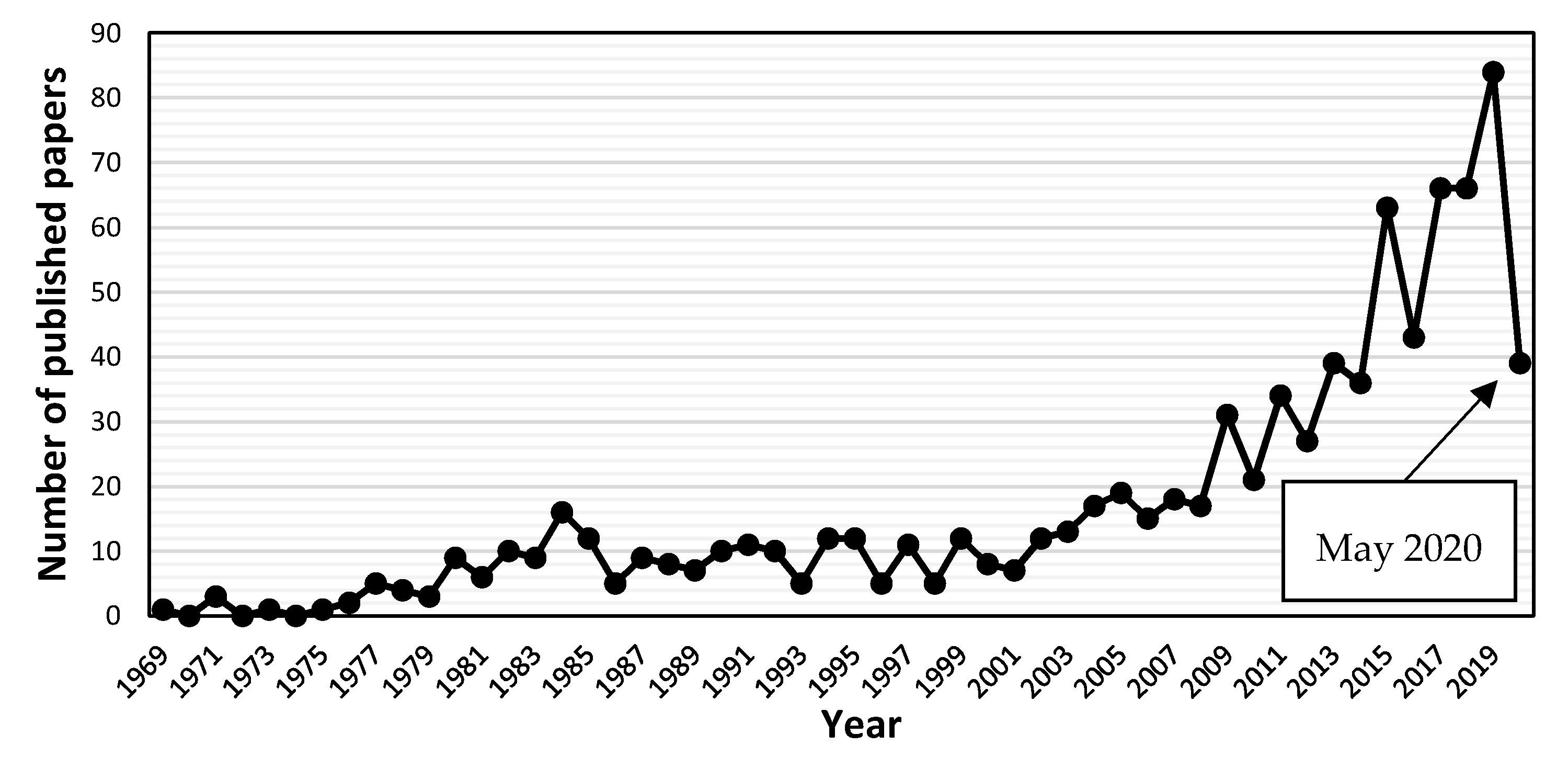



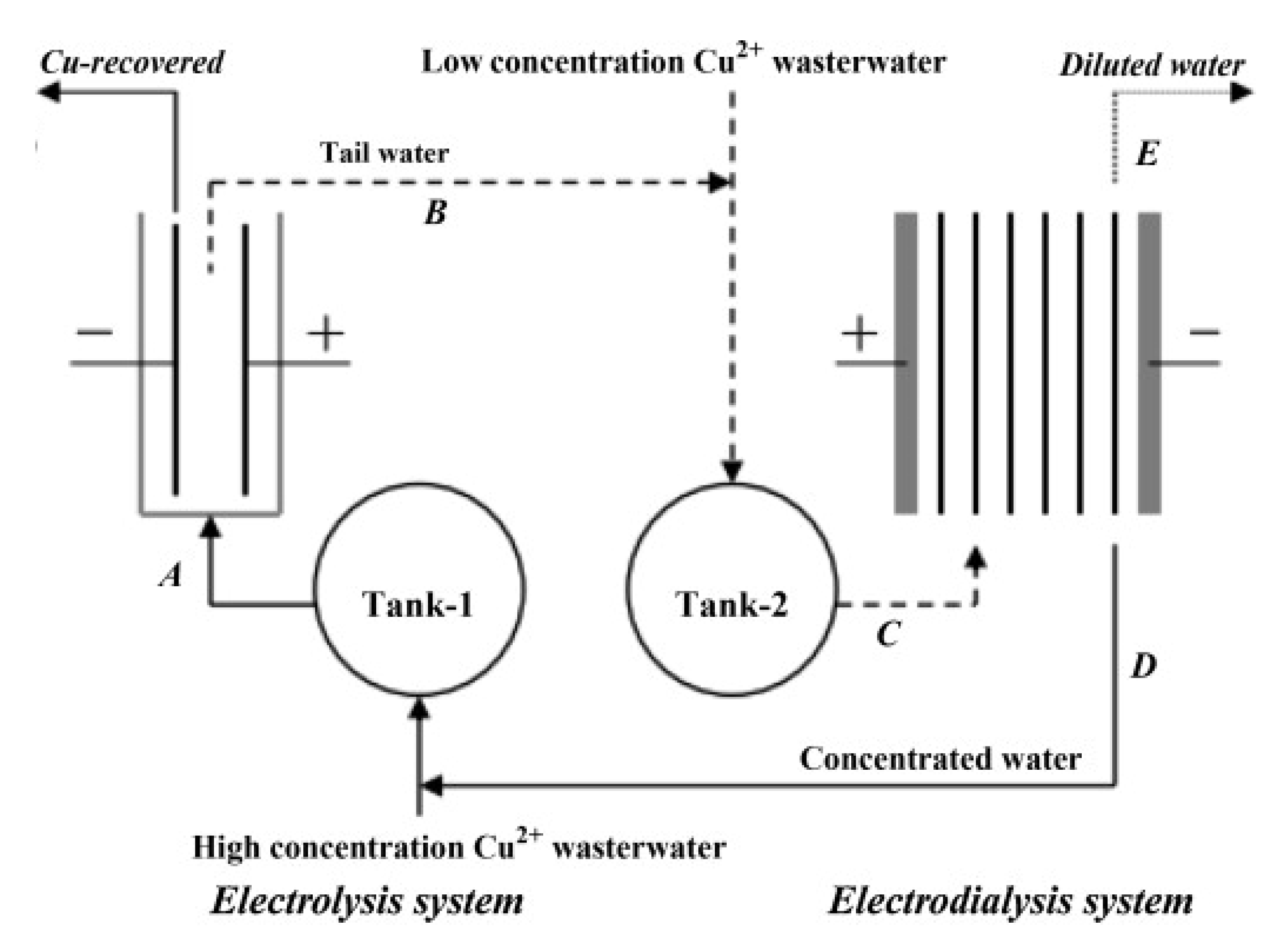
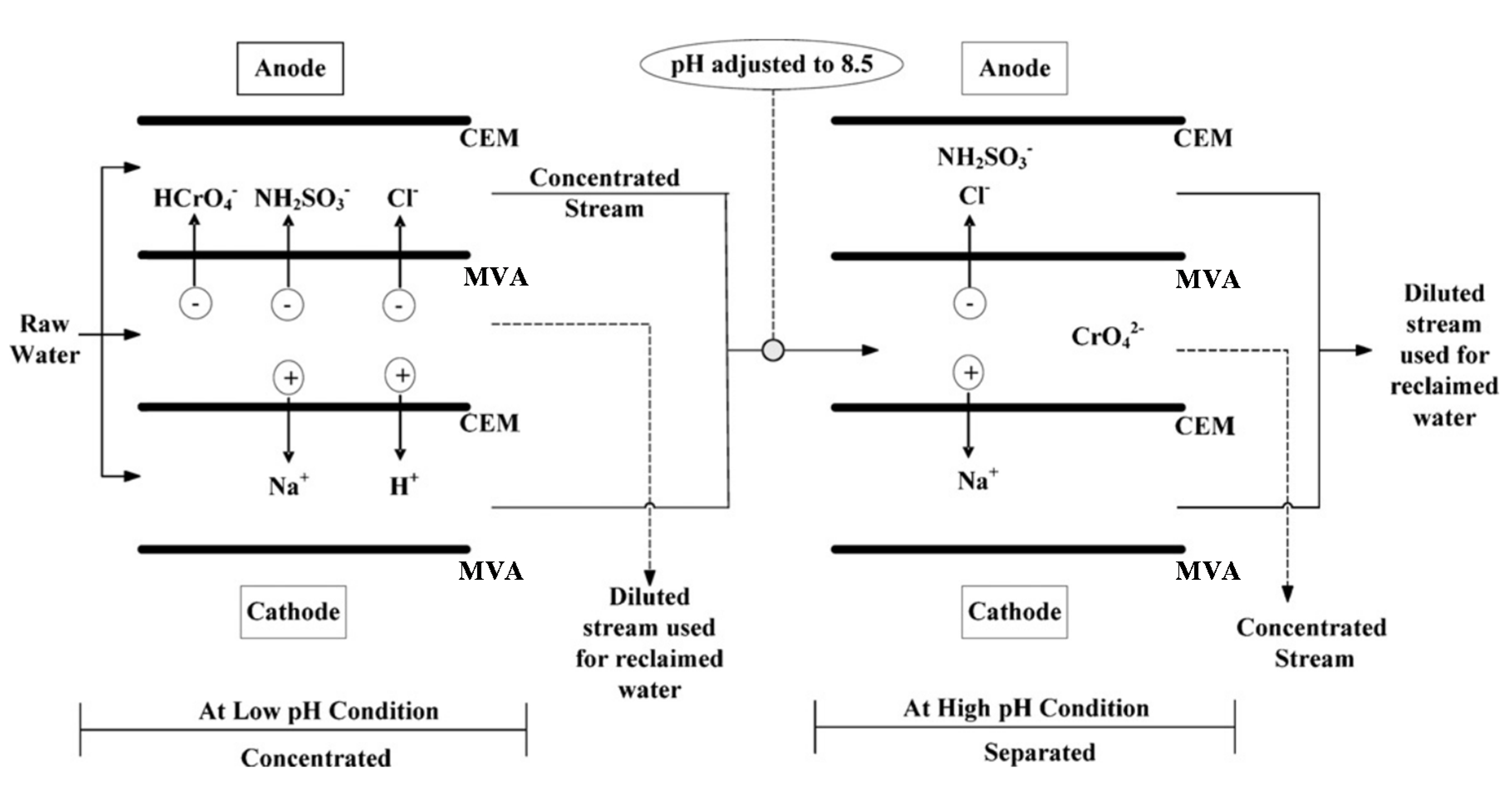
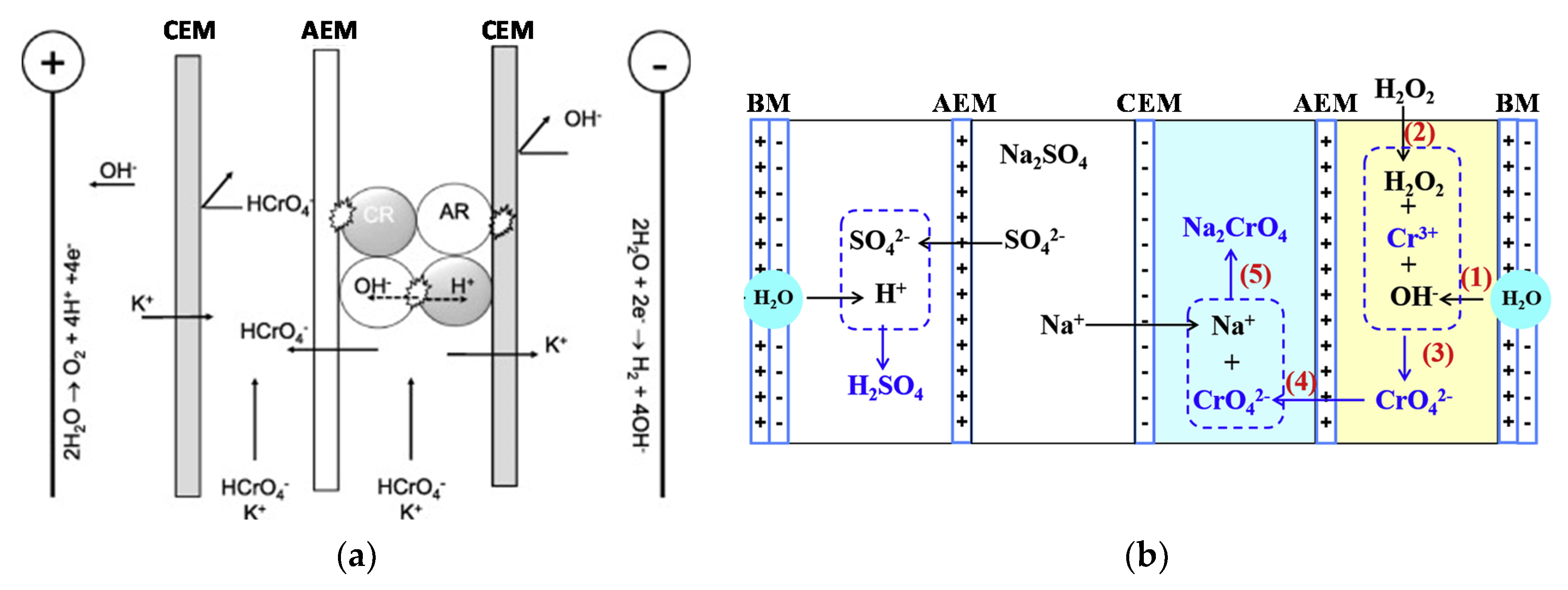

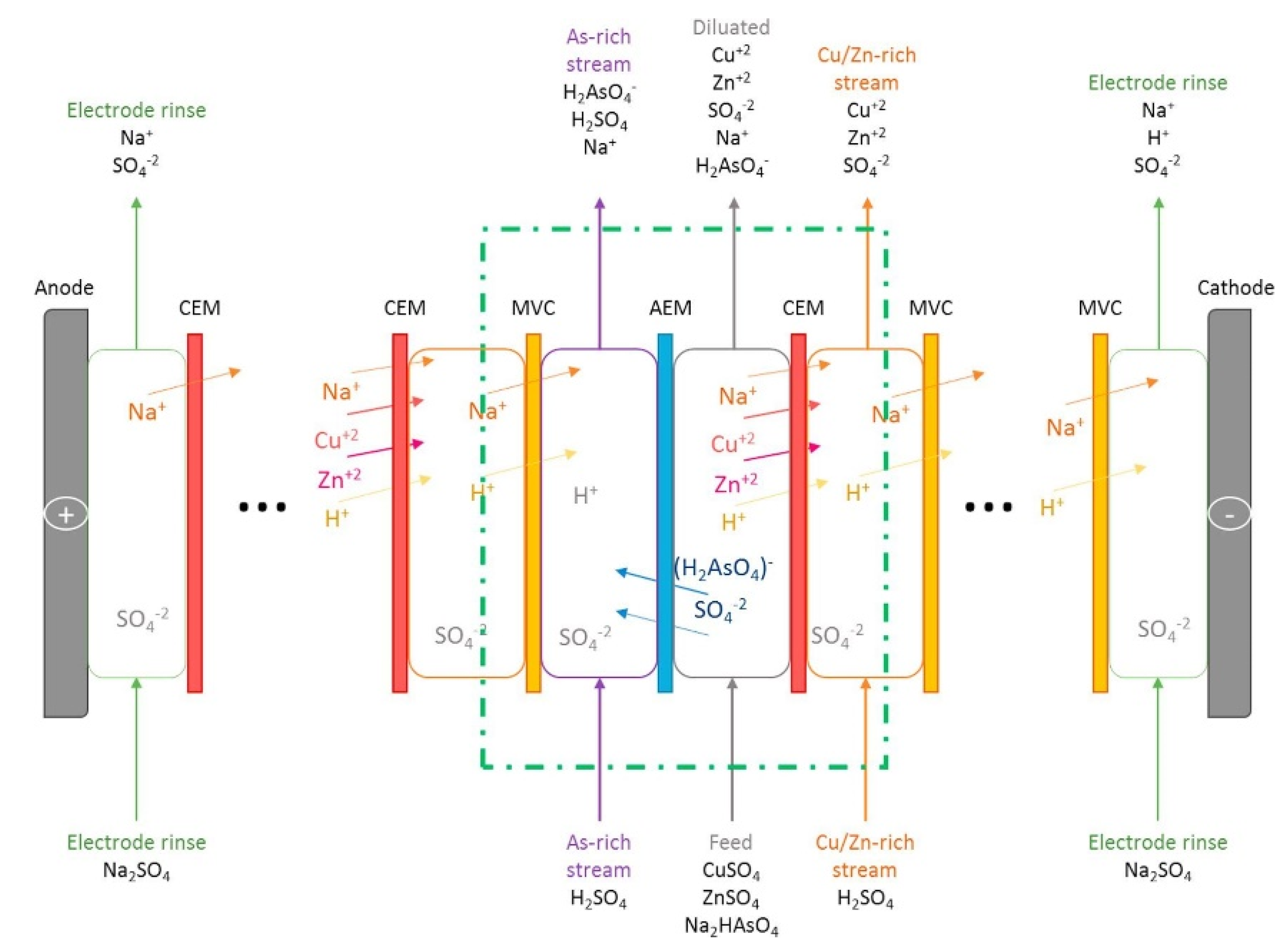


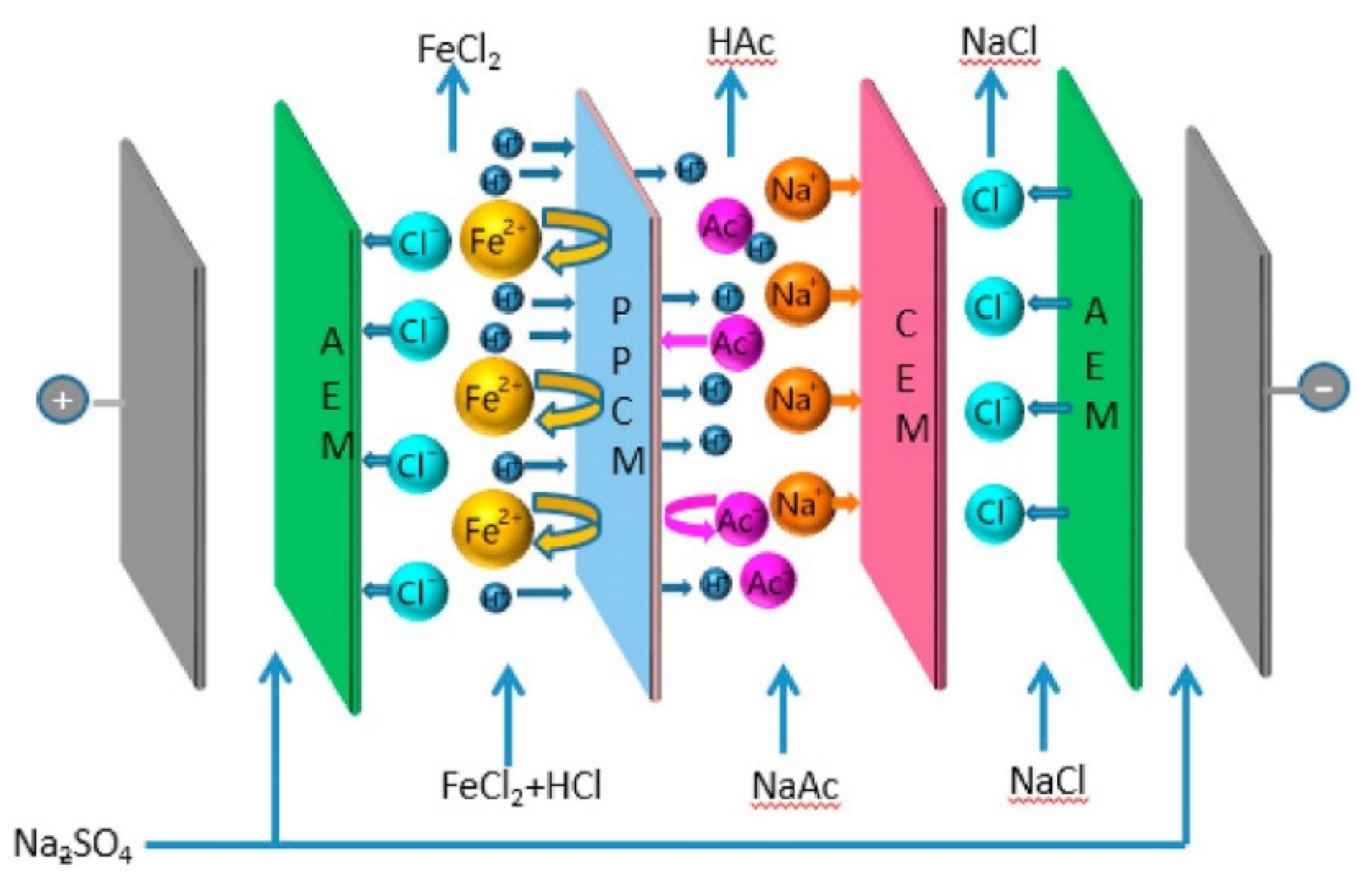
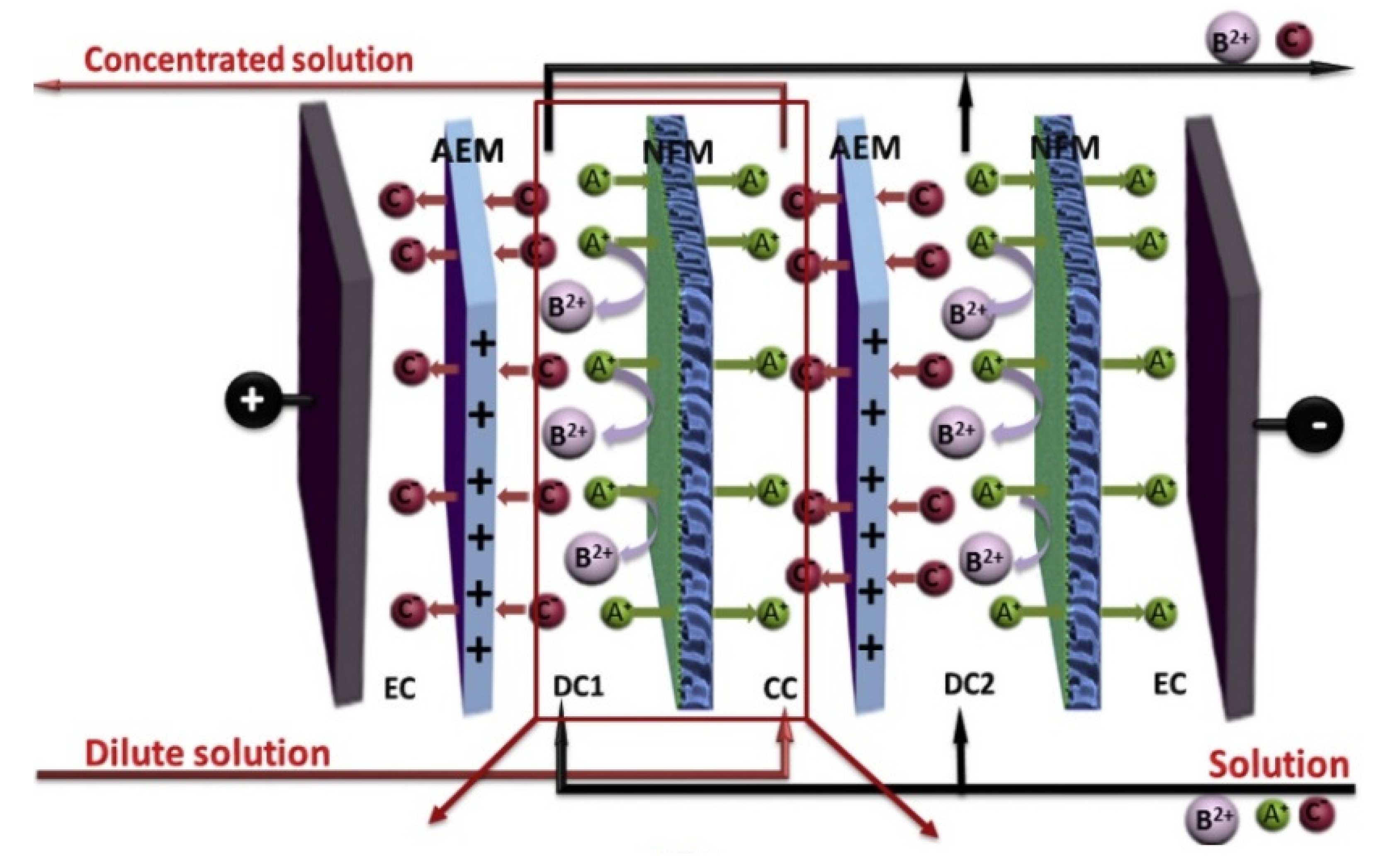
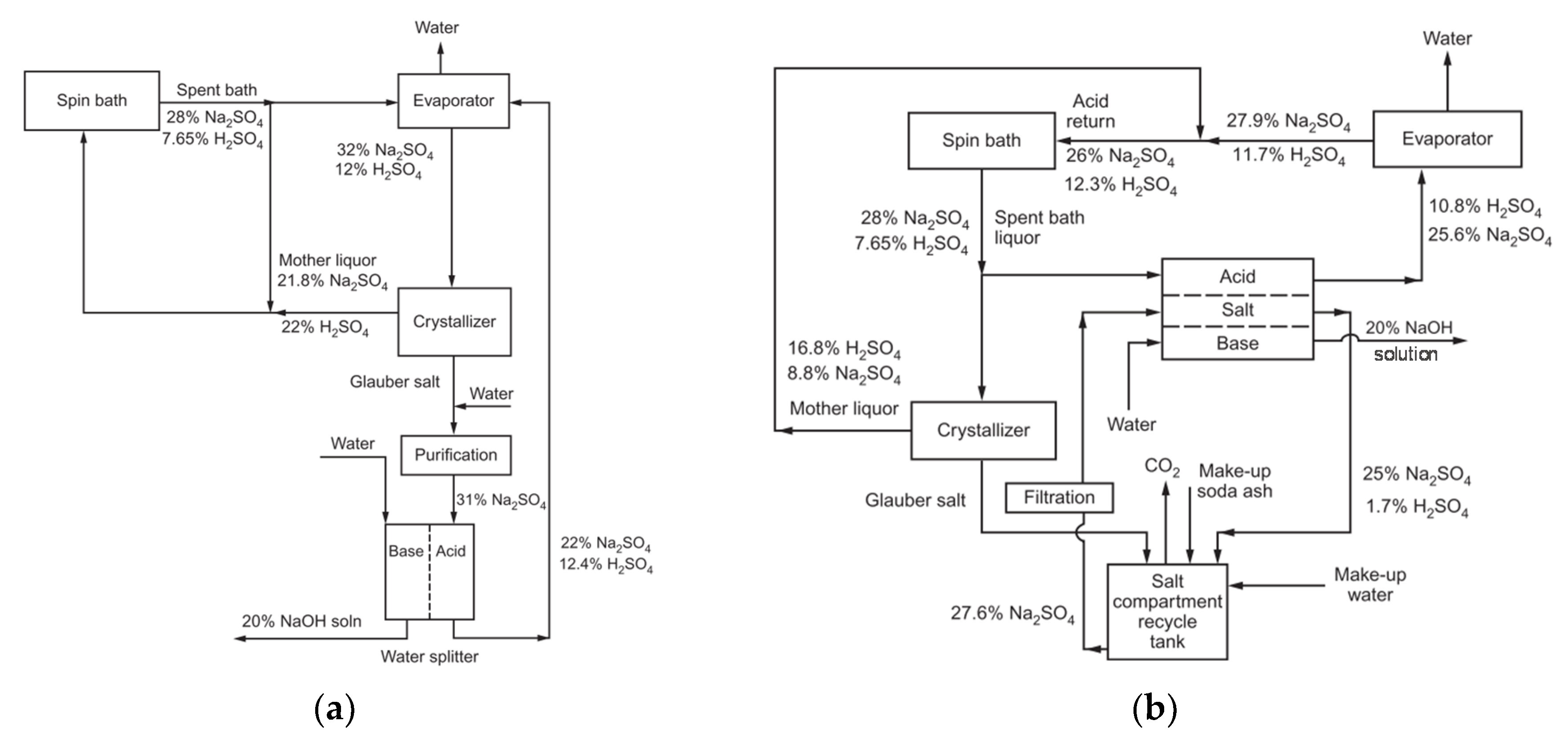
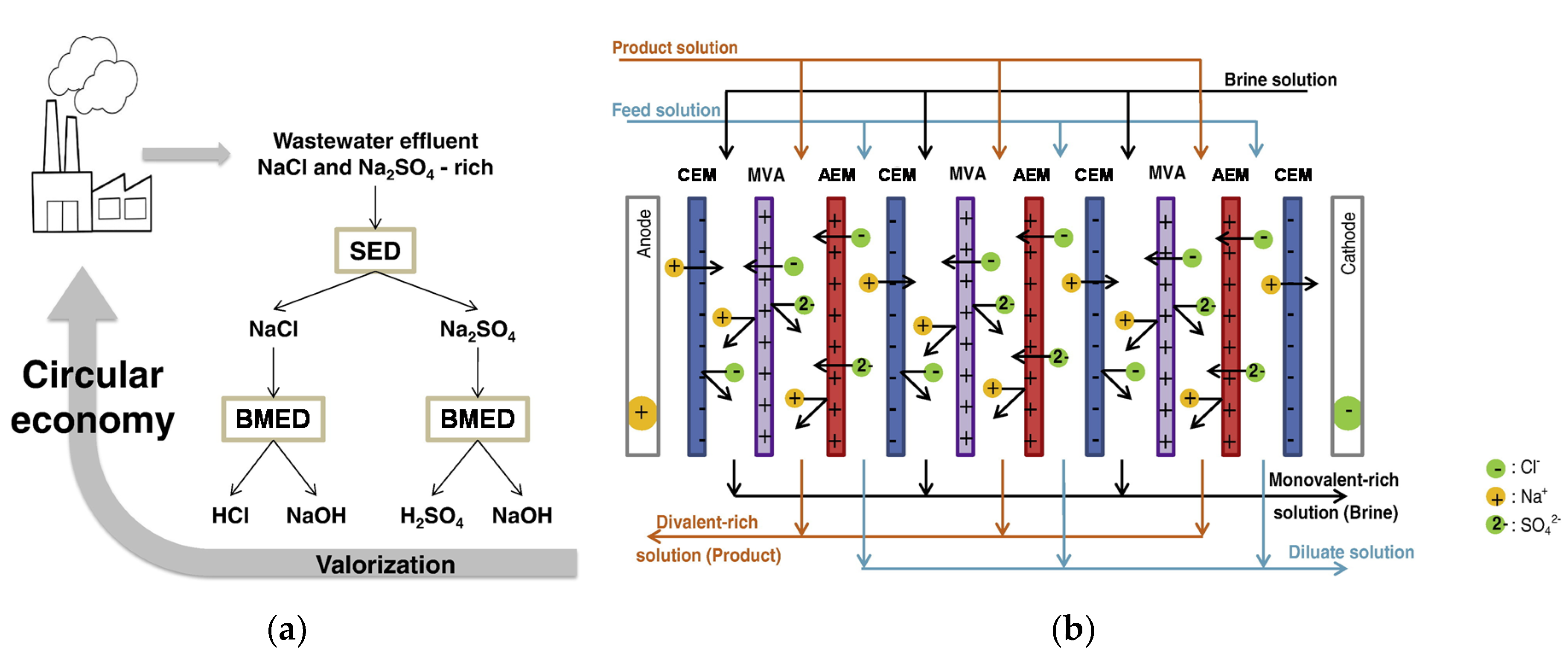

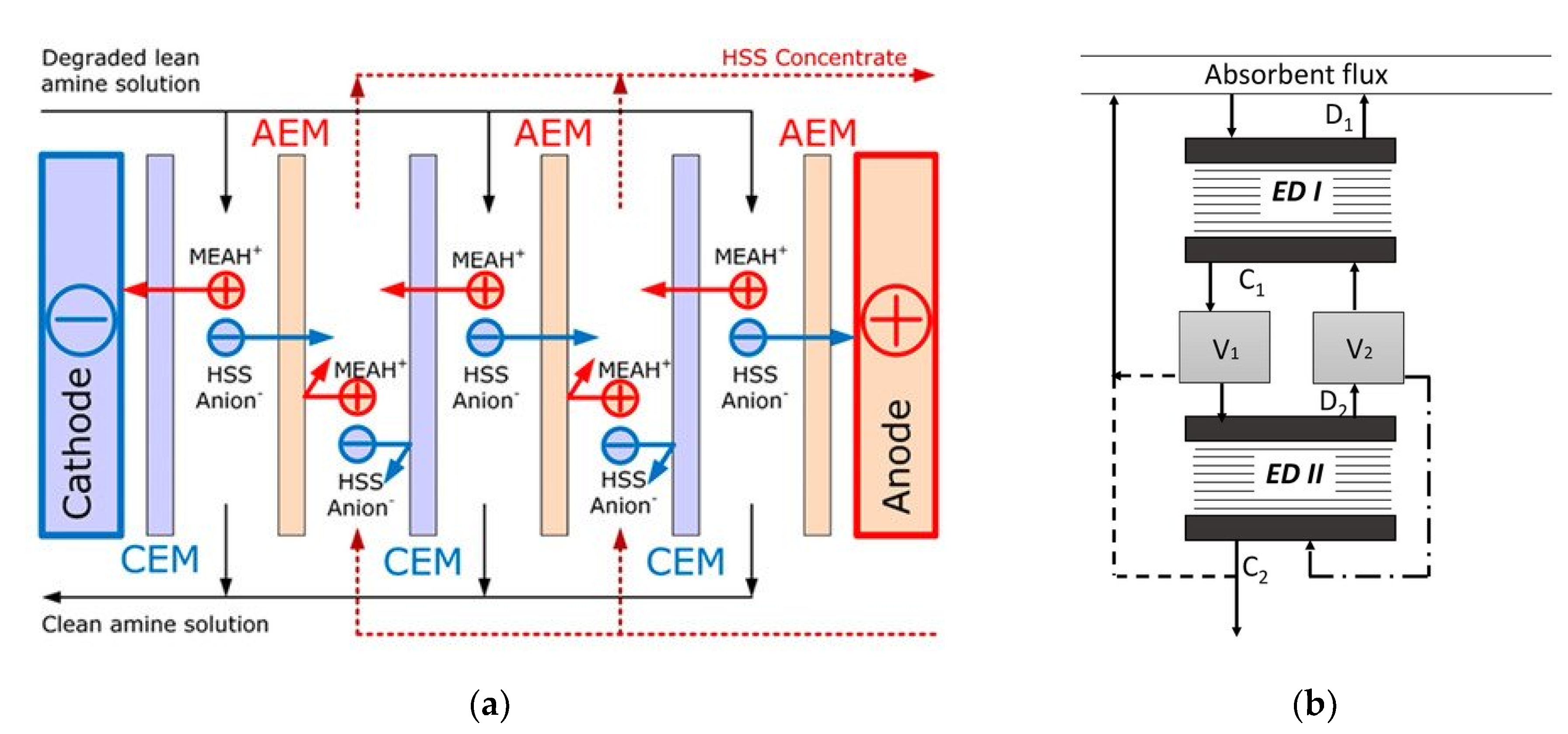









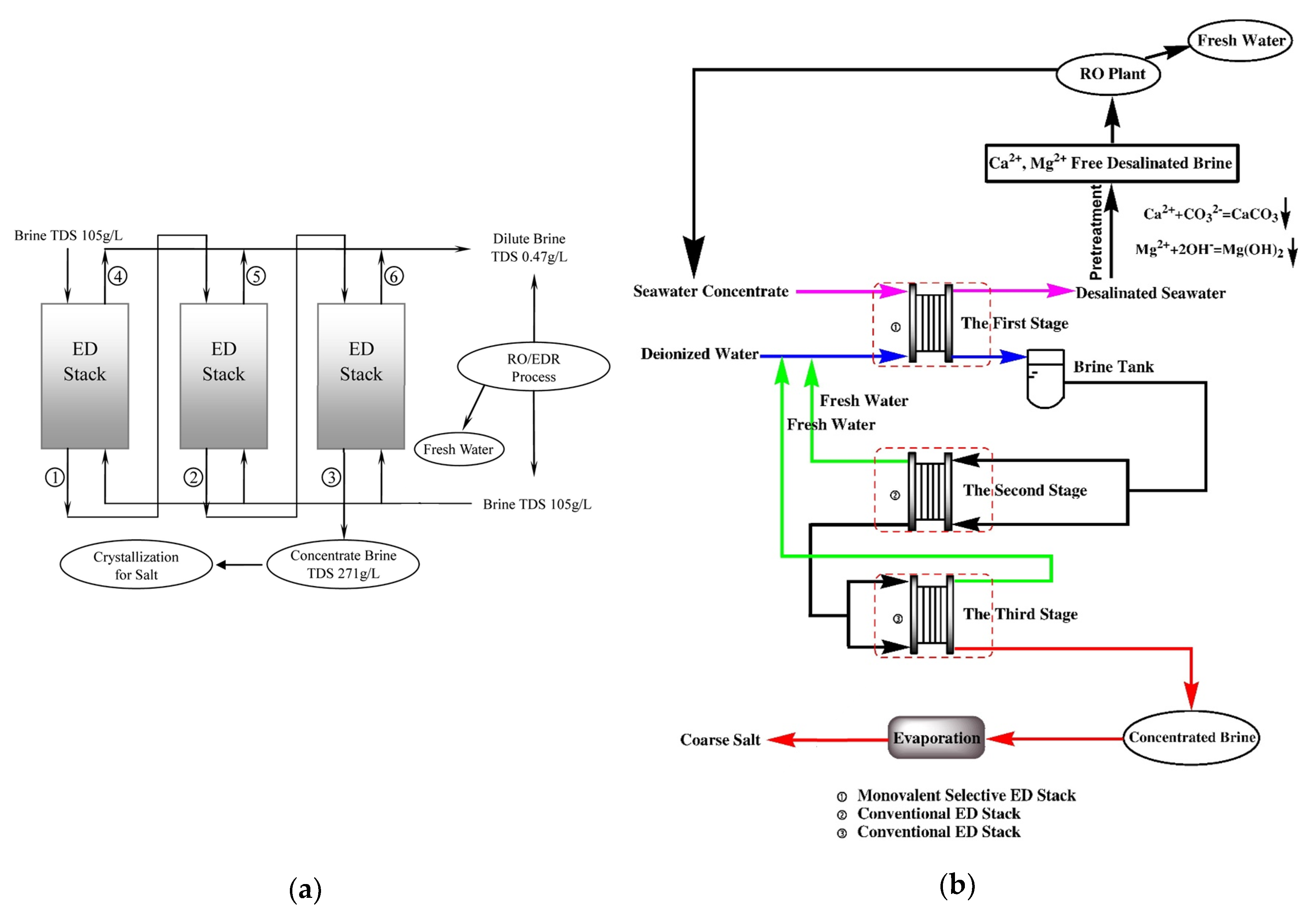






| Search Word | Search Word | |
|---|---|---|
| Electrodialysis | AND | Wastewater |
| Bipolar membrane electrodialysis | Effluent | |
| Selective electrodialysis | Spent solution | |
| Selectrodialysis | Recovery | |
| Electrodialysis metathesis | Reclamation | |
| Electrodeionisation | Reuse | |
| Continuous electrodeionisation | Valorisation | |
| Reverse electrodialysis | Regeneration | |
| Zero liquid discharge |
| Wastewater | Treatment Process | Main Remarks | Ref. |
|---|---|---|---|
| NaCl + Na2SO4 solution, 0.01 M each | ED with MVAs | Layer-by-layer composite AEM, = 11.5 | [312] |
| NaCl + Na2SO4 solution, 0.05 M each | ED with MVAs | MVAs with hydrophobic alkyl side chain, max = 13.1, long-term stability | [362] |
| NaCl + MgCl2 or LiCl + MgCl2 solutions, 0.1 M each | ED with MVCs | Zwitterion structure MVCs, | [363] |
| NaCl+MgCl2 solution, 0.1 M each | ED with MVCs | Zwitterion structure MVCs with hydrophobic alkyl side chain, max | [364] |
| Model solutions with two salts with the same counter-ion among NaCl, Na2SO4, MgCl2, MgSO4 and NaNO3, 0.01 M each | ED or ED with MVMs | Max separation efficiency ~68% for cations by MVMs (comparable to NF), but lower for anions | [365] |
| NaCl + Na2SO4 solution, 8 mM each | SED | purity > 85%, η ≈ 50% | [39] |
| MgCI2 + Na2SO4 solutions, 0.3–0.5 M each | EDM | η > 100%, Espec ≈ 0.9–1.6 kWh/kg, MgSO4 purity ~98% | [40] |
| Na2SO4 solutions, 0.01 M/0.3 M | RED-alkaline polymer electrolyte water electrolysis | VOC ≈ 12 V (200 cell pairs), Pd,max = 0.04–0.11 W/m2 by changing solutions velocity and temperature, H2 production 50 cm3/(h·cm2) | [366] |
| Two-stage ED with MVCs | On-line membrane modification, reduced from 0.36 to 0.11, from 0.81 to 0.12, η = 75–92%, 1 g/L diluate, stable long-run, but larger water transport, membrane resistance, and Espec (up to ~35% more) | [367] | |
| Photovoltaic industry simulated wastewater, 120–180 mg/L NaF and/or 750–2000 mg/L NaNO3 | ED | With single salt, max removal efficiency ~60% and 75% for in 6 min, under optimal conditions Espec = 0.25–0.36 kWh/m3; with mixture, ion competition affected only F− removal | [368] |
| F− solutions: single salt at 25–200 mg/L, binary and ternary mixtures with 100 mg/L at same equivalent concentration | ED | High removal efficiencies, Espec = 0.02–0.49 kWh/m3, Cl− affected F− separation, did not | [369] |
| Synthetic secondary effluent of graphite industry, 10–30 mg/L NaF, 6 g/L NaCl | ED | Response surface methodology, F− removal 99.69% with Espec = 0.76 kWh/m3 under optimal conditions | [370] |
| B artificial wastewater, 25–100 mg/L; binary or ternary mixtures with 100 mg/L B + at same or doubled equivalent concentration | ED | Max removal of B ~80%, enhanced at high pH (10.5) due to a predominance of , hindered by Cl− and not by , Espec = 0.02–1.24 kWh/m3 | [371] |
| Acidic model solution from B-selective sorbents regeneration, 0.2 M HCl or 0.1 M H2SO4 + 1.0 or 5.2 g/L H3BO3 | Two-stage ED with pH increase | Regenerating acid (HCl or H2SO4) recovered in the concentrate ~90%, ~93% of H3BO3 (non-ionic) retained within the diluate and concentrated in the 2nd stage after alkalinisation, reusable solutions | [372] |
| B-containing industrial landfill leachate, 62.8–76.5 mg/L B (+ , Cl−, Ca2+ and Mg2+) | Two-stage ED with pH increase | Desalination in the 1st stage 80%, removal in the 2nd stage 97% under alkaline conditions, max η = 25–28%, estimated cost 1.27 $/m3 | [373,374] |
| Model nuclear power plant effluent, 60–400 mg/L H3BO3 | Three-compartment EDI | Max removal ~45%, optimal pH = 10 | [375] |
| NH4NO3 model wastewater from fertilizer production, 0.012 M | ED | Thin heterogeneous IEMs vs. commercial ones: higher limiting current density due to larger back-diffusion and electroconvection; lower alkalisation due to lower water dissociation | [376] |
| Synthetic solutions with single acid or salt: H2SO4, HNO3, NH4NO3, NaCl, LiCl, Na2SO4, 0.06–0.3 M | ED or BMED-ED | ED concentrator without flow through concentrate chambers, acid concentration 1.16 M at η = 89% for BMED and 26% for ED, Espec = 0.83 kWh/mol | [377] |
| Alkaline liquid from bauxite solid residue (Bayer process) washing (2.4 g/L …) | ED with aeration | NaOH recovery, NaAl(OH)4 separation, TDS and OH− removal 61.3% and 76.6%, η = 60%, Espec = 11.15 kWh/kg | [378] |
| Synthetic or real wastewater from mineral carbonation for CO2 sequestration, 0.05–1.0 M (NH4)2SO4, 0.05–0.54 M (NH4)HSO4, (+MgSO4, NH3, Fe(II), Fe(III)…) | BMED | Different setups for regenerating rock-derived solutions after leaching or after carbonation, | [379] |
| Model solution from Li-ion waste batteries, Li+ and Co2+ 0.02 M each | BMED with complexation | Co-EDTA chelated anions and Li+ separated in the acid and base compartments, respectively, removals 99%, but Co absorption in AEM; metal recovery enhanced in semi-batch operation for the feed | [380] |
| Wastewater | Treatment Process/Exp. Device | Main Remarks | Ref. |
|---|---|---|---|
| Solutions with octanoic acid or anionic surfactants; alkaline bleach plant filtrate from sulphate pulp mill, 1370 mg/L COD | IEM resistance measurement cell | Slight inorganic fouling on CEM by bleach plant filtrate, significant organic fouling on AEM by all solutes | [29] |
| Solutions with carboxylic acids (propanoic, octanoic and decanoic acid); alkaline bleach plant filtrate from sulphate pulp mill, 1850 mg/L COD | IEM resistance measurement cell, ED | No CEM fouling, AEM fouling due to organic anions, especially compounds with longer chain, and at higher currents | [381] |
| Solutions with 16 charged or neutral trace organic contaminants, 0.1 mg/L with 100 g/L NaCl | ED | Adsorption governed by electrostatic interactions, transport mostly diffusion driven, migration of charged components only at very low NaCl concentration | [382] |
| Solutions with NaCl, Na2SO4 or MgCl2 and acetic acid, phenol or glucose, 0.8 eq/L salts and 0.1 M organics | ED | Phenomenological model: convection-diffusion of neutral organics affected by steric effects and ion hydration | [383] |
| Solutions with NaCl, Na2SO4 or MgCl2 and acetic acid, phenol, glucose or acetate | ED | Phenomenological model: transport of several organics larger with than with Cl−, opposite trend for phenol | [384] |
| Wastewater from bisphenol A diphenyl phosphate production, 4.5–4.8% total salt (NaCl and sodium phenolate), pH = 13.2–13.5, diluted with pure water | RED-ED | Ultrapure water fed into the RED diluate, VOC up to 1.65 V (10 cell pairs) and Pd,max,net up to 1.12 W/m2 in RED at dilution ratio 1.0:0.5, Espec lower than that of standalone ED (17.65 vs. 25.32 kWh/m3) with 27.4% pre-desalination in RED | [385] |
| NaCl-glycerol solution, 1.11–1.67 M NaCl and 0.06–0.6 M glycerol | ED | 7 membrane pairs tested, phenomenological model: C3H8O3 electro-osmotic co-transport 38–64%, osmotic co-transport 16–41%, diffusion 9–28%, low glycerol/NaCl flux at low glycerol/NaCl and NaCl concentrations | [386] |
| Simulated dairy wastewater, 10 mM citrate, 1 mM lactate, 30 mM NaCl… | ED | Guanidinium groups in AEM as functional moiety binding oxyanions, enhanced transport of phosphate and citrate | [387] |
| Diluted effluent from sodium dithionate processing, 35 g/L HCOONa, 30 g/L Na2S2O3… | ED with MVAs | Recovery of HCOONa 69%, with 87% purity, η = 70%, Espec = 96 kWh/m3 | [388] |
| Steel manufacturing wastewater (Cl−, , Na+, Mg2+, Ca2+), 2.8–4.0 mS/cm, 36–72 mg/L COD | Sand filtration-EDR | Water recovery 75%, desalination 92%, concentrate COD below discharge limit, Espec = 0.85 kWh/m3, operation cost 0.146 $/m3 | [389] |
| Secondary effluent from spinning processes, chemical industries, and metal processors (Cl−, …), 7.3 mS/cm, 41.5 g/L COD | Sand filtration-EDR | Lower techno-economic efficiency compared to fiber filtration-UF-RO | [390] |
| ZnO washing wastewater (Na+, K+, Cl−, Ca2+ and ), ~0.35 M, 1.2 mM TOC | ED with MVMs | Overall η ≈ 80%, divalent ions retained, thus scaling prevented, stable long-term performance of pilot plant with removal target of 50% (before evaporation) | [281] |
| Kraft pulp mill dissolved electrostatic precipitator dust (Cl−, , Na+, K+), 137 g/L TDS (0.1 wt% TOC in the dust) | ED with MVMs | Selective removal of Cl− at η = 60–78% and Espec ≈ 1 kWh/kg, organics in the dust recycled with the sulphate-rich diluate, no fouling, accumulated dust simply flushed, successful long-term operation, operation saving of 800 $/1000 ton Kraft pulp | [391] |
| Paper mill effluent, 6046 mg/L TDS, 390 mg/L COD | MF-ED | Max TDS removal ~90%, water recovery 80%, Espec ≈ 0.5 kWh/m3, concentrate usable as biomass | [392] |
| Primary textile effluent, 2,980 mg/L TDS, 220 mg/L COD | UF-ED | Desalination ~96%, Espec = 0.9 kWh/m3, reusable water | [393] |
| Model textile effluent with 1 g/L reactive blue 194 and 40 g/L Na2SO4 | Tight UF-based diafiltration-BMED | Pre-concentration at a factor of 8 and diafiltration with 8 diavolumes, UF permeate with low dye content (2.7 mg/L) and 21.06 g/L Na2SO4, 99.5% dye recovery, ~99% salt conversion into 99% pure 0.29 M acid and 0.4 M base without fouling, Espec = 4.2 kWh/kg | [394] |
| Model textile effluent with 0.25 g/L Remazol Brilliant Blue R and 50 g/L Na2SO4 | BMED | Effect of zeta potential of dye molecule on fouling, fouling controlled by the identification of a “critical salt concentration” below which desalination cannot proceed due to fouling, η =39%, desalination 74%, 72% of Na+ and 66.9% of converted into base and acid, respectively | [395] |
| Tannery unhairing effluent, pH = 12, 576 mg/L S2−, 23,289 mg/L COD, 436 mg/L Ca2+, 429.6 mg/L Cl− | ED with protective UF membrane on AEM | Anti-fouling solution against proteins and peptides, desalination 56%, 90% of organics retained within the diluate, thus water recycling | [396] |
| Almond processing treated wastewater (electrocoagulation and electrooxidation), 7.2 mS/cm, 296 mg/L TOC | ED | Concentration factor of 10 in the concentrate, diluate target 0.5 mS/cm, TOC removal ~70%, water recovery 94%, no fouling, scale-up at pre-industrial scale, Espec = 1.1–2.9 kWh/m3 | [397] |
| Waste brine from olive pickling process, 103.3 mS/cm, pH = 3.5, 8033 mg/L dissolved organic carbon; coupled with storm water, 3.6 mS/cm | RED | VOC = 1.37 V (~70% of the ideal one, 10 cell pairs), Pd,max = 0.59 W/m2 enhanced (with respect to NaCl solutions at the same conductivities, 0.44 W/m2) by pH gradient and organic acids (lower resistance) | [398] |
| Lysine fermentation effluent, 152 mS/cm, 17,800 g/L , 102,300 ppm TOC | MF-ED | Separation of 73.1% and 83.5% , Espec = 106 kWh/m3, pulsed electric field effective against fouling, demineralized waste usable as animal feed, concentrate as fertilizer | [399] |
| Bio-refinery effluents: molasses effluents, lignocellulosic stream, sugar cane juice, 3.2–72.4 mS/cm, 38–380 g/L COD | ED | Salt removal 96% and 63% from lignocellulosic and molasses effluents, lower from sugar cane juice, low COD loss (< 6.3%), η = 69–104%, Espec = 0.44–1.59 kWh/kg salt | [400] |
| Bio-refinery effluents: synthetic salt mixtures with sorbitol, molasses effluent | ED | Simplified process model, predictions in good agreement with experimental results | [401] |
| Vinasse from a distillery producing ethanol from sugar can juice, 30,500 mg/L COD, 11.5 mS/cm | UF-ED with MVMs | K+ recovery 72%, Espec = 9 kWh/m3, η = 54%, concentrated stream for fertigation, diluate stream for fertigation or biogas production (anaerobic digestion) | [402] |
| Solutions of 3-chloro-1,2-propanediol (α-monochlorohydrin or 3-MCH) (model effluent from biodiesel production or other sources), 10 or 30% wt + 0.1 M KCl | BMED | Recovery of glycidol by dehydrohalogenation caused by OH− in the base compartment, selectivity 96%, η = 64%, glycidol distilled with 75.6% yield | [403] |
| Model antibiotic effluent with 0.95 g/L penicillin, 1 g/L and 1 g/L bovine serum albumin | ED with UF membrane, 3-comp. (AEM-UF-CEM) | Penicillin recovery ~20%, removal of from feed and antibiotic product 90%, no fouling, Espec = 0.058–0.082 kWh/g, estimated profit 6850 $/ton produced penicillin (8 L/day wastewater) | [404] |
| Effluent from anaerobic digester–decanter, 13,800 mg/L COD, 1700 mg/kg total N, 1800 mg/kg Cl−, 2,900 mg/kg Na+… | ED | Separation 70–96% for monovalent ions, < 50% for divalent ions, Espec = 6–11 kWh/m3 for water recovery 50–95% | [405] |
| Simulated supernatant of excess sludge mixed with influent from anaerobic-aerobic biological treatment, 100 mg/L P * | ED or ED-BMED | recovery 95.8%, ED-BMED: 0.075 M H3PO4 recovered, η ≈ 70–80%, Espec = 5.3–29.3 kWh/kg | [406] |
| SED-struvite precipitator | 6.8 mM phosphate in SED product (from 0.8 mM initial product, struvite effluent), average overall η ≈ 70%, desalination 95%, phosphate recovery 93%, Espec = 16.7 kWh/kg phosphate | [407] |
| High-Salinity Sol. | Low-Salinity Sol. | Performance | Ref. |
|---|---|---|---|
| SWRO brine 1 or 2 M NaCl | Secondary effluent 0.02 M NaCl | VOC (5 cell pairs), Pd,max: 1 M–0.02 M → 0.90 V, ~0.48 W/m2 2 M–0.02 M → 1.02 V, ~0.57 W/m2 | [423] Figure 33a |
| • SWRO brine 1.2 M NaCl • FO brine 2.4 M NaCl | • River water 0.01 M NaCl • Seawater 0.6 M NaCl | Pd,max, estimated maximum reduction of Espec: 1.2 M–0.01 M → 1.48 W/m2, 7.8%; 2.4 M–0.01 M → 1.86 W/m2, 13.5%; 1.2 M–0.6 M → 0.09 W/m2, 0.5%; 2.4 M–0.6 M → 0.37 W/m2, 2.2% | [540] |
| BWRO brine 31.3 mS/cm (~0.4 M), 19.5 mg/L dissolved organic carbon | Brackish groundwater 8.3 mS/cm (~0.095 M), 4.1 mg/L dissolved organic carbon | VOC = 0.53 V (10 cell pairs, 78% permselectivity), Pd,max = 0.07 W/m2; with NaCl solutions at the same conductivity, Pd,max = 0.09 W/m2 indicated effects of NOM and divalent ions | [398] |
| MD brine 4, 5 or 5.4 M NaCl (from 1 M feed, i.e., SWRO retentate) | Seawater 0.5 M NaCl | At 20 °C and 0.7 cm/s, VOC = 1.23–2.1 V (25 cell pairs), Pd,max = 0.45–1.1 W/m2, water recovery 92%; at 10–50 °C, 5 M and 0.7 cm/s, VOC ≈ 1.7 and Pd,max ≈ 0.5–1.05 W/m2; at 20 °C, 5 M and 1.1 cm/s, Pd,max ≈ 1.1 W/m2 and Pd,max,net ≈ 0.67 W/m2 | [541] Figure 33b |
| • SWRO brine 1 M NaCl • MD brine 5 M NaCl | • Brackish water 0.1 M NaCl • Seawater 0.5 M NaCl | VOC (25 cell pairs), Pd,max: 1 M–0.1 M → 2.1 V, 0.39 W/m2; 5 M–0.1 M → 3.4 V, 1.5 W/m2 (Pd,max,net ≈ 1.2 W/m2, H2 production by alkaline polymer electrolyte water electrolysis cell 44 cm3/(h·cm2)); 1 M–0.5 M → 0.71 V, 0.05 W/m2; 5 M–0.5 M → 1.9 V, 0.55 W/m2 | [542] |
| MD brine 2–5 M NaCl (from 1 M feed, i.e., SWRO retentate) | Seawater 0.5 M NaCl | At 20–60 °C, water recovery 75–95%, VOC = 1.26–1.95 V (25 cell pairs), Pd,max ≈ 0.22–1.1 W/m2, exergetic efficiency 49% under best conditions, electrical energy consumption (1.3 kWh/m3) reduced by 23% and Espec (4.4 kWh/m3) reduced by 16.6% through RED inclusion | [536] Figure 33b |
| SWRO brine 1.1–1.5 M NaCl (RO with 30–50% water recovery from 43 g/L feed, i.e., high salinity seawater) | MCDI brine ~0.023 M NaCl (MCDI with 50–80% water recovery from ~0.85 g/L feed, i.e., SWRO permeate) | Pd,max ≈ 2.45–2.83 W/m2, Espec = 2.0 kWh/m3 reduced by ~39% compared to RO-RO and by ~17% compared to RO-RO-RED | [537] Figure 33c |
| • ED diluate 38.1 mS/cm (from ED of real seawater in salt production plant) • Seawater 48.7 mS/cm | Distilled water 0.2 mS/cm (from evaporation of ED brine in salt production plant) | VOC (10 cell pairs), Pd,max: 38.1–0.2 mS/cm → 2.02 V, ~0.23 W/m2; 48.7–0.2 mS/cm → 2.1 V, ~0.26 W/m2 | [543] |
| ED brine (from ED of simulated seawater at 30 g/L sea crystal) | Simulated wastewater 0.8 g/L NaCl, 0.1 g/L KH2PO4, 0.1 g/L NH4Cl, 0.5 g/L glucose | Partial desalination of seawater (~60%) by consuming only the electrical energy produced by RED | [538] Figure 33d |
| Desalination brine 1.2 M NaCl | • Brackish water 0.02 M NaCl • Seawater 0.6 M NaCl | Partial desalination of brackish water (~75%) or seawater (~50%) by consuming only the self-produced electrical energy, the developed model predicted enhanced desalination by changing the operating conditions | [539] Figure 33e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. https://doi.org/10.3390/membranes10070146
Gurreri L, Tamburini A, Cipollina A, Micale G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes. 2020; 10(7):146. https://doi.org/10.3390/membranes10070146
Chicago/Turabian StyleGurreri, Luigi, Alessandro Tamburini, Andrea Cipollina, and Giorgio Micale. 2020. "Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives" Membranes 10, no. 7: 146. https://doi.org/10.3390/membranes10070146
APA StyleGurreri, L., Tamburini, A., Cipollina, A., & Micale, G. (2020). Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes, 10(7), 146. https://doi.org/10.3390/membranes10070146