Continuous Production of Galacto-Oligosaccharides by an Enzyme Membrane Reactor Utilizing Free Enzymes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Enzyme Activity
2.3. Batch Conversion
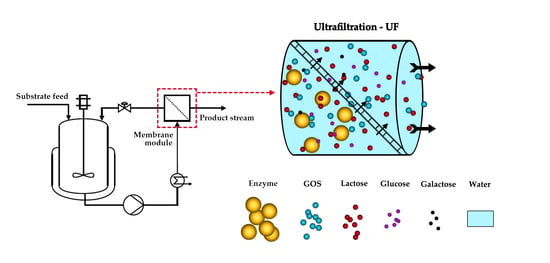
2.4. Enzyme Membrane Reactor (EMR)
2.5. Terminology
- Relative mass fraction () was calculated as the ratio of the mass of a saccharide fraction i () to the total mass of saccharides present in the solution:
- Relative mass percentage was the relative mass fraction ( ) expressed in percentage;
- Residence time (τ) was given as the weight of the reaction liquor in the reactor (mR) divided by the mass flow rate of the permeate (q):
- Yield (Y) was defined as the concentration of the generated DP3-6 fractions ( divided by the concentration of lactose in the feed ( ):
- Biocatalyst productivity (P) was the total quantity of DP3-6 formed by one unit of crude enzyme preparation per hour:
2.6. Preliminary Filtration Tests
2.6.1. Pressure-Scan
2.6.2. Determination of Limiting Flux
2.6.3. Membrane Cleaning
- The membrane plant was drained and flashed several times with deionized water.
- Membrane cleaning was carried out by circulating a NaOH solution (pH = 10–11) for 1–2 h at 40–50 °C under 0.5–1 bar pressure.
- The plant was drained and flushed several times with water to remove the cleaning agent.
- Permeability of the cleaned membrane was measured with DI water. In certain cases, when the original permeability of the membrane (<25%) was not recovered by the alkaline cleaning procedure, then additional cleaning with citric acid and/or Ultrasil (Ecolab, Paul, MN, USA) was performed (1 w/w%, 40–50 °C, 0.5–1 bar, 0.5–1 h).
2.7. Short-Term Catalytic Runs
2.8. Long-Term Catalyst Runs
2.9. Statistical Test
2.10. High Performance Liquid Chromatography
3. Results and Discussion
3.1. Preliminary Filtration Experiments
3.1.1. Pressure-Scan
3.1.2. Limiting Flux
3.2. Catalytic Performance
3.2.1. Batch Conversion in STR
3.2.2. Short-Term Runs in EMR
3.2.3. Long-Term Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GRAS | generally recognized as safe |
| QPS | qualified presumption of safety |
| DP | degree of polymerization |
| DP2 | disaccharides (lactose and non-lactose) |
| DP3-6 | galacto-oligosaccharide fractions with a degree of polarization between 3 and 6 |
| EMR | enzymatic membrane reactor |
| GOS | galacto-oligosaccharides |
| RMSE | root mean squared error |
| SSE | sum of squares due to error |
| STR | stirred tank reactor |
| UF ONPG | ultrafiltration ortho-Nitrophenyl-β-galactoside |
| List of symbols | |
| cb | bulk concentration of retained compounds (g·kg−1) |
| cE | enzyme concentration in reaction liquid (U·g−1) |
| cL | lactose concentration in feed (g·kg−1) |
| clim | limiting concentration of retained compounds (g·kg−1) |
| Jlim | permeate flux in Equation (5) (kg·h−1·m−2) |
| k | mass transfer coefficient (kg·h−1·m−2) |
| P | biocatalyst productivity (g·U−1·h−1) |
| q | permeate mass flow rate (kg·h−1) |
| t | operational time (h) |
| Y | yield of DP3-6 (w/w%) |
| Greek letters | |
| τ | residence time (h) |
References
- Carol, T.C.; Linda, T. Generally Recognized as Safe (GRAS) Determination for the Use of Galacto-Oligosaccharides as a Food Ingredient. In GRAS Notice 000489; FDA: Silver Spring, MD, USA, 2013. [Google Scholar]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.; Tomar, S.; Singh, R.; Singh, A.; Ali, B. Galactooligosaccharides: Novel components of designer foods. J. Food Sci. 2011, 76, R103–R111. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Forsythe, S.J.; El-Nezami, H. Probiotics interaction with foodborne pathogens: A potential alternative to antibiotics and future challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 3320–3333. [Google Scholar] [CrossRef] [PubMed]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [Green Version]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Global Galactooligosaccharibeds (GOS) Market Insights, Forecast to 2025. Available online: https://www.360marketupdates.com/global-galactooligosaccharides-gos-market-13729874 (accessed on 16 June 2020).
- Chen, X.Y.; Gänzle, M.G. Lactose and lactose-derived oligosaccharides: More than prebiotics? Int. Dairy J. 2017, 67, 61–72. [Google Scholar] [CrossRef]
- Park, A.-R.; Oh, D.-K. Galacto-oligosaccharide production using microbial β-galactosidase: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Warmerdam, A.; Zisopoulos, F.K.; Boom, R.M.; Janssen, A.E. Kinetic characterization of galacto-oligosaccharide (GOS) synthesis by three commercially important β-galactosidases. Biotechnol. Prog. 2014, 30, 38–47. [Google Scholar] [CrossRef]
- Martins, G.N.; Ureta, M.M.; Tymczyszyn, E.E.; Castilho, P.; Gomez-Zavaglia, A. Technological aspects of the production of fructo and galacto-oligosaccharides. Enzymatic synthesis and hydrolysis. Front. Nutr. 2019, 6, 78. [Google Scholar] [CrossRef]
- Su, Z.; Luo, J.; Li, X.; Pinelo, M. Enzyme membrane reactors for production of oligosaccharides: A review on the interdependence between enzyme reaction and membrane separation. Sep. Purif. Technol. 2020, 243. [Google Scholar] [CrossRef]
- Tymczyszyn, E.; Santos, M.; Costa, M.d.C.; Illanes, A.; Gómez-Zavaglia, A. History, synthesis, properties, applications and regulatory issues of prebiotic oligosaccharides. In Carbohydrates Applications in Medicine; Research Signpost: Kerala, India, 2014. [Google Scholar]
- Kovács, Z.; Benjamins, E.; Grau, K.; Rehman, A.U.; Ebrahimi, M.; Czermak, P. Recent developments in manufacturing oligosaccharides with prebiotic functions. In Biotechnology of Food and Feed Additives; Springer: Berlin, Heidelberg, Germany, 2013; pp. 257–295. [Google Scholar] [CrossRef]
- Illanes, A.; Guerrero, C.; Vera, C.; Wilson, L.; Conejeros, R.; Scott, F. Enzymatic production of galacto-oligosaccharides. In Lactose-Derived Prebiotics, 1st ed.; Academic Press: Valparaíso, Chile, 2016. [Google Scholar]
- Satyawali, Y.; Vanbroekhoven, K.; Dejonghe, W. Process intensification: The future for enzymatic processes? Biochem. Eng. J. 2017, 121, 196–223. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Athanasopoulos, V.I.; Niranjan, K.; Rastall, R.A. Synthesis of galacto-oligosaccharide from lactose using β-galactosidase from Kluyveromyces lactis: Studies on batch and continuous UF membrane-fitted bioreactors. Biotechnol. Bioeng. 2005, 89, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Czermak, P.; Ebrahimi, M.; Grau, K.; Netz, S.; Sawatzki, G.; Pfromm, P.H. Membrane-assisted enzymatic production of galactosyl-oligosaccharides from lactose in a continuous process. J. Membr. Sci. 2004, 232, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, M.; Placido, L.; Engel, L.; Shams-Ashaghi, K.; Czermak, P. Two-Stage Integrated Ceramic Membrane Reactor System For The Continuous Enzymatic Synthesis Of Oligosaccharides. In Proceedings of the WFC10: Discover the Future of Filtration & Separation, Leipzig, Germany, 14–18 April 2008; pp. II-492–II-496. [Google Scholar]
- Foda, M.I.; Lopez-Leiva, M. Continuous production of oligosaccharides from whey using a membrane reactor. Process. Biochem. 2000, 35, 581–587. [Google Scholar] [CrossRef]
- Gonzalez, R.; Ebrahimi, M.; Czermak, P. Experimental and modeling study of Galactosyl-Oligosaccharides formation in continuous recycle membrane reactors (CRMR). Open Food Sci. J. 2009, 3, 1–9. [Google Scholar] [CrossRef]
- Pocedičová, K.; Čurda, L.; Mišún, D.; Dryáková, A.; Diblíková, L. Preparation of galacto-oligosaccharides using membrane reactor. J. Food Eng. 2010, 99, 479–484. [Google Scholar] [CrossRef]
- Ren, H.; Fei, J.; Shi, X.; Zhao, T.; Cheng, H.; Zhao, N.; Chen, Y.; Ying, H. Continuous ultrafiltration membrane reactor coupled with nanofiltration for the enzymatic synthesis and purification of galactosyl-oligosaccharides. Sep. Purif. Technol. 2015, 144, 70–79. [Google Scholar] [CrossRef]
- Córdova, A.; Astudillo, C.; Guerrero, C.; Vera, C.; Illanes, A. Assessment of the fouling mechanisms of an ultrafiltration membrane bioreactor during synthesis of galacto-oligosaccharides: Effect of the operational variables. Desalination 2016, 393, 79–89. [Google Scholar] [CrossRef]
- Córdova, A.; Astudillo, C.; Vera, C.; Guerrero, C.; Illanes, A. Performance of an ultrafiltration membrane bioreactor (UF-MBR) as a processing strategy for the synthesis of galacto-oligosaccharides at high substrate concentrations. J. Biotechnol. 2016, 223, 26–35. [Google Scholar] [CrossRef]
- Matella, N.; Dolan, K.; Lee, Y.S. Comparison of galactooligosaccharide production in free-enzyme ultrafiltration and in immobilized-enzyme systems. J. Food Sci. 2006, 71, C363–C368. [Google Scholar] [CrossRef]
- Das, R.; Sen, D.; Sarkar, A.; Bhattacharyya, S.; Bhattacharjee, C. A comparative study on the production of galacto-oligosaccharide from whey permeate in recycle membrane reactor and in enzymatic batch reactor. Ind. Eng. Chem. Res. 2011, 50, 806–816. [Google Scholar] [CrossRef]
- Splechtna, B.; Nguyen, T.-H.; Haltrich, D. Comparison between discontinuous and continuous lactose conversion processes for the production of prebiotic galacto-oligosaccharides using β-galactosidase from Lactobacillus reuteri. J. Agric. Food Chem. 2007, 55, 6772–6777. [Google Scholar] [CrossRef] [PubMed]
- Petzelbauer, I.; Splechtna, B.; Nidetzky, B. Development of an ultrahigh-temperature process for the enzymatic hydrolysis of lactose. III. Utilization of two thermostable β-glycosidases in a continuous ultrafiltration membrane reactor and galacto-oligosaccharide formation under steady-state conditions. Biotechnol. Bioeng. 2002, 77, 394–404. [Google Scholar] [CrossRef]
- Warmerdam, A.; Boom, R.M.; Janssen, A.E. β-galactosidase stability at high substrate concentrations. SpringerPlus 2013, 2, 402. [Google Scholar] [CrossRef] [Green Version]
- Pázmándi, M.; Maráz, A.; Ladányi, M.; Kovács, Z. The impact of membrane pretreatment on the enzymatic production of whey-derived galacto-oligosaccharides. J. Food Process. Eng. 2018, 41, e12649. [Google Scholar] [CrossRef]
- Paulen, R.; Foley, G.; Fikar, M.; Kovács, Z.; Czermak, P. Minimizing the process time for ultrafiltration/diafiltration under gel polarization conditions. J. Membr. Sci. 2011, 380, 148–154. [Google Scholar] [CrossRef]
- Élysée-Collen, B.; Lencki, R.W. Protein ultrafiltration concentration polarization layer flux resistance I. Importance of protein layer morphology on flux decline with gelatin. J. Membr. Sci. 1997, 129, 101–113. [Google Scholar] [CrossRef]
- Yazdanshenas, M.; Tabatabaeenezhad, A.; Roostaazad, R.; Khoshfetrat, A. Full scale analysis of apple juice ultrafiltration and optimization of diafiltration. Sep. Purif. Technol. 2005, 47, 52–57. [Google Scholar] [CrossRef]
- Ma, S.; Kassinos, S.C.; Kassinos, D. Direct simulation of the limiting flux: I. Interpretation of the experimental results. J. Membr. Sci. 2009, 337, 81–91. [Google Scholar] [CrossRef]
- Palai, T.; Mitra, S.; Bhattacharya, P.K. Kinetics and design relation for enzymatic conversion of lactose into galacto-oligosaccharides using commercial grade β-galactosidase. J. Biosci. Bioeng. 2012, 114, 418–423. [Google Scholar] [CrossRef] [PubMed]







| Component | No3 | No5 | No2 | No7 | No4 | No6 | No1 | No8 | Batch |
|---|---|---|---|---|---|---|---|---|---|
| τ [h] | 1.1 | 2.1 | 2.2 | 2.6 | 1.1 | 2.1 | 2.2 | 2.8 | 6.0 |
| cE [U·g−1] | 19.1 | 17.3 | 19.1 | 19.1 | 190.6 | 173.4 | 190.6 | 190.6 | 5.7 |
| τ × cE [U·h·g−1] | 21.5 | 36.1 | 41.9 | 49.8 | 215.4 | 360.8 | 423.6 | 537.5 | 34.3 |
| P [g·h−1·U−1] × 10−3 | 3.42 | 2.28 | 2.32 | 1.87 | 0.46 | 0.28 | 0.23 | 0.18 | 3.28 |
| DP2 | 63.8 | 61.7 | 50.5 | 53.8 | 45.0 | 41.9 | 40.2 | 41.7 | 44.2 |
| Glu | 11.7 | 10.5 | 17.1 | 14.3 | 18.6 | 20.8 | 20.8 | 22.2 | 17.2 |
| Gal | 0.0 | 0.4 | 0.0 | 0.9 | 3.3 | 3.8 | 6.2 | 4.2 | 1.0 |
| DP3 | 19.7 | 22.0 | 22.9 | 22.9 | 21.6 | 20.6 | 20.6 | 20.7 | 25.0 |
| DP4 | 4.4 | 5.4 | 7.5 | 6.8 | 7.9 | 8.8 | 8.3 | 8.6 | 10.5 |
| DP5 | 0.6 | 0.0 | 1.9 | 1.3 | 2.7 | 4.0 | 3.7 | 2.6 | 2.0 |
| DP6 | 0.0 | 0.0 | 0.2 | 0.0 | 1.0 | 0.0 | 0.1 | 0.0 | 0.1 |
| DP3-6 | 24.6 | 27.4 | 32.4 | 31.0 | 33.2 | 33.4 | 32.8 | 31.9 | 37.6 |
| Response Variable | Model Parameters | Goodness of Fit | ||||
|---|---|---|---|---|---|---|
| b1 | b2 | SSE | R2 | Adjusted-R2 | RMSE | |
| DP2 | 3.932 | 0.06594 | 65.39 | 0.9779 | 0.9754 | 2.695 |
| DP3-6 | 5.224 | 0.1547 | 22.26 | 0.9769 | 0.9744 | 1.573 |
| Glu | 0.951 | 0.04417 | 28.26 | 0.9307 | 0.923 | 1.772 |
| Gal | 0.01835 | 0.002243 | 28.26 | 0.9319 | 0.9244 | 0.5721 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, T.; Pázmándi, M.; Galambos, I.; Kovács, Z. Continuous Production of Galacto-Oligosaccharides by an Enzyme Membrane Reactor Utilizing Free Enzymes. Membranes 2020, 10, 203. https://doi.org/10.3390/membranes10090203
Cao T, Pázmándi M, Galambos I, Kovács Z. Continuous Production of Galacto-Oligosaccharides by an Enzyme Membrane Reactor Utilizing Free Enzymes. Membranes. 2020; 10(9):203. https://doi.org/10.3390/membranes10090203
Chicago/Turabian StyleCao, Teng, Melinda Pázmándi, Ildikó Galambos, and Zoltán Kovács. 2020. "Continuous Production of Galacto-Oligosaccharides by an Enzyme Membrane Reactor Utilizing Free Enzymes" Membranes 10, no. 9: 203. https://doi.org/10.3390/membranes10090203
APA StyleCao, T., Pázmándi, M., Galambos, I., & Kovács, Z. (2020). Continuous Production of Galacto-Oligosaccharides by an Enzyme Membrane Reactor Utilizing Free Enzymes. Membranes, 10(9), 203. https://doi.org/10.3390/membranes10090203